Roles of Syzygium in Anti-Cholinesterase, Anti-Diabetic, Anti-Inflammatory, and Antioxidant: From Alzheimer’s Perspective
Abstract
:1. Introduction
2. Multifactorial AD and Multitarget Agents as a Strategy for Neuroprotection
3. Ethnobotany and Ethnomedicinal Properties of Syzgium
4. Neuroprotective Agents from Syzygium
4.1. Anti-Cholinesterase Activity
| Species | Plant Part/Compound | Test | Activity | Reference | |
|---|---|---|---|---|---|
| 1 | Syzygium cumini (L.) Skeels. | Ethanol leaf extract | In vitro AChE | 44.54 µg/mL of IC50 | [36] |
| Ex vivo AChE | No significant effect | ||||
| Leaf essential oil | In vitro AChE | 32.9 µg/mL of IC50 | [22] | ||
| Polyphenol-rich leaf extract | In vitro AChE and BChE | Significant reduction in cholinesterase activities; bound polyphenolic extract showed better inhibitory activity than free polyphenolic extract | [37] | ||
| Polyphenol-rich leaf extract | In vivo AChE and BChE from alloxan-induced diabetic rats | Enzyme activities were significantly reduced after 14 days (400 mg/kg oral dose) | [37] | ||
| Methanol seed extract | In vivo AChE from scopolamine-induced rats | Significant reduction in AChE activity (400 mg/kg oral dose) | [29] | ||
| Leaf extract | In vitro AChE | No significant activity | [38] | ||
| 2 | Syzygium aqueum Alston | Methanol leaf extract | In vitro ACHE and BChE | 16.04 µg/mL and 13.95 µg/mL of IC50, respectively | [43] |
| 3 | Syzygium polyanthum (Wight) Walp. | Methanol and ethyl acetate extracts from leaves | In vitro ACHE | 47.30 and 45.10 µg/mL of IC50, respectively | [38] |
| Methanol leaf and stem extracts | In vitro ACHE and BChE | >80% inhibition at 200 µg/mL concentration (8.28 and 6.54 µg/mL of IC50 in the leaf extract, respectively) | [41] | ||
| 4 | Syzygium aromaticum (L.) Merrill and Perry | Methanol, ethyl acetate, and hexane extracts from leaves; methanol bud extract | In vitro ACHE | 42.10, 55.9, and 62.05 µg/mL of IC50, respectively (leaves); 45.25 µg/mL of IC50 (bud) | [38] |
| Methanol extract, clove oil, and eugenol | In vitro ACHE and BChE using TLC bioautography | Eugenol (42.44 and 63.51 µg/mL of IC50) showed better inhibition than extract (61.5 and 103.53 µg/mL of IC50) and oil (49.73 and 88.14 µg/mL of IC50), respectively | [18] | ||
| Clove bud essential oil | In vitro ACHE and BChE | 1.5 μL/L and 18.2 μL/L of IC50, respectively | [27] | ||
| Ethanol extract | HPTLC-densitometry | Showed efficiency in AChE inhibition | [44] | ||
| Ethanol bud extract | In vitro AChE isolated from human erythrocytes | No inhibitory effect | [45] | ||
| Ethanol bud extract | In vitro AChE of parasite C. cotylophorum | 86.86% inhibition at 0.5 mg/mL after 8 hr exposure | [34] | ||
| Clove oil (eugenol) encapsulated with a nanostructured lipid carrier | In vitro ACHE and BChE from D. melanogaster tissue | 4.3 and 3.5 mM of IC50, respectively | [35] | ||
| Aqueous and hydroalcoholic extract of clove buds | In vitro AChE | 253.29 µg/mL of IC50 in aqueous extract | [40] | ||
| Clove oil | In vitro AChE from AlCl3-induced rats | Significant reduction in AChE activity | [46] | ||
| Ethanol bud extract | In vivo AChE from CeCI3-induced memory-impaired rats | Corrected the AChE rate caused by CeCI3 toxicity and improved cholinergic neural transmission | [42] | ||
| Eugenol derivatives | In vitro ACHE and BChE | 4-Allyl-2-methoxyphenyl-4-ethyl benzoate inhibited AChE with 5.64 µg/mL of IC50 | [47] | ||
| Isoeugenol | In vitro ACHE | 77 nM of IC50 | [48] | ||
| 5 | Syzygium antisepticum (Blume) Merr. and L.M.Perry | Methanol leaf extract; ursolic acid; gallic acid | In vitro ACHE | 61.9% at 300 µg/mL concentration; 81.64% at 200 µg/mL concentration; 73.39% at 200 µg/mL concentration | [24] |
| 6 | Syzygium samarangense (Blume) Merr. and L.M.Perry | Essential oil | In vitro ACHE and BChE | 4.83 and 5.69 mg GALAE/g, respectively | [39] |
| Dihydrochalcone | In vitro ACHE and BChE | 98.5% inhibition at 0.25 mM and 68% inhibition at 0.20 mM, respectively | [49] | ||
| 7 | Syzygium coriaceum Bosser and J. Guého | Essential oil | In vitro ACHE and BChE | 4.79 and 7.10 mg GALAE/g, respectively | [39] |
| 8 | Syzygium jambos (L.) Alston | Aqueous leaf extract | In vitro ACHE from homogenized tissue of rat brain | No significant activity | [30] |
| Methanol stem and leaf extracts | In vitro ACHE and BChE | >80% inhibition at 200 µg/mL concentration (16.05 and 15.25 µg/mL of IC50 from stem extract, respectively) | [41] | ||
| 9 | Syzygium grande (Wight) Walp. | Methanol leaf extract | In vitro ACHE and BChE | >80% inhibition at 200 µg/mL concentration | [41] |
| 10 | Syzygium lineatum (DC.) Merr. and L.M.Perry | Methanol leaf extract | In vitro ACHE and BChE | >80% inhibition at 200 µg/mL concentration (20.69 µg/mL of IC50 for BChE) | [41] |
4.2. Anti-Diabetic Activity
4.3. Anti-Inflammatory Activity
| Species | Plant Part/Compound | Test | Activity | Reference | |
|---|---|---|---|---|---|
| 1 | S. malaccense (L.) Merr. and L.M. Perry | Methanol leaf extract | In vitro LPS-induced neuroinflammatory assay on murine BV-2 microglial cells; in vivo croton oil-induced ear edema test | Neuroprotective activity by a reduction in nitric oxide production in vitro; decreased mice ear edema in vivo | [63] |
| 2 | S. cumini | Methanol fruit extract | In vitro membrane stabilization, egg albumin denaturation, and bovine serum albumin denaturation assays; in vivo murine models of carrageenan, formaldehyde, and PGE2 induced paw edema. | Showed inflammatory activities both in vitro and in vivo | [64] |
| Betulinic acid | In vivo Fx1A antiserum-induced passive Heymann nephritis (PHN) in Sprague-Dawley rats | Ameliorated mRNA and protein expression of NF-κB, iNOS, TNF-α, Nrf2, HO-1, and NQO1 in the kidney, reducing inflammation | [65] | ||
| Polyphenol-rich leaf extract | In vivo Alloxan-induced diabetic rats | NF-κB and inflammatory cytokines such as TNF-α and IL-1α were regulated | [37] | ||
| Anthocyanins di-glucosides from pulp | In vitro determination of cytokine production in LPS-induced RAW264.7 macrophages | Inhibited pro-inflammatory mediators such as IL-6, IL-1β, and TNF-α | [66] | ||
| Aqueous seed extract | In vivo high cholesterol diet-streptozotocin-induced diabetes in rats | Exhibited significant anti-inflammatory and β-cell salvaging activity via overexpression of PPARγ and PPARα activity and a significant decrease in TNF-α levels when treated with 100, 200, 400 mg/kg/day doses | [67] | ||
| Methanol seed extract | In vitro high glucose (HG) diabetic cardiomyopathy in H9C2 cardiomyoblast cells | HG-induced activation of NF-κB, TNF-α, and IL-6 was remarkably reduced | [68] | ||
| Seed extract | In vivo Aβ1-40-infused AD model rats | Reduced the levels of Aß burdens and oligomers by suppressing the levels of TNFα and LPO in the corticohippocampal tissues | [62] | ||
| 3 | Syzygium caryophyllatum (L.) Alston | Aqueous root extract | In vitro anti-inflammatory test using heat-induced albumin denaturation assay | 6.229 µg/mL of IC50 | [69] |
| 4 | S. aqueum | Polyphenol-rich leaf extract | In vitro lipoxygenase inhibitor screening assay, membrane stabilizing activity (hypotonic solution-induced hemolysis), and in vivo carrageenan-induced hind-paw edema in rats | Inhibited LOX, COX-1, and COX-2 with higher COX-2 selectivity reduced the extent of lysis of erythrocytes and markedly reduced leukocyte numbers in rats challenged with carrageenan. | [70] |
| Leaf extract | In vivo STZ-induced oxidative stress and inflammation in pancreatic beta cells in rats | Significantly decreased levels of TLR-4, MYD88, pro-inflammatory cytokines TNF-α, and TRAF-6 in pancreatic tissue homogenates, which correlated well with minimal pancreatic inflammatory cell infiltration | [56] | ||
| 5 | Syzygium mundagam (Bourd.) Chithra | Methanol bark extract | In vivo carrageenin- and egg albumin-induced paw edema, cotton pellet implanted granuloma in rats | Effective anti-inflammation at 200 mg/kg dose | [71] |
| 6 | Syzygium calophyllifolium (Wight) Walp. | Methanol bark extract | In vivo carrageenin- and egg albumin-induced paw edema, cotton pellet implanted granuloma | 200 mg/kg dose significantly reduced the paw edema in carrageenan (96.71%) and egg albumin models (54.24%) compared to the control. Chronic inflammation was also inhibited by up to 70.46% | [72] |
| 7 | S. aromaticum | Ethanol/water extract | In vivo carrageenan-induced paw edema inflammatory in rats | Pretreatment at different doses (100, 200, and 400 mg/kg) produced a significant (p < 0.001) reduction in paw inflammation up to 5 h of carrageenan injection | [73] |
| Essential oil | In vivo formalin-induced and carrageenan-induced paw edema inflammation in rats | 26.9 ± 2.5 μg/paw of EC50 | [74] | ||
| Aqueous clove extract | In vivo LPS-induced lung inflammation in mice. | Inhibited matrix metalloproteinases: MMP-2 (15%) and MMP-9 (18%) activity in lung homogenates, reducing inflammation | [75] | ||
| Ethanol extract | In vitro TNF-α induced inflammation in dental pulp stem cells | Prevented the increase in IL-6 levels | [76] | ||
| Eugenol | Cytochrome c reduction assay to measure superoxide anion generation in human neutrophils | Inhibited the generation of superoxide anion by neutrophils via the inhibition of Raf/MEK/ERK1/2/p47phox-phosphorylation pathway | [77] | ||
| Eugenol | In vivo ethanol-induced ulcer in rats | Decreased TNF-α and IL-6 cytokine concentrations responsible for inflammation | [78] | ||
| Essential oil | Isbolographic study using the formalin test in rats | S. aromaticum in combination with ketorolac, showed an antinociceptive effect in the treatment of inflammatory pain | [79] | ||
| 8 | S. samarangense | Polyphenol vescalagin | In vivo methylglyoxal-induced inflammation in diabetic rats | The pancreatic levels of NF-κB, ICAM-1, and TNF-α protein, were reduced | [80] |
| Lyophilized fruit powder | In vivo STZ-induced pancreatic beta cells apoptosis in rats | Pancreatic ß-cell apoptosis was alleviated with significantly down-regulated cleaved caspase-3 and Bax and upregulated Bcl-2 and Bcl-xl protein expression | [81] | ||
| 9 | S. polyanthum | Leaf extract | In vivo coronary artery ligation-induced myocardial infarction in rats | Reduced levels of C-reactive protein (CRP) and myeloperoxidase (MPO) in the rats started from day 4 after the induction of myocardial infarction. | [82] |
| 10 | S. jambos | Bark extract | In vivo streptozotocin-induced inflammation in diabetic rats | Significantly reduced TNF-α and increased IL-10 (p < 0.05) in pancreatic tissues | [61] |
4.4. Antioxidant Activity
| Species | Plant Part/Compound | Reference | |
|---|---|---|---|
| 1 | S. cumini | Leaf | [36,37,89] |
| Fruit | [64,88,90] | ||
| Bark | [91] | ||
| Polyphenol-rich extract | [92,93] | ||
| Seed kernels powder | [94] | ||
| 2 | S. polyanthum | Leaf | [38] |
| 3 | S. aromaticum | Flower | [86] |
| Bud | [42,95] | ||
| Bud essential oil | [27,86,87,96,97,98] | ||
| Eugenol | [87] | ||
| All parts | [99] | ||
| 4 | S.antisepticum | Leaf | [24] |
| Gallic acid, myricitrin, and quercitrin | [24] | ||
| 5 | S. caryophyllatum | Leaf | [100] |
| Fruit | [100,101] | ||
| Fruit pulp healthy snack | [102] | ||
| 6 | Syzygium paniculatum Gaertn. | Leaf | [103] |
| Fruit | [104] | ||
| Volatile oil from the aerial part | [105] | ||
| 7 | S. malaccense | Leaf | [63,88,106] |
| Myricetin derivatives | [107] | ||
| 8 | S. aqueum | Stem | [108] |
| Bark | [108] | ||
| 9 | S. polyanthum | Leaf | [109] |
| 10 | S. jambos | Fruit | [110] |
| Bark | [61] | ||
| 11 | S. samarangense | Vescalagin | [80] |
| 12 | Syzygiumcymosum (Lam.) DC. | Leaf | [111] |
4.5. In Vivo Neuroprotection Studies
| Species | Plant Part/Compound | Test | Activity | Reference | |
|---|---|---|---|---|---|
| 1 | S. cumini | Seed extract | Eight-arm radial maze task for learning-related memory | Improved learning-related memory through the antioxidative defense by a reduction in corticohippocampal levels of lipid peroxide | [112] |
| Seed extract | Aß1-40-infused AD model rats | Significantly increased the memory-related learning ability of the AD model rats with reductions in the levels of corticohippocampal Aβ1-40-burden and Aβ1-40-oligomers, and increased the levels of brain cognition and memory-related proteins, including BDNF, TrKB, PSD-95 and SNAP-25 | [62] | ||
| 2 | S. aqueum | Methanol leaves | POBCCA surgery in rats | Improved short- and long-term recognition memory in NOR test, improved spatial learning in MWM test at 200 mg/kg dose | [43] |
| 3 | S. aromaticum | Aqueous bud extract | AlCl3-induced neurotoxic rats | Restored the parameters (Al, Ca2+, MDA, nitrite/nitrate, Mg+, Na+, GSH, GPx) to the near-normal levels, significantly normalized expression of the SOD1 gene | [46] |
| Clove oil | Amyloid1-42-induced spatial memory-impaired rats | Improved spatial memory in Shuttle box test and apoptosis, PRDX6, and GCN5L1 levels were recovered through swimming training and clove consumption | [28] | ||
| Clove oil | MCAO-stroke-induced rats | The pre-treated and post-treated groups with clove oil showed improvement in neurological deficit score | [96] | ||
| Ethanol bud extract | CeCI3-induced memory-impaired mice | Symptoms of retracted neurons with condensed chromatin undergoing necrosis or apoptosis and vacuolated space were alleviated, which improved the state of memory in mice | [42] | ||
| Clove essential oil | C. elegans model | Extended lifespan and promoted production and health of C. elegans by inducing DAF-16/FOXO nuclear translocation from the cytoplasm and causing apoptosis of germ cells in ACEP-1 and DAF-16 | [85] | ||
| 4 | S. malaccense | Freeze-dried fruit | HFD-induced cognitive impaired rats | Improved AKT signaling in the hippocampus that prevented the activation of GSK3-β, lowered tau phosphorylation, and improved brain antioxidant enzyme activities. | [114] |
5. Bioactive Phytoconstituents
| Compound Name | Chemical Structure | Biological Activity | Reference | |
|---|---|---|---|---|
| 1 | Betulinic acid (in powder) |  | Anti-inflammatory and antioxidant | [65] |
| 2 | Eugenol (in MeOH) | 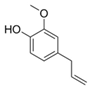 | Anti-cholinesterase, anti-inflammatory, and antioxidant | [18,77,78] |
| 3 | Isoeugenol (in EtOH) | 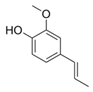 | Anti-cholinesterase, and anti-diabetic | [48] |
| 4 | Vescalagin (in lyophilized powder) |  | Anti-inflammatory, antioxidant, and anti-diabetic | [57,80] |
| 5 | 2′,4′-Dihydroxy-6′- methoxy-3′,5′-dimethyl-dihydrochalcone | 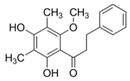 | Anti-cholinesterase | [49] |
| 6 | Ursolic acid (in DMSO, tween 20, or MeOH) | 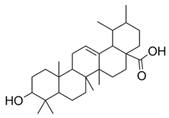 | Anti-cholinesterase | [24] |
| 7 | Gallic acid (in DMSO, tween 20, or MeOH) | 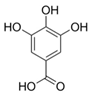 | Anti-cholinesterase | [24] |
| 8 | Myricitin (in DMSO, tween 20, or MeOH) | 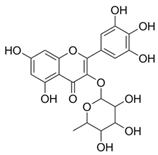 | Antioxidant and anti-diabetic | [24,58] |
| 9 | Quercitin (in DMSO, tween 20, or MeOH) | 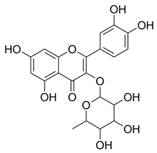 | Antioxidant | [24] |
| 10 | 6-Heptadeca-8Z,11Z,14Z-trienyl salicylic acid (in DMSO) | 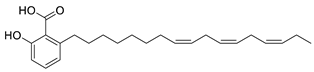 | Anti-cholinesterase | [115] |
| 11 | 6-Heptadeca-9Z,12Z-dienyl salicylic acid (in DMSO) | 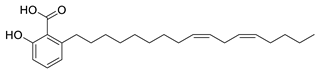 | Anti-cholinesterase | [115] |
| 12 | (E)-β-Caryophyllene (in essential oil) | 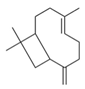 | Anti-cholinesterase and anti-diabetic | [22] |
| 13 | ß-Pinene (in essential oil) | 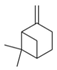 | Anti-cholinesterase and anti-diabetic | [39] |
| 14 | (E)-ß-Ocimene (in essential oil) | 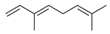 | Anti-cholinesterase and anti-diabetic | [39] |
6. Syzygium aromaticum
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The Global Prevalence of Dementia: A Systematic Review and Metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, K.; Govindaraju, T. Current Progress, Challenges and Future Prospects of Diagnostic and Therapeutic Interventions in Alzheimer’s Disease. RSC Adv. 2018, 8, 23780–23804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, S.; Rangra, N.; Pradhan, K. A Comprehensive Review on Phytopharmacological Investigations of Acacia auriculiformis A.Cunn. Ex Benth. Asian Pac. J. Trop. Biomed. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Samanta, S.; Ramesh, M.; Govindaraju, T. Alzheimer’s Is a Multifactorial Disease. In Alzheimer’s Disease: Recent Findings in Pathophysiology, Diagnostic and Therapeutic Modalities; Royal Society of Chemistry: Cambridge, UK, 2022. [Google Scholar]
- Li, X.-T. Alzheimer’s Disease Therapy Based on Acetylcholinesterase Inhibitor/Blocker Effects on Voltage-Gated Potassium Channels. Metab. Brain Dis. 2022, 37, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhu, Q.; Lu, J. Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies. Cells 2022, 11, 479. [Google Scholar] [CrossRef]
- Aung, E.E.; Kristanti, A.N.; Aminah, N.S.; Takaya, Y.; Ramadhan, R. Plant Description, Phytochemical Constituents and Bioactivities of Syzygium Genus: A Review. Open Chem. 2020, 18, 1256–1281. [Google Scholar] [CrossRef]
- Cock, I.E.; Cheesman, M. Plants of the Genus Syzygium (Myrtaceae): A Review on Ethnobotany, Medicinal Properties and Phytochemistry. In Bioactive Compounds of Medicinal Plants; Apple Academic Press: Waretown, NJ, USA, 2018; pp. 75–124. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The Amyloid Hypothesis in Alzheimer Disease: New Insights from New Therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J.F. Selective Loss of Central Cholinergic Neurons in Alzheimer’s Disease. Lancet 1976, 308, 1403. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Akter, K.; Lanza, E.A.; Martin, S.A.; Myronyuk, N.; Rua, M.; Raffa, R.B. Diabetes Mellitus and Alzheimer’s Disease: Shared Pathology and Treatment? Br. J. Clin. Pharmacol. 2011, 71, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterfield, D.A.; Lauderback, C.M. Lipid Peroxidation and Protein Oxidation in Alzheimer’s Disease Brain: Potential Causes and Consequences Involving Amyloid β-Peptide-Associated Free Radical Oxidative Stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- McGeer, P.L.; Schulzer, M.; McGeer, E.G. Arthritis and Anti-Inflammatory Agents as Possible Protective Factors for Alzheimer’s Disease. Neurology 1996, 47, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Moeini, R.; Memariani, Z.; Asadi, F.; Bozorgi, M.; Gorji, N. Pistacia Genus as a Potential Source of Neuroprotective Natural Products. Planta Med. 2019, 85, 1326–1350. [Google Scholar] [CrossRef] [PubMed]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective Effects of Berry Fruits on Neurodegenerative Diseases. Neural Regen. Res. 2014, 9, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Dalai, M.K.; Bhadra, S.; Chaudhary, S.K.; Bandyopadhyay, A.; Mukherjee, P.K. Anti-Cholinesterase Activity of the Standardized Extract of Syzygium aromaticum L. Pharmacogn. Mag. 2014, 10, S276. [Google Scholar]
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: A Review of Its Phytochemical Constituents and Traditional Uses. Asian Pac. J. Trop. Biomed. 2012, 2, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Chhikara, N.; Kaur, R.; Jaglan, S.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive Compounds and Pharmacological and Food Applications of Syzygium cumini—A Review. Food Funct. 2018, 9, 6096–6115. [Google Scholar] [CrossