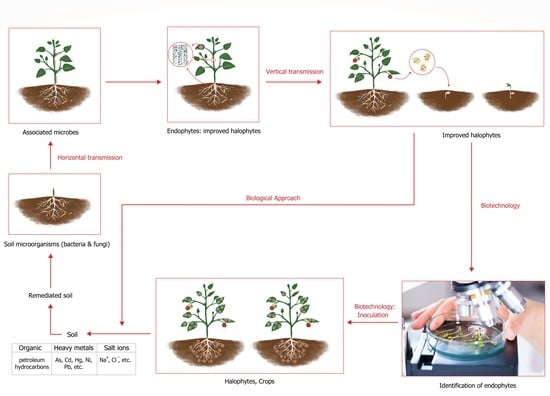
Endophytes and Halophytes to Remediate Industrial Wastewater and Saline Soils: Perspectives from Qatar
Abstract
:1. Introduction
2. Mechanisms of Nature: A Brief Glance
2.1. Avoidance Mechanisms
2.1.1. Exclusion Mechanisms
2.1.2. Extrusion Mechanism
2.1.3. Inclusion Mechanism
2.2. Tolerance Mechanism
3. Phytoremediation in Saline and Polluted Soils
3.1. Desalination of Soil
| Plants | Habitat & Distribution | Remarks & Roles | References | |
|---|---|---|---|---|
| Remarks | Roles | |||
| Aerluropus spp. (Monocot) | Highly saline sandy soil, shallow Sabkhas | Not succulent, extrusion mechanism with high selectivity to Na+ | Efficient Na+ accumulator, recommended remediator | [46,47] |
| Anabasis setifera (Dicot) | Periphery of Sabkhas, stressed in dry and saline soils | Succulent leaves, it is a facultative halophyte, inclusion mechanism | Accumulates substantial amount of Na+ & Cl− | [20,48,49] |
| Arthrocnemum meridionale * (Dicot) | Tidal zone and Sabkha depressions | Succulent shoots, inclusion mechanism | Efficient Na+ & Cl− accumulator | [36,50] |
| Atriplex leucoclada (Dicot) | Saline sandy soil, Sabkhas, and coastlines | Not succulent, extrusion mechanism | Reduces soil salts (desalination), efficient Na+ & Cl− absorption | [51] |
| Avicennia marina (Dicot) | Muddy tidal zone | Not succulent, much accumulation of Na+ and Cl−, sugar accumulation | Restoration program & desalination | [52] |
| Cleome spp. (Dicot) | Sandy coastal soil | Not succulent | Needs to be evaluated | [53] |
| Cressa cretica (Dicot) | Moist saline soils & Sabkhas | Not succulent, high salt tolerance | Herbal medicine (antibacterial and anti-fungi), possible role of associated bacteria | [54] |
| Cyperus spp. (Monocot) | Coastal saline areas, Agric. fields | Not succulent, tolerance mechanism is operating, medicinal plants | Possible desalination role, revegetation of salt affected lands | [55] |
| Frankenia pulverulenta (Dicot) | Moist saline soils | Not succulent, medicinal plant | Accumulates Na+ & Cl−, less K+ | [56] |
| Halocnemum strobilaceum (Dicot) | Salt flats | Succulent shoots | Accumulates Na+ & Cl−, and remediates saline soil | [14,57] |
| Halodule uninervis (Monocot) | Marine, shallow depths | Not succulent, accumulates Na+, Cl−, and K+ | Remediates sea water | [33,58] |
| Halopeplis perfoliata (Dicot) | Highly saline Sabkhas with sandy shelly soil | Succulent shoots, high Na+ and Cl− content, accumulation of compatible solutes | Remediate saline patches | [43,59] |
| Halopyrum mucronatum (Monocot) | Coastal dunes | Not succulent, seawater inhibits its germination | Possible remediation role at vegetative stage and bioenergy crops | [60] |
| Haloxylon sp. (Dicot) | Highly saline patches | Succulent stems, highly salt-tolerant, some species are xerophytes | Accumulates Na+ & Cl−, phytoremediation role is possible | [61] |
| Heliotropium spp. (Dicot) | Saline sandy soil, fields and gardens | Not succulent, found at saline, alkaline, and dry soils | Phytoremediation role is possible | [14,62] |
| Juncus rigidus (Monocot) | Swamp brackish waters | Not succulent | Phytoremediation of organic compounds, heavy metals, and saline soil | [63] |
| Limonium axillare (Dicot) | Coastline with saline shelly soil | Succulent leaves, extrusion mechanism is operating, succulent plant | Useful in Phytoremediation of saline soil | [22,64] |
| Polypogon monspeliensis (Monocot) | Gardens and fields, near the sea shores and salt marshes | Not succulent, suitable for saline soils and rich of Zn | Salinity can alleviate the toxicity of Zn | [65] |
| Salicornia europaea (Dicot) | Muddy salty tidal zones | Succulent, model for salt tolerance studies | Possible saline crop, phytoremediation of salts at constructed wetlands | [66,67] |
| Salsola sp. (Dicot) | Moist saline soil, coastal sand dunes | Succulent, inclusion mechanism is operating, high content of Na+ and Cl− | Possible phytoremediation of saline soils | [62] (This article covered many halophytes) |
| Seidlitzia rosmarinus (Dicot) | Very well adapted at dry and saline lands | Succulent shoots, inclusion mechanism is operating, high content of Na+ and Cl− | Phytoremediation of saline soils | [59,68] |
| Sporobolus spp. (Monocot) | Moist saline sandy soils | Succulent, efficient extrusion & inclusion mechanisms are operating | Accumulate compatible solutes at cytoplasm, accumulate Na+ & Cl−, high root content of K+ | [69,70,71] |
| Suaeda spp. (Dicot) | Moist saline soil in Sabkhas | Succulent, inclusion mechanism is operating, high content of Na+ and Cl− | Possible phytoremediation of saline soils | [72] |
| Tamarix spp. (Dicot) | Moist saline soils, fields and depressions | Not succulent, extrusion mechanism is operating, high accumulation of salts | Phytoremediator of saline soils | [73,74] |
| Tetraena qatarensis (Dicot) | Found at many locations of Qatar, coastline, disturbed rocky and sandy areas | Succulent, inclusion mechanism is operating, high content of Na+ and Cl− | phytoremediator of saline soils | [14,48] |
| Teucrium polium (Dicot) | Saline and shallow depressions | Not succulent, needs confirmation about its phytoremediation activities | Medicinal plant, antimicrobial effects against some microbes | [13,75] |
3.2. Selective Absorption of Toxic Ions
4. Detoxification of Polluted Soils
4.1. Bio-Mining of Polluted Soils
4.2. Petroleum Hydrocarbons
5. Endophytic Microorganisms
| Plants | Endophytes | Roles & Characterizations | References |
|---|---|---|---|
| Aerluropus spp. (Monocot) | No reports | No reports | No reports |
| Anabasis. spp. (Anabasis setifera), (Dicot) | Amycolatopsis anabasis; Aurantimonas endophytica, Glycomyces anabasis | Isolated from roots | [156] |
| Arthrocnemum meridionale (Dicot) | Bradyrhizobium sp., Chromohalobacter canadensis, Halomonas sp., Psychrobacter sp., Rudaea cellulosilytica, Bacilli species | Bacterial consortia: isolated from different parts of the plant, many functions | [97,149,157] |
| Atriplex leucoclada (Dicot) | Various phyla, halotolerant bacteria: Bacillus, Halobacillus, and Kocuria | Nitrogen fixation | [158] |
| Avicennia marina (Dicot) | Large number of microbes: bacteria and fungi | Nitrogen fixation, phosphate solubilization, growth promotion in saline conditions, produces useful biological molecules | [159,160,161,162] |
| Cleome spp. (Dicot) | Enterobacter cloacae, Klebsiella pneumoniae, Kluyvera cryocrescens | Improves growth, establishes sustainable crop production | [163] |
| Cressa cretica (Dicot) | Bacteria and fungi, Planctomyces, Halomonas, Jeotgalibacillus | Rhizosphere and non-rhizosphere sources, Salt tolerant, mitigating saline stress | [54] |
| Cyperus spp. (Monocot) | Endophytic bacteria mercury resistant | Resistance to Hg, accumulate mercury | [107] |
| Frankenia pulverulenta (Dicot) | No reports | No reports | No reports |
| Halocnemum strobilaceum (Dcot) | Bacteria phyla: Actinobacteria and Firmicutes | Potential enzyme producers | [136] |
| Halodule uninervis (Monocot) | Bacteria such as: Bacillus, Jeotgalicoccus, Planococcus, Staphylococcus | Bacteria against pathogenic fungi: Phytophthora capsici, Pyricularia oryzae Pythium ultimum, Rhizoctonia solani | [164] |
| Halopeplis perfoliata (Dicot) | Some bacteria found in the soil associated with this species | Plays roles to improve Agriculture and industrial practices | [153] |
| Halopyrum mucronatum (Monocot) | Possible, needs investigation | No reports | No references |
| Haloxylon sp. (Dicot) | Bacteria: Streptomyces spp. and Inquilinus sp., fungi: Penicillium spp. are found at rhizosphere | Some other microbes thrive during phytoremediation of oil-contaminated soil | [165] |
| Heliotropium spp. (Dicot) | Endophytic fungi of various genera | Pharmaceutically significant, Natural products | [166] |
| Juncus rigidus (Monocot) | The family Sphingomonadaceae is the most abundant in the root endophytic community, other microorganisms involved | Phytoremediation: Petroleum compounds, heavy metal | [31,167] |
| Limonium axillare, spp. (Dicot) | Endophytic fungi: Alternaria and Fusarium | Might be a source of growth-promoting regulators (e.g., Gibberellines) | [168] |
| Polypogon monspeliensis (Monocot) | Rhizosphere microorganisms | Many physiological and biochemical parameters are activated, growth, and nutrition | [116,117] |
| Salicornia europaea (Dicot) | Endophytes such as Bacillus spp., Planococcus rifietoensis, Variovorax paradoxus, Arthrobacter agilis | Assistance to cope with salinity, producing 1-aminocyclopropane-1-carboxylate deaminase, Indole-3-acetic acid, Phosphate-solubilizing activities | [169,170] |
| Salsola sp. (Dicot) | Endophytes and rhizosphytes, bacteria: Actinobacteria & and possibly others | Bioactive secondary metabolites, production of antifungal metabolites, medical significance | [171,172] |
| Seidlitzia rosmarinus (Dicot) | Endophytes: Roots: Brevibacterium, Kocuria, Paenibacillus, Pseudomonas, Rothia, Staphylococcus Shoot: Brevibacterium, Halomonas, Planococcus Planomicrobium Pseudomonas Rothia, Staphylococcus, Stenotrophomonas | Improves plant fitness in saline soils, salt resistance, production of IAA, ACC (1-aminocyclopropane-1-carboxylate) deaminase, etc. | [173] |
| Sporobolus spp. (Monocot) | Fungal endophytes in the root system | Necessary for plant success in harsh environment | [174] |
| Suaeda spp. (Dicot) | Dominant phyla were Actinobacteria. Proteobacteria, Firmicutes, endophytic fungi such as Alternaria spp. and Phoma spp. were found in some species | Survival and stress resistance of the plant species. | [76] |
| Tamarix spp. (Dicot) | Various bacteria and fungi species in rhizosphere and endosphere. Bacteria: novel nickel (Ni)-resistant endophytic bacteria: Stenotrophomonas sp. S20, Pseudomonas sp. P21, and Sphingobium sp. S42, Fungi: Aspergillus sydowii, Eupenicillium crustaceum, Fusarium spp., Penicillium chrysogenum | Possible roles against bacteria, biotechnology roles, medical and agricultural roles | [175,176] |
| Tetraena spp. (Dicot) | Endophytic and rhizosphytic bacteria | The isolation and identification of populations of endophytic and rhizosphere bacteria, having antimicrobial potential | [136] |
| Teucrium polium (Dicot) | Two bacteria bacilli species, two fungi species, Penicillium spp. | Plays a role in growth and health | [177] |
6. Modern Approaches
- (1)
- Salinity problems: The selection of native plants able to regulate particular toxic ions has been considered as a new trend to desalinize soils. This subject is being investigated and surely needs biotechnological efforts in the coming years. Moreover, additional serious work is needed to find and recognize the microbial species that can boost native plants to alleviate salt stress in Sabkhas and saline patches. Some evidence was presented that some Bacilli species, adjacent to, or associated with, some halophytes, are promising in increasing the ability to accumulate Na+ ions and improving phytoextraction capacity during the restoration of saline lands.
- (2)
- Heavy metal pollution: Many halophytes proved efficient in resisting heavy metals by avoidance and tolerance mechanisms. Further investigations are needed to identify more native plants that are able to select particular heavy metals from polluted soils at either oil or gas fields. Regarding As and Hg metals, at least four halophytes proved efficient to accumulate As and Hg in gas fields; these are: Cyperus spp., Juncus rigidus, Polypogon monspeliensis, and Salicornia europaea. Therefore, more studies are needed to identify some microbes that might help in increasing the capacity of native plants to accumulate these heavy metals. Moreover, other halophytes with their endophytes were reported to accumulate heavy metals, and adopting modern technology might help in increasing their capacity to deal with heavy metals in polluted soils.
- (3)
- Organic and petroleum hydrocarbon pollution: Recent studies have shown many vital roles of some bacterial endophytes in the bioremediation (detoxification) of pollutants (organic and inorganic), plant litter, and other volatile compounds. For example, Singh et al. [184] have suggested that endophytes adapt, assemble, and colonize to promote plant growth by producing plant growth-promoting enzymes, making the host plants resistant to various environmental conditions. These enzymes include hydrolases, oxidoreductases, oxygenase, and peroxidases. These enzymes proved efficient in the degradation of pollutants [197].
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Epstein, E.; Norlyn, J.D.; Rush, D.W.; Kingsbury, R.W.; Kelley, D.B.; Cunningham, G.A.; Wrona, A.F. Saline culture of crops: A genetic approach. Science 1980, 210, 399–404. [Google Scholar] [CrossRef]
- Valipour, M.; Krasilnikof, J.; Yannopoulos, S.; Kumar, R.; Deng, J.; Roccaro, P.; Mays, L.; Grismer, M.E.; Angelakis, A.N. The evolution of agricultural drainage from the earliest times to the present. Sustainability 2020, 12, 416. [Google Scholar] [CrossRef] [Green Version]
- Yasseen, B.T.; Al-Thani, R.F. Ecophysiology of Wild Plants and Conservation Perspectives in the State of Qatar. Agric. Chem. 2013, 10, 55305. [Google Scholar] [CrossRef] [Green Version]
- Qadir, M.; Tubeileh, A.; Akhtar, J.; Larbi, A.; Minhas, P.S.; Khan, M.A. Productivity enhancement of salt-affected environments through crop diversification. Land Degrad. Dev. 2008, 19, 429–453. [Google Scholar] [CrossRef]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Hussain, T.; Huchzermeyer, B.; Ajmal Khan, M. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environ. Exp. Bot. 2013, 91, 22–29. [Google Scholar] [CrossRef]
- Karakas, S.; Dikilitas, M.; Tıpırdamaz, R. Phytoremediation of Salt-Affected Soils Using Halophytes. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Suganya, K.; Poornima, R.; Selvaraj, P.S.; Parameswari, E.; Kalaiselvi, P. The potential of halophytes in managing soil salinity and mitigating climate change for environmental sustainability. Environ. Conserv. J. 2021, 22, 103–110. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Phytoremediation of polluted soils and waters by native Qatari plants: Future perspectives. Environ. Pollut. 2020, 259, 13694. [Google Scholar] [CrossRef]
- Rabhi, M.; Karray-Bouraoui, N.; Medini, R.; Attia, H.; Athar, H.-u.-R.; Abdelly, C.; Smaoui, A. Seasonal variations in phytodesalination capacity of two perennial halophytes in their natural biotope. J. Biol. Res. Thessaloniki. 2010, 14, 181–189. [Google Scholar]
- Montesinos, E. Plant-associated microorganisms: A view from the scope of microbiology. Int. Microbiol. 2003, 6, 221–223. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfar, S.A.; Böer, B.; Al Khulaidi, A.W.; El-Keblawy, A.; Alateeqi, S. Plants of Sabkha Ecosystems of the Arabian Peninsula. Chapter 5. In Sabkha Ecosystems, Tasks for Vegetation Science; Gul, B., Böer, B., Khan, M., Clüsener-Godt, M., Hameed, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 49, pp. 55–80. [Google Scholar] [CrossRef]
- Abdel-Bari, E.M. The Flora of Qatar, The Dicotyledons, The Monocotyledons; Environmental Studies Centre, Qatar University: Doha, Qatar, 2012; Volumes 1, 2. [Google Scholar]
- Abdel-Bari, E.M.; Yasseen, B.T.; Al-Thani, R.F. Halophytes in the State of Qatar; Environmental Studies Center, University of Qatar: Doha, Qatar, 2007; ISBN 99921-52-98-2. [Google Scholar]
- Yasseen, B.T. Traits of wild plants in Qatar peninsula and research perspectives. J. Biol. Nat. 2016, 5, 52–66. [Google Scholar]
- Larcher, W. Physiological Plant Ecology: Eco-Physiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Agarie, S.; Shimoda, T.; Shimizu, Y.; Baumann, K.; Sunagawa, H.; Kondo, A.; Ueno, O.; Nakahara, T.; Nose, A.; Cushman, J.C. Salt tolerance, salt accumulation and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J. Exper. Bot. 2007, 58, 1957–1967. [Google Scholar] [CrossRef]
- Aslam, R.; Bostan, N.; Amen, N.-E.; Maria, M.; Safdar, W.A. Critical review on halophytes: Salt tolerant plants. J. Med. Plants Res. 2011, 5, 7108–7118. [Google Scholar]
- Longstreth, D.J.; Nobel, P.S. Salinity effects on leaf anatomy: Consequences for photosynthesis. Plant Physiol. 1979, 63, 700–703. [Google Scholar] [CrossRef] [Green Version]
- Abulfatih, H.A.; Abdel Bari, E.M.; Alsubaey, A.; Ibrahim, Y.M. Halophytes and Soil Salinity in Qatar. Qatar Univ. Sci. J. 2002, 22, 119–135. [Google Scholar]
- Flowers, T.J.; Yeo, A.R. Ion relations of plants under drought and salinity. Aust. J. Plant Physiol. 1986, 13, 75–91. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Abu-Al-Basal, M.A. Ecophysiology of Limonium axillare and Avicennia marina from the Coastline of Arabian Gulf-Qatar. J. Coast. Conserv. Plan. Manag. 2008, 12, 35–42. [Google Scholar] [CrossRef]
- Paleg, L.G.; Aspinall, D. The Physiology and Biochemistry of Drought Resistance in Plants; Academic Press: Sydney, Australia; New York, NY, USA; London, UK, 1981. [Google Scholar]
- Youssef, A.M.; Hassanein, R.A.; Hassanein, A.A.; Morsy, A.A. Changes in quaternary ammonium compounds, proline and protein profiles of certain halophytic plants under different habitat conditions. Pak. J. Biol. Sci. 2003, 6, 867–882. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Foolad, M.R. Roles of glycinebetaine and proline in improving plant abiotic resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Al-Thani, R.F.; Alhadi, F.A.; Abbas, R.A.A. Soluble sugars in plants under stress at the Arabian Gulf region: Possible roles of microorganisms. J. Plant Biochem. Physiol. 2018, 6, 224. [Google Scholar] [CrossRef]
- Wyn Jones, R.G.; Storey, R. Betaines. In Physiology and Biochemistry of Drought Resistance in Plants; Paleg, L.G., Aspinall, D., Eds.; Academic Press: Sydney, Australia, 1981; pp. 171–204. [Google Scholar]
- Ohnishi, N.; Murata, N. Glycinebetaine counteracts the inhibitory effects of salt stress on the degradation and synthesis of D1 protein during photoinhibition in Synechococcus sp. PCC 7942. Plant Physiol. 2006, 141, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Trovato, M.; Mattioli, R.; Costantino, P. Multiple roles of proline in plant stress tolerance and development. Rend. Lincei. 2008, 19, 325–346. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Solutes in native plants in the Arabian Gulf region and the role of microorganisms: Future research. J. Plant Ecol. 2018, 11, 671–684. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Perspectives of future water sources in Qatar by phytoremediation: Biodiversity at ponds and modern approach. Int. J. Phytoremediat. 2021, 23, 866–889. [Google Scholar] [CrossRef]
- Korphage, M.L.; Langhus, B.G.; Campbell, S. Technical Report: Project Final Report, Remediation of Leon Water Flood, Butler County, Kansas. Kansas Corporation Commission, Wichita, Kansas, USA; Kansas Corporation Commission, Oil and Gas Division; ALL Consulting; Kansas Biological Survey. 2003. Available online: www.osti.gov/servlets/purl/819317 (accessed on 1 September 2021).
- Khalafallah, A.A.; Geneid, Y.A.; Shaetaey, S.A.; Shaaban, B. Responses of the seagrass Halodule uninervis (Forssk.) Aschers.to hypersaline conditions. Egypt. J. Aquat. Res. 2013, 39, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Alavi, N.; Parseh, I.; Ahmadi, M.; Jafarzadeh, N.; Yari, A.R.; Chehrazi, M.; Chorom, M. Phytoremediation of total petroleum hydrocarbons (TPHs) from highly saline and clay soil using Sorghum halepenes (L.). pers. and Aeluropus littoralis (Guna) Parl. Soil Sedim. Contam. 2017, 26, 127–140. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Ajmal Khan, M.; Gul, B. Arthrocnemum macrostachyum: A potential case for agriculture using above seawater salinity. In Prospects for Saline Agriculture. Tasks for Vegetation Science; Ahmad, R., Malik, K.A., Eds.; Springer: Dordrecht, The Nederlands, 2002; Volume 37. [Google Scholar] [CrossRef]
- Liang, L.; Liu, W.; Sun, Y.; Huo, X.; Li, S.; Zhou, Q. Phytoremediation of heavy metal contaminated saline soils using halophytes: Current progress and future perspectives. Environ. Rev. 2017, 25, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M. Potential use of halophytes to remediate saline soils. BioMed Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef]
- Caparrós, P.G.; Ozturk, M.; Gul, A.; Batool, T.S.; Pirasteh-Anosheh, H.; Unal, B.T.; Altay, V.; Toderich, K.N. Halophytes have potential as heavy metal phytoremediators: A comprehensive review. Environ. Exp. Bot. 2022, 193, 104666. [Google Scholar] [CrossRef]
- Aljuburi, H.J.; Al-Masry, H.H. Effects of salinity and indole acetic acid on growth and mineral content of date palm seedlings. Fruits 2000, 55, 315–323. [Google Scholar]
- Yasseen, B.T. An Analysis of the Effects of Salinity on Leaf Growth in Mexican Wheats. Ph.D. Thesis, The University of Leeds, Leeds, UK, 1983. [Google Scholar]
- Gairola, S.; Bhatt, A.; El-Keblawy, A.A. Perspective on potential use of halophytes for reclamation of salt-affected lands. Wulfenia J. 2015, 22, 88–97. [Google Scholar]
- Lew, D. Mechanism of Salt Tolerance in Glycophytes and Halophytes. In Plant Adaptation; 2019; Available online: www.drdarrinlew.us/plant-adaptation/mechanism-of-salt-tolerance-in-glycophytes-and-halophytes.html (accessed on 1 June 2021).
- Al-Easa, H.S.; Risk, A.M.; Abdel-Bari, E.M. Chemical Constituents and Nutritive Values of Range Plants in Qatar; Qatar University, Scientific & Applied Research Centre, The Doha Modern Printing Press Ltd.: Doha, Qatar, 2003; p. 746. ISBN 99921-52-59-1. [Google Scholar]
- Gulzar, S.; Ajmal Khan, M.; Ungar, I.A. Effects of salinity on growth, ionic content and plant—water status of Aeluropus lagopoides. Commun. Soil Sci. Plant Analy. 2003, 34, 1657–1668. [Google Scholar] [CrossRef]
- Barhoumi, Z.; Djebali, W.; Abdelly, C.; Chaïbi, W.; Smaoui, A. Ultrastructure of Aeluropus littoralis leaf salt glands under NaCl stress. Protoplasma 2008, 233, 195–202. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Al-Thani, R.F. Halophytes and associated properties of natural soils in the Doha area, Qatar. AEHMS 2007, 10, 320–326. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Gairola, S.; Bhatt, A. Maternal salinity environment affects salt tolerance during germination in Anabasis setifera: A facultative desert halophyte. J. Arid Land. 2016, 8, 254–263. [Google Scholar] [CrossRef]
- Ajmal Khan, M.; Ungar, I.A.; Showalter, A.M. Salt stimulation and tolerance in an intertidal stem-succulent halophyte. J. Plant Nutr. 2005, 28, 1365–1374. [Google Scholar] [CrossRef]
- Mann, E.; Rutter, A.; Zeeb, B. Evaluating the efficacy of Atriplex spp. in the phytoextraction of road salt (NaCl) from contaminated soil. Environ. Pollut. 2020, 265, 11496. [Google Scholar] [CrossRef]
- Zouhaier, B.; Hussain, A.A.; Saleh, K.A. Traits allowing Avicennia marina propagules to overcome seawater salinity. Flora 2018, 242, 16–21. [Google Scholar] [CrossRef]
- Kulya, J.; Lontom, W.; Bunnag, S.; Theerakulpisut, P. Cleome gynandra L. (C4 plant) shows higher tolerance of salt stress than its C3 close relative, C. viscosa L. AAB Bioflux 2011, 3, 59–66. Available online: http://www.aab.bioflux.com.ro (accessed on 14 January 2022).
- Etemadi, N.; Müller, M.; Etemadi, M.; Brandón, M.G.; Ascher-Jenull, J.; Insam, H. Salt tolerance of Cressa cretica and its rhizosphere microbiota. Biologia 2020, 75, 355–366. [Google Scholar] [CrossRef]
- Mumtaz, S.; Saleem, M.H.; Hameed, M.; Batool, F.; Parveen, A.; Amjad, S.F.; Mahmood, A.; Arfan, M.; Ahmed, S.; Yasmin, H.; et al. Anatomical adaptations and ionic homeostasis in aquatic halophyte Cyperus laevigatus L. Under high salinities. Saudi J. Biol. Sci. 2021, 28, 2655–2666. [Google Scholar] [CrossRef]
- Bueno, M.; Lendínez, M.L.; Calero, J.; Cordovilla, M.P. Salinity responses of three halophytes from inland saltmarshes of Jaén (southern Spain). Flora 2020, 266, 151589. [Google Scholar] [CrossRef]
- Trofimova, T.A.; Hossain, A.; da Silva, J.A.T. The ability of medical halophytes to phytoremediate soil contaminated by salt and heavy metals in Lower Volga, Russia. Asian Australas. J. Plant Sci. Biotech. 2012, 6, 108–114. [Google Scholar]
- Al-Arbash, A.; Al-Bader, D.; Suleman, P. Structural and biochemical responses of the seagrass Halodule uninervis to changes in salinity in Kuwait Bay, Doha area. Kuwait J. Sci. 2016, 43, 131–142. [Google Scholar]
- Youssef, A.M. Salt tolerance mechanisms in some halophytes from Saudi Arabia and Egypt. Res. J. Agric. Biol. Sci. 2009, 5, 191–206. [Google Scholar]
- Sharma, R.; Wungrampha, S.; Singh, V.; Pareek, A.; Sharma, M.K. Halophytes as bioenergy crops. Front. Plant Sci. 2016, 7, 1372. [Google Scholar] [CrossRef] [Green Version]
- Ajmal Khan, M.; Ungar, I.; Showalter, A. Effects of sodium chloride treatments on growth and ion accumulation of the halophyte Haloxylon recurvum. Comm. Soil Sci. Plant Anal. 2000, 31, 2763–2774. [Google Scholar] [CrossRef]
- Devi, S.; Rani, C.; Datta, K.S.; Bishoni, S.K.; Mahala, S.C.; Angrish, R. Phytoremediation of soil salinity using salt hyperaccumulator plants. Indian J. Plant Physiol. 2008, 13, 347–356. [Google Scholar]
- Syranidou, E.; Christofilopoulos, S.; Kalogerakis, N. Juncus spp.—The helophyte for all (phyto) remediation purposes? New Biotech. 2017, 38, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Alhaddad, F.A.; Abu-Dieyeh, M.H.; Elazazi, E.M.; Ahmed, T. Salt tolerance of selected halophytes at the two initial growth stages for future management options. Sci. Rep. 2021, 11, 10194. [Google Scholar] [CrossRef]
- Ouni, Y.; Mateos-Naranjo, E.; Abdelly, C.; Lakhdar, A. Interactive effect of salinity and zinc stress on growth and photosynthetic responses of the perennial grass, Polypogon monspeliensis. Ecol. Eng. 2016, 95, 171–179. [Google Scholar] [CrossRef]
- Katschnig, D.; Broekman, R.; Rozema, J. Salt tolerance in the halophyte Salicornia dolichostachya moss: Growth, morphology and physiology. Environ. Exper. Bot. 2013, 92, 32–42. [Google Scholar] [CrossRef]
- Farzi, A.; Borghei, S.M.; Vossoughi, M. The use of halophytic plants for salt phytoremediation in constructed wetlands. Int. J. Phytoremediat. 2017, 19, 643–650. [Google Scholar] [CrossRef]
- Hadi, M.R. Biotechnological potentials of Seidlitzia rosmarinus: A mini review. Afr. J. Biotech. 2009, 8, 2429–2431. [Google Scholar]
- Naidoo, G.; Naidoo, Y. Salt tolerance in Sporobolus virginicus: The importance of ion relations and salt secretion. Flora 1998, 193, 337–344. [Google Scholar] [CrossRef]
- Mishra, N.K.; Sangwan, A. Phytoremediation of salt-affected soils: A review of processes, applicability, and the impact on soil health in Hisar. Haryana. Int. J. Engin. Sci. Inven. Res. Dev. 2016, 2. Available online: www.ijesird.com (accessed on 11 March 2021).
- Weragodavidana, P.S.B. Salt Gland Excretion Efficiency and Salinity Tolerance of Sporobolus Species. Master’s Thesis, United Arab Emirates University, College of Science, Department of Biology, Al Ain, United Arab Emirates, 2016. [Google Scholar]
- Ravindran, K.C.; Venkatesan, K.; Balakrishnan, V.; Chellappan, K.P.; Balasubramanian, T. Restoration of saline land by halophytes for Indian soils. Soil Biol. Biochem. 2007, 39, 2661–2664. [Google Scholar] [CrossRef]
- Imada, S.; Acharya, K.; Li, Y.-p.; Taniguchi, T.; Iwanaga, F.; Yamamoto, F.; Yamanaka, N. Salt dynamics in Tamarix ramosissima in the lower Virgin River floodplain, Nevada. Trees 2013, 27, 949–958. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Tian, Y.; Guo, K.; Sun, H.; Liu, X.; Chu, H.; Liu, B. Long-term phytoremediation of coastal saline soil reveals plant species-specific patterns of microbial community recruitment. mSystem 2020, 5, e00741-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariq, M.; Ageel, A.M.; al-Yahya, M.A.; Mossa, J.S.; al-Said, M.S. Anti-inflammatory activity of Teucrium polium. Int. J. Tissue React. 1989, 11, 185–188. [Google Scholar] [PubMed]
- Sun, Y.; Wang, Q.; Lu, X.D.; Okane, I.; Kakishima, M. Endophytic fungi associated with two Suaeda species growing in alkaline soil in China. Mycosphere 2011, 2, 239–248. [Google Scholar]
- Yasseen, B.T.; Jurjees, J.A.; Dawoud, H.S. Response of sugar beet leaf growth and its ionic composition to sodium chloride. J. Agric. Water Reso. Res. 1988, 7, 47–59. [Google Scholar]
- Flowers, T.J.; Läuchli, A. Sodium versus potassium: Substitution and compartmentation. In Inorganic Plant Nutrition; Läuchli, A., Bieleski, R.L., Eds.; Springer: Berlin, Germany, 1983; Volume 15, pp. 651–681. [Google Scholar]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Blackwood, G.C.; Miflin, B.J. The effects of salt on NADH malate dehydrogenase activity in maize and barley. Plant Sci. Lett. 1976, 7, 435–446. [Google Scholar] [CrossRef]
- Abu-Al-Basal, M.A.; Yasseen, B.T. Changes in growth variables and potassium content in leaves of black barley in response to NaCl. Braz. J. Plant Physiol. 2009, 21, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Schachtman, D.; Liu, W. Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 1999, 4, 281–287. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2007, 133, 651–669. [Google Scholar] [CrossRef]
- Jeschke, W.D. Net K+—Na+ exchange across the plasmalemma of meristematic root tissues. Z. Pflanzenphysiol. Bd. 1979, 94, 325–330. [Google Scholar] [CrossRef]
- Teaster, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Gassmann, W.; Schroeder, J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 1995, 270, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Matoh, T.; Watanabe, J.; Takahashi, E. Effects of sodium and potassium salts on the growth of a halophyte Atriplex gmelina. Soil Sci. Plant Nutr. 1986, 32, 451–459. [Google Scholar] [CrossRef]
- Kotula, L.; Garcia Caparros, P.; Zörb, C.; Colmer, T.D.; Flowers, T.J. Improving crop salt tolerance using transgenic approaches: An update and physiological analysis. Plant Cell Environ. 2020, 43, 2932–2956. [Google Scholar] [CrossRef]
- Wang, H.-L.; Tian, C.-Y.; Jiang, L.; Wang, L. Remediation of heavy metals contaminated saline soils: A halophyte choice? Environ. Sci. Technol. 2014, 48, 21–22. [Google Scholar] [CrossRef]
- Osuji, L.C.; Onojake, C.M. Trace heavy metals associated with crude oil: A case study of Ebocha-8 oil-spill-polluted site in Niger Delta, Nigeria. Chem. Biodivers. 2004, 1, 1708–1715. [Google Scholar] [CrossRef]
- Al-Sulaiti, M.Y.; Al-Shaikh, I.M.; Yasseen, B.; Ashraf, S.; Hassan, H.M. Ability of Qatar’s native plant species to phytoremediate industrial wastewater in an engineered wetland treatment system for beneficial water re-use. In Qatar Foundation Annual Research Forum Proceedings; Hamad bin Khalifa University Press (HBKU Press): Doha, Qatar, 2013; Volume 2013, p. EEO 010. [Google Scholar] [CrossRef]
- Yasseen, B.T. Phytoremediation of industrial wastewater from oil and gas fields using native plants: The research perspectives in the State of Qatar. Cent. Eur. J. Exp. Biol. 2014, 3, 6–23. [Google Scholar]
- Al-Khateeb, S.A.; Leilah, A.A. Heavy metals accumulation in the natural vegetation of eastern province of Saudi Arabia. J. Biol. Sci. 2005, 5, 707–712. [Google Scholar]
- Rezvani, M.; Zaefarian, F. Bioaccumulation and translocation factors of cadmium and lead in Aeluropus littoralis. Aust. J. Agric. Eng. 2011, 2, 114–119. [Google Scholar]
- Rafiee, M.; Rad, M.J.; Afshari, A. Bioaccumulation and translocation factors of petroleum hydrocarbons in Aeluropus littoralis. Environ. Health Engin. Manag. J. 2017, 4, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Andrades-Moreno, L. Accumulation and tolerance characteristics of cadmium in a halophytic Cd-hyperaccumulator, Arthrocnemum macrostachyum. J. Hazard. Mater. 2010, 184, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Torre, S.; Mateos-Naranjo, E.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Isolation of plant-growth-promoting and metal-resistant cultivable bacteria from Arthrocnemum macrostachyum in the Odiel marshes with potential use in phytoremediation. Mar. Pollut. Bull. 2016, 110, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Youghly, N.A.; Taher, H.S.; Youssef, H.H.; Elbous, M.M.; Fayez, M.; Hegazi, N.A.; El-Bana, M.I. Assessment of heavy metal contamination and tolerant bacteria associated with halophyte Arthrocnemum Macrostachyum in Lake Manzala, Egypt. Alfarama J. Basic Appl. Sci. 2021, 2, 263–284. [Google Scholar] [CrossRef]
- Lutts, S.; Lefèvre, I.; Delpérée, C.; Kivits, S.; Dechamps, C.; Robledo, A.; Correal, E. Heavy metal accumulation by the halophyte species Mediterranean Saltbush. J. Environ. Qual. 2004, 33, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.T.A.; Oliveira, O.M.C.; Triguis, J.A.; Queiroz, A.F.S.; Ferreira, S.L.C.; Martins, C.M.S.; Silva, A.C.M.; Falcão, B.A. Phytoremediation in mangrove sediments impacted by persistent total petroleum hydrocarbons (TPH’s) using Avicennia schaueriana. Mar. Pollut. Bull. 2013, 67, 130–136. [Google Scholar] [CrossRef]
- Toledo-Bruno, A.G.I.; Aribal, L.G.; Lustria, M.G.M.; Rico, A.; Marin, R.A. Phytoremediation potential of mangrove species at Pangasihan Mangrove Forest reserve in Mindanao, Philippines. J. Biodivers. Environ. Sci. 2016, 9, 142–149. [Google Scholar]
- He, J.; Strezov, J.V.; Kumar, R.; Weldekidan, H.; Jahan, S.; Dastjerdi, B.H.; Zhou, X.; Kan, T. Pyrolysis of heavy metal contaminated Avicennia marina biomass from phytoremediation: Characterisation of biomass and pyrolysis products. J. Clean. Prod. 2019, 234, 1235–1245. [Google Scholar] [CrossRef]
- Haribabu, T.E.; Sudha, P.N. Effect of heavy metals copper and cadmium exposure on the antioxidant properties of the plant Cleome gynandra. Int. J. Plant Environ. Sci. 2011, 1, 80–87. [Google Scholar]
- Abdul Latiff, A.A.; Abd Karim, A.T.; Ahmad, A.S.; Ridzuan, M.B.; Hung, Y.-T. Phytoremediation of metals in industrial sludge by Cyperus Kyllingia-Rasiga, Asystassia Intrusa and Scindapsus Pictus Var. Argyaeus plant species. Int. J. Integ. Eng. 2012, 4, 1–8. [Google Scholar]
- Badr, N.; Fawzy, M.; Al-Qahtani, K.M. Phytoremediation: An ecological solution to heavy metal-polluted soil and evaluation of plant removal ability. World Appl. Sci. J. 2012, 16, 1292–1301. [Google Scholar]
- Chayapan, P.; Kruatrachue, M.; Meetam, M.; Pokethitiyook, P. Phytoremediation potential of Cd and Zn by wetland plants, Colocasia esculenta L. Schott., Cyperus malaccensis Lam. and Typha angustifolia L., grown in hydroponics. J. Environ. Biol. 2015, 36, 1179–1183. [Google Scholar] [PubMed]
- Perez, A.; Martinaz, D.; Barraza, Z.; Marrugo-Negrete, J.L. Endophytic bacteria associated to genus Cyperus and Paspalum in soils with mercury contamination. Rev. UDCA Actual. Divulg. Científica 2016, 19, 67–76. [Google Scholar]
- Galfati, I.; Bilal, E.; Beji Sassi, A.; Abdallah, H.; Zaier, A. Accumulation of heavy metals in native plants growing near the phosphate treatment industry, Tunisia. Carpathian J. Earth Environ. Sci. 2011, 6, 85–100. [Google Scholar]
- Badr, N.; Faraj, M.; Mohamed, A.; Alshelmani, N.; El-Kailany, R.; Bobtana, F. Evaluation of the phytoremediation performance of Hammada scoparia and Halocnemum Strobilaceum for Cu, Fe, Zn and Cr accumulation from the industrial area in Benghazi, Libya. J. Med. Chem. Sci. 2020, 3, 138–144. [Google Scholar]
- Bobtana, F.; Elabbar, F.; Bader, N. Evaluation of Halocnemum Strobilaceum and Hammada Scoparia plants performance for contaminated soil phytoremediation. J. Medic. Chem. Sci. 2019, 3, 126–129. [Google Scholar]
- Durako, M.J.; Kenworthy, W.J.; Fatemy, S.M.R.; Valavi, H.; Thayer, G.W. Assessment of the toxicity of Kuwait crude oil on the photosynthesis and respiration of seagrasses of the northern Gulf. Mar. Pollut. Bull. 1993, 27, 223–227. [Google Scholar] [CrossRef]
- Bu-Olayan, A.H.; Thomas, B.V. Accumulation and translocation of trace metals in Halodule uninervis, in the Kuwait coast. Biosci. Biotechnol. Res. Asia 2010, 7, 25–32. [Google Scholar]
- Mujeeb, A.; Aziz, I.; Ahmed, M.Z.; Alvi, S.K.; Shafiq, S. Comparative assessment of heavy metal accumulation and bio-indication in coastal dune halophytes. Ecotoxico. Environ. Saf. 2020, 195, 110486. [Google Scholar] [CrossRef]
- Aurangzeb, N.; Irshad, M.; Hussain, F.; Mahmood, Q. Comparing heavy metals accumulation potential in natural vegetation and soil adjoining wastewater canal. J. Chem. Soc. Pak. 2011, 33, 661–665. [Google Scholar]
- Al-Jaloud, A.A.; Al-Saiady, M.Y.; Assaeed, A.M.; Chaudhary, S.A. Some halophyte plants of Saudi Arabia, their composition and relation to soil properties. Pak. J. Biol. Sci. 2001, 4, 531–534. [Google Scholar]
- Farzamisepeher, M.; Norouzi Haj Abdal, F.; Farajzadeh, M.A. Phytoremdiation potential of Polypogon monspeliensis L. in remediation of petroleum polluted soils. J. Plant Environ. Physiol. 2013, 8, 75–87. [Google Scholar]
- Farzamisepehr, M.; Nourozi, F. Physiological responses of Polypogon monspeliensis L. in petroleum-contaminated soils. Iranian J. Plant Physiol. 2018, 8, 2391–2401. [Google Scholar] [CrossRef]
- García-Mercado, H.D.; Villagómez, G.F.; Garzón-Zúñiga, M.A.; Duran, M.C. Fate of mercury in a terrestial biological lab process using Polypogon monspeliensis and Cyperus odoratus. Int. J. Phytoremediat. 2019, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Samreen, S.; Khan, A.A.; Khan, M.R.; Ansari, S.A.; Khan, A. Assessment of phytoremediation potential of seven weed plants growing in chromium- and nickel-contaminated soil. Water Air Soil Pollut. 2021, 232, 209. [Google Scholar] [CrossRef]
- Polypogon. Available online: https://en.wikipedia.org/wiki/Polypogon (accessed on 14 June 2021).
- Song, U.; Hong, J.E.; An, J.H.; Chung, J.S.; Moon, J.W.; Lim, J.H.; Lee, E.J. Heavy metal accumulation in halophyte Salicornia europaea and salt marsh in west-coast of Korea. J. Environ. Sci. 2011, 20, 483–491. [Google Scholar]
- Khalilzadeh, R.; Pirzad, A.; Sepehr, E.; Khan, S.; Anwar, S. The Salicornia europaea potential for phytoremediation of heavy metals in the soils under different times of wastewater irrigation in northwestern Iran. Environ. Sci. Pollut. Res. 2021, 28, 47605–47618. [Google Scholar] [CrossRef]
- Dragovic, R.; Zlatkovic, B.; Dragovic, S.; Petrovic, J.; Mandic, L.T. Accumulation of heavy metals in different parts of Russian thistle (Salsola tragus, chenopodiaceae), a potential hyperaccumulator plant species. Biol. Nyssana 2014, 5, 83–90. [Google Scholar]
- Centofanti, T.; Bañuelos, G. Evaluation of the halophyte Salsola soda as an alternative crop for saline soils high in selenium and boron. J. Environ. Mang. 2015, 157, 96–102. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Yang, H.; Li, X.; Cui, Z. Physiological responses of Suaeda glauca and Arabidopsis thaliana in phytoremediation of heavy metals. J. Environ. Manag. 2018, 223, 132–139. [Google Scholar] [CrossRef]
- Al-Taisan, W.A. Suitability of using Phragmites australis and Tamarix aphylla as vegetation filters in industrial areas. Am. J. Environ. Sci. 2009, 5, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Betancur-Galvis, L.A.; Carrillo, H.; Luna-Guido, M.; Marsch, R.; Dendooven, L. Enhanced dissipation of polycyclic aromatic hydrocarbons in the rhizosphere of the Athel Tamarisk (Tamarix Aphylla L. Karst.) grown in saline-alkaline soils of the former lake Texcoco. Int. J. Phytoremediat. 2012, 14, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Suska-Malawska, M.; Sulwiński, M.; Wilk, M.; Otarov, A.; Mętrak, M. Potential eolian dust contribution to accumulation of selected heavy metals and rare earth elements in the aboveground biomass of Tamarix spp. from saline soils in Kazakhstan. Environ. Monit. Assess. 2019, 191, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper and nickel tolerance and bioaccumulation by shrub plant Tetraena qatarensis. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaman, M. Teucrium as a novel discovered hyperaccumulator for the phytoextraction of Ni-contaminated soils. Ekoloji 2014, 23, 81–89. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; pp. 305–342. [Google Scholar]
- Al-Shalchi, W. Determination of Traces in Natural Gas; Baghdad. 2005. Available online: http://www.scribd.com/doc/3915859/Determination-of-Traces-in-Natural-Gas (accessed on 14 February 2019).
- Molina, J.A.; Oyarzun, R.; Esbrí, J.M.; Higueras, P. Mercury accumulation in soils and plants in the Almadén mining district, Spain: One of the most contaminated sites on Earth. Environ. Geochem. Health. 2006, 28, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Kachout, S.S.; Ben Mansoura, A.; Mechergui, R.; Leclerc, J.C.; Rejeb, M.N.; Ouerghi, Z. Accumulation of Cu, Pb, Ni and Zn in the halophyte plant Atriplex grown on polluted soil. J. Sci. Food Agric. 2012, 92, 336–342. [Google Scholar] [CrossRef]
- MacFarlane, G.R.; Pulkownik, A.; Burchett, M.D. Accumulation and distribution of heavy metals in the grey mangrove, Avicennia marina (Forsk.)Vierh.: Biological indication potential. Environ. Pollut. 2003, 123, 139–151. [Google Scholar] [CrossRef]
- Bibi, F.; Strobel, G.A.; Naseer, M.I.; Yasir, M.; Al-Ghamdi, A.A.K.; Azhar, E.I. Halophytes-associated endophytic and rhizospheric bacteria: Diversity, antagonism and metabolite production. Biocon. Sci. Technol. 2018, 28, 192–213. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Sheokand, S.; Dhansu, P.; Kumar, A.; Mann, A.; Kumar, N.; Devi, S.; Rani, B.; Kumar, A.; Meena, B.L. Halophyte Growth and Physiology Under Metal Toxicity, Chapter 5. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019. [Google Scholar] [CrossRef]
- Lefèevre, I.; Marchal, G.; Meerts, P.; Correal, E.; Lutts, S. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environ. Exp. Bot. 2009, 65, 142–152. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Kadukova, J.; Manousaki, E.; Kalogerakis, N. Pb and Cd accumulation and phytoexcretion by salt cedar (Tamarix smyrnensisBunge). Int. J. Phytoremediation. 2008, 10, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Manousaki, E.; Kalogerakis, N. Halophytes present new opportunities in phytoremediation of heavy metals and saline soils. Ind. Eng. Chem. Res. 2011, 50, 656–660. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Halophytes–An emerging trend in phytoremediation. Int. J. Phytoremediation. 2011, 13, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, R.F.; Yasseen, B.T. Biological soil crusts and extremophiles adjacent to native plants at Sabkhas and Rawdahs, Qatar: The Possible Roles. Front. Environ. Microbiol. 2018, 4, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Mushtaq, H.; Ali, U.; Hakim, M.; Ali, E.; Mubeen, S.; Mushtaq, H. Screening, isolation, biochemical and plant growth promoting characterization of endophytic bacteria. Microbiol. Curr. Res. 2018, 2, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Bacon, C.W.; White, J.F. Microbial Endophytes; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 341–388. [Google Scholar]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Frank, A.C.; Guzmán, J.P.S.; Shay, J.E. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.; Hassan, S.E.; Mahgoub, H.; Eid, A.; Ewais, E. Plant growth promoting activities of endophytic bacteria associated with the halophyte Arthrocnemum macrostachyum (Moric) K. Koch. Plant. N. Egypt. J. Microbiol. 2018, 49, 123–141. [Google Scholar]
- Yacine, G.; Zamoum, M.; Sabaou, N.; Zitouni, A. Endophytic actinobacteria from native plants of Algerian Sahara: Potential agents for biocontrol and promotion of plant growth, Chapter 7. In New and Future Developments in Microbial Biotechnology and Bioengineering. Actinobacteria: Diversity and Biotechnological Applications; Singh, B., Gupta, V., Passari, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 109–124. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Lucero, M.E.; Unc, A.; Cooke, P.; Dowd, S.; Sun, S. Endophyte microbiome diversity in micropropagated Atriplex canescens and Atriplex torreyi var griffithsii. PLoS ONE 2011, 6, e17693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeshen, M.N.; Moussa, T.A.A.; Ahmed, F.; Abdulfaraj, A.A.; Jalal, R.S.; Majeed, M.A.; Baeshen, N.A.; Huelsenbeck, J.P. Diversity profiling associated bacteria from the soils of stress tolerant plants from seacoast of Jeddah, Saudi Arabia. Appl. Environ. Res. 2020, 18, 8217–8231. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Halo-thermophilic bacteria and heterocyst cyanobacteria found adjacent to halophytes at Sabkhas —Qatar: Preliminary study and possible roles. Afr. J. Microb. Res. 2017, 11, 1346–1354. [Google Scholar]
- Al-Fayyad, H.J. Isolation, Characterization and Identification of Endophytic Microorganisms from Mangrove plants (Avicennia marina). BIOL 497-Senior Project, (2020–2021). Master’s Dissertation, Biological and Environmental Sciences Department, College of Arts and Sciences, Qatar University, Doha, Qatar.
- Wang, H.F.; Li, X.Y.; Gao, R.; Xie, Y.G.; Xiao, M.; Li, Q.L.; Li, W.J. Amycolatopsis anabasis sp. nov., a novel endophytic actinobacterium isolated from roots of Anabasis elatior. Int. J. Syst. Evol. Microbiol. 2020, 70, 3391–3398. [Google Scholar] [CrossRef]
- Mora-Ruiz, M.D.R.; Font-Verdera, F.; Orfila, A.; Rita, J.; Rosselló-Móora, R. Endophytic microbial diversity of the halophyte Arthrocnemum macrostachyum across plant compartments. FEMS Microbiol. Ecol. 2016, 92, fiw145. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, S.; Mehnaz, S.; Mirza, M.S.; Malik, K.A. Isolation and characterization of bacteria associated with the rhizosphere of halophytes (Salsola stocksii and Atriplex amnicola) for production of hydrolytic enzymes. Braz. J. Microbiol. 2019, 50, 85–97. [Google Scholar] [CrossRef]
- Janarthine, S.R.S.; Eganathan, P. Plant growth promoting of eEndophytic Sporosarcina aquimarina SjAM16103 isolated from the pneumatophores of Avicennia marina L. Int. J. Microbiol. 2012, 2012, 532060. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Shahzad, R.; Khan, A.L.; Halo, B.A.; Al-Yahyai, R.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Endophytic bacterial diversity of Avicennia marina helps to confer resistance against salinity stress in Solanum lycopersicum. J. Plant Interact. 2017, 12, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.K.; Gupta, M.K.; Sarma, V.V. Bioactive metabolites of endophytic fungi of Avicennia marina (Forssk.) Vierh. KAVAKA 2020, 55, 39–49. [Google Scholar] [CrossRef]
- Khalil, A.M.A.; Abdelaziz, A.M.; Khaleil, M.M.; Hashem, A.H. Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett. Appl. Microbiol. 2021, 72, 263–274. [Google Scholar] [CrossRef]
- Shipoh, P.M. Isolation, Identification and Characterization of Cleome gynandra L. Bacterial Seed Endophytes from Northern Namibia. Master’s Thesis, The University of Namibia, Wenhaok, Namibia, 2021. [Google Scholar]
- Bibi, F.; Naseer, M.I.; Hassan, A.M.; Yasir, M.; Al-Ghamdi, A.A.K.; Azhar, E.I. Diversity and antagonistic potential of bacteria isolated from marine grass Halodule uninervis. 3 Biotech 2018, 8, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ateeqi, S.S. Phytoremediation of Oil-Polluted Desert Soil in Kuwait Using Native Plant Species. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2014. [Google Scholar]
- Kuralarasi, R.; Gayalhri, M.; Lingakumar, K. Diversity of endophytic fungi from Heliotropium indicum L. leaf. World J. Pharmaceut. Res. 2018, 7, 1040–1047. [Google Scholar]
- Syranidou, E.; Thijs, S.; Avramidou, M.; Weyens, N.; Venieri, D.; Pintelon, I.; Vangronsveld, J.; Kalogerakis, N. Responses of the endophytic bacterial communities of Juncus acutus to pollution with metals, eEmerging organic pollutants and to bioaugmentation with indigenous strains. Front. Plant Sci. 2018, 9, 1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalmuratova, I.; Choi, D.-H.; Yoon, H.-J.; Yoon, T.-M.; Kim, J.-G. Diversity and plant growth promotion of fungal endophytes in five halophytes from the Buan salt marsh. J. Microbiol. Biotechnol. 2021, 31, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, N.; Zhao, Z.-Y.; Zhang, K.; Wu, G.-H.; Tian, C.-Y. Isolation of endophytic plant growth-promoting bacteria associated with the halophyte Salicornia europaea and evaluation of their promoting activity under salt stress. Curr. Microbiol. 2016, 73, 574–581. [Google Scholar] [CrossRef]
- Furtado, B.U.; Gołębiewski, M.; Skorupa, M.; Hulisz, P.; Hrynkiewicz, K. Bacterial and Fungal Endophytic Microbiomes of Salicornia europaea. Appl. Environ. Microbiol. 2019, 85, e00305-19. [Google Scholar] [CrossRef] [Green Version]
- Bibi, F.; Strobel, G.A.; Naseer, M.I.; Yasir, M.; Al-Ghamdi, A.A.K.; Azhar, E.I. Microbial flora associated with the halophyte—Salsola imbricate and its biotechnical potential. Front. Microbiol. 2018, 9, 65. [Google Scholar] [CrossRef]
- Razghandi, M.; Mohammadi, A.; Ghorbani, M.; Mirzaee, M.R. New fungal pathogens and endophytes associated with Salsola. J. Plant Protect. Res. 2020, 60, 362–368. [Google Scholar] [CrossRef]
- Shurigin, V.; Egamberdieva, D.; Li, L.; Davranov, K.; Panosyan, H.; Birkeland, N.-K.; Wirth, S.; Bellingrath-Kimura, S.D. Endophytic bacteria associated with halophyte Seidlitzia rosmarinus Ehrenb. ex Boiss. from saline soil of Uzbekistan and their plant beneficial traits. J. Arid Land 2020, 12, 730–740. [Google Scholar] [CrossRef]
- Khidir, H.H.; Eudy, D.M.; Porras-Alfaro, A.; Natvig, D.O.; Sinsabaugh, R.L. A general suite of fungal endophytes dominates roots of two dominant grasses in a semiarid grassland. J. Arid Environ. 2010, 74, 35–42. [Google Scholar] [CrossRef]
- Gashgari, R.; Gherbawy, Y.; Ameen, F.; Alsharari, S. Molecular characterization and analysis of antimicrobial activity of endophytic-from medicinal plants in Saudi Arabia. Jundishapur J. Microbiol. 2016, 9, e26157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, N.; Han, S.; Sun, Y.; Wang, L.; Qu, Z.; Dai, M.; Zhao, G. Characterization and bioremediation potential of nickel-resistant endophytic bacteria isolated from the wetland plant Tamarix chinensis. FEMS Microbiol. Lett. 2020, 367, fnaa098. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Advanced Res. 2017, 8, 687–695. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Barcia-Piedras, J.M.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Camacho, M.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Assessing the role of endophytic bacteria in the halophyte Arthrocnemum macrostachyum salt tolerance. Plant Bio. 2016, 19, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Torre, S.; Barcia-Piedras, J.M.; Caviedes, M.A.; Pajuelo, E.; Redondo-Gómez, S.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Bioaugmentation with bacteria selected from the microbiome enhances Arthrocnemum macrostachyum metal accumulation and tolerance. Mar. Pollut. Bull. 2017, 117, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Janarthine, S.R.S.; Eganathan, P.; Balasubramanian, T.; Vijayalakshmi, S. Endophytic bacteria isolated from the pneumatophores of Avicennia marina. Afr. J. Microbiol. 2011, 5, 4455–4466. [Google Scholar] [CrossRef]
- De Souza, M.P.; Huang, C.P.A.; Chee, N.; Terry, N. Rhizosphere bacteria enhance the accumulation of selenium and mercury in wetland plants. Planta 1999, 209, 259–263. [Google Scholar] [CrossRef]
- Koocheki, A.; Mahalati, M.N. Feed Value of Some Halophytic Range Plants of Arid Regions of Iran; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Bencherif, K.; Dalpé, Y.; Lounès-Hadj Sahraoui, A. Influence of Native Arbuscular Mycorrhizal Fungi and Pseudomonas fluorescens on Tamarix Shrubs Under Different Salinity Levels. In Microorganisms in Saline Environments: Strategies and Functions. Soil Biology; Giri, B., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 56, pp. 265–283. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Sidhu, G.K.; Datta, S.; Kumar, S.; Singh, J. Endophytic microbes in abiotic stress management. In Microbial Endophytes; Kumar, A., Singh, V.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 91–123. [Google Scholar]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biology. 2018, 60, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Qurashi, A.W.; Sabri, A.N. Osmoadaptation and plant growth promotion by salt tolerant bacteria under salt stress. Afr. J. Microbiol. Res. 2011, 5, 3546–3554. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, H.B.; Müller, R. Possibility of bacterial recruitment of plant genes associated with the biosynthesis of secondary metabolites. Plant Physiol. 2003, 132, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Karenlampi, S.; Schat, H.; Vangronsveld, J.; Verkleij, J.A.C.; van der Lelie, D.; Mergeay, M.; Tervahauta, A.I. Genetic engineering in the improvement of plants for phytoremediation of metal polluted soils. Environ. Pollut. 2000, 107, 225–231. [Google Scholar] [CrossRef]
- Gratão, P.L.; Prasad, M.N.V.; Cardoso, P.F.; Lea, P.J.; Azevedo, R.A. Phytoremediation: Green technology for the clean-up of toxic metals in the environment. Braz. J. Plant Physiol. 2005, 17, 53–64. [Google Scholar] [CrossRef]
- Fulekar, M.H.; Singh, A.; Bhaduri, A.M. Genetic engineering strategies for enhancing phytoremediation of heavy metals. Afr. J. Biotech. 2009, 8, 529–535. [Google Scholar]
- Van Aken, B.; Correa, P.A.; Schnoor, J.L. Phytoremediation of polychlorinated biphenyls: New trends and promises. Environ. Sci. Technol. 2010, 44, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Singh, M.K.; Singh, V.K.; Modi, A.; Jaiswal, P.; Rashmi, K.; Kumar, A. Microbial enzymes and their exploitation in remediation of environmental contaminants, Chapter 6. In Microbe Mediated Remediation of Environmental Contaminants; Kumar, A., Singh, V.K., Singh, P., Mishra, V.K., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 59–71. [Google Scholar] [CrossRef]


| Plants | Phytoremediation | References | |
|---|---|---|---|
| Inorganic | Organic | ||
| Aeluropus spp. (Monocot) | Cd, Pb | Petroleum hydrocarbons | [92,94,95] |
| Anabasis setifera (Dicot) | Mn, Cu | No reports | [93] |
| Arthrocnemum meridionale (Dicot) | Al, Cd, Cu, Fe, Mn, Zn | * | [96,97,98] |
| Atriplex leucoclada (Dicot) | Cd, Cu, Ni, Pb, Zn | * | [99] |
| Avicennia marina (Dicot) | Cd, Co, Cr, Cu, Fe, Ni, Zn | Petroleum hydrocarbons | [100,101,102] |
| Cleome spp. (Dicot) | Efficient: (Cd, Cu) | * | [103] |
| Cressa cretica (Dicot) | Some heavy metals | Possible petroleum hydrocarbons | [37] |
| Cyperus spp. (Monocot) | Al, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Zn, (Phyto-stabilization of Ni) | Petroleum hydrocarbons | [104,105,106,107] |
| Frankenia pulverulenta (Dicot) | Cd, Cr, Cu, Ni, Sr, Zn | Petroleum hydrocarbons | [108] |
| Halocnemum strobilaceum (Dicot) | Cd, Cu, Fe, Mn, Ni, Pb, Zn | * | [93,109,110] |
| Halodule uninervis (Monocot) | Cu, Fe, Ni, Pb | Petroleum hydrocarbons | [111,112] |
| Halopeplis perfoliata ** (Dicot) | Some heavy metals | Possible petroleum hydrocarbons | [12,14] |
| Halopyrum mucronatum (Monocot) | Some heavy metals, bioindicator for: Cr, Fe, Pb, Zn | No reports | [113] |
| Haloxylon sp. (Dicot) | Heavy metals: Cu, Fe, Mn, Zn | Possible petroleum hydrocarbons | [93] |
| Heliotropium spp. (Dicot) | Cd, Cr, Cu, Fe, Mn, Pb, Zn | * | [114] |
| Juncus rigidus (Monocot) | Cd, Cu, Fe, Hg, Mn | Denitrification & buffering methane emission. petroleum hydrocarbons | [37,63,115] |
| Limonium axillare * (Dicot) | Cd, Co, Cr, Cu, Fe, Ni, Zn | No reports | [14] |
| Polypogon monspeliensis (Monocot) | Cr, Hg, Ni, Zn | Petroleum hydrocarbons, TOG# | [116,117,118,119,120] |
| Salicornia europaea (Dicot) | Pb, Zn, Root stabilization: Cd, Cu, Ni | No reports | [121,122] |
| Salsola sp. (Dicot) | B, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Se, Zn | No reports | [93,123,124] |
| Seidlitzia rosmarinus (Dicot) | Some heavy metals | No reports | [14,68] |
| Sporobolus spp. (Monocot) | Some heavy metals, and toxic ions | Petroleum hydrocarbons | [70,92] |
| Suaeda spp. (Dicot) | Cd, Cu, Fe, Mn, Pb, Zn | No reports | [93,125] |
| Tamarix spp. (Dicot) | Cd, Cu, Fe, Mn, Ni, Pb, Zn | Polycyclic aromatic hydrocarbons | [126,127,128] |
| Tetraena qatarensis (Dicot) | Cd, Cr, Cu, Fe, Ni, Zn | Possible petroleum hydrocarbons | [14,31,129] |
| Teucrium polium (Dicot) | Co, Ni | Possible petroleum hydrocarbons | [130] |
| Metal | Plant Species | |
|---|---|---|
| Monocot | Dicot | |
| Al | Cyperus spp. | Arthrocnemum meridionale |
| B | - | Salsola sp. |
| Cd | Aeluropus spp., Cyperus spp., Juncus rigidus | Arthrocnemum meridionale, Atriplex leucoclada, Avicennia marina, Cleome spp., Frankenia pulverulenta, Halocnemum strobilaceum, Heliotropium spp. Limonium axillare, Salicornia europaea, Salsola sp., Tamarix spp., Tetraena qatarensis |
| Co | Cyperus spp. | Avicennia marina, Limonium axillare, Salsola sp., Teucrium polium |
| Cr | Cyperus spp., Halopyrum mucronatum, Polypogon monspeliensis | Avicennia marina, Frankenia pulverulenta, Heliotropium spp., Limonium axillare, Salsola sp., Tetraena qatarensis |
| Cu | Cyperus spp., Halodule uninervis, Juncus rigidus | Anabasis setifera, Arthrocnemum meridionale, Atriplex leucoclada, Avicennia marina, Cleome spp., Frankenia pulverulenta, Haloxylon sp., Heliotropium spp., Limonium axillare, Salicornia europaea Salsola sp., Suaeda spp., Tamarix spp., Tetraena qatarensis |
| Fe | Cyperus spp., Halodule uninervis, Halopyrum mucronatum, Juncus rigidus | Arthrocnemum meridionale, Avicennia marina, Halocnemum strobilaceum, Haloxylon sp., Heliotropium spp., Limonium axillare, Salsola sp., Suaeda spp., Tamarix spp., Tetraena qatarensis |
| Hg | Cyperus spp., Juncus rigidus, Polypogon monspeliensis | - |
| Mn | Cyperus spp., Juncus rigidus | Anabasis setifera, Arthrocnemum meridionale, Halocnemum strobilaceum, Haloxylon sp., Heliotropium spp., Salsola sp., Suaeda spp., Tamarix spp. |
| Ni | Cyperus spp., Halodule uninervis, Polypogon monspeliensis | Atriplex leucoclada, Avicennia marina, Frankenia pulverulenta, Halocnemum strobilaceum, Limonium axillare, Salicornia europaea, Salsola sp., Tamarix spp., Tetraena qatarensis, Teucrium polium |
| Pb | Aeluropus spp., Cyperus spp., Halodule uninervis, Halopyrum mucronatum | Atriplex leucoclada, Halocnemum strobilaceum, Heliotropium spp., Salicornia europaea, Salsola sp., Suaeda spp., Tamarix spp. |
| Se | - | Salsola sp. |
| Sr | - | Frankenia pulverulenta |
| Zn | Cyperus spp., Halopyrum mucronatum, Polypogon monspeliensis | Arthrocnemum meridionale, Atriplex leucoclada, Avicennia marina, Frankenia pulverulenta, Halocnemum strobilaceum, Haloxylon sp., Heliotropium spp., Limonium axillare, Salicornia europaea, Salsola sp., Suaeda spp., Tamarix spp., Tetraena qatarensis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasseen, B.T.; Al-Thani, R.F. Endophytes and Halophytes to Remediate Industrial Wastewater and Saline Soils: Perspectives from Qatar. Plants 2022, 11, 1497. https://doi.org/10.3390/plants11111497
Yasseen BT, Al-Thani RF. Endophytes and Halophytes to Remediate Industrial Wastewater and Saline Soils: Perspectives from Qatar. Plants. 2022; 11(11):1497. https://doi.org/10.3390/plants11111497
Chicago/Turabian StyleYasseen, Bassam T., and Roda F. Al-Thani. 2022. "Endophytes and Halophytes to Remediate Industrial Wastewater and Saline Soils: Perspectives from Qatar" Plants 11, no. 11: 1497. https://doi.org/10.3390/plants11111497
APA StyleYasseen, B. T., & Al-Thani, R. F. (2022). Endophytes and Halophytes to Remediate Industrial Wastewater and Saline Soils: Perspectives from Qatar. Plants, 11(11), 1497. https://doi.org/10.3390/plants11111497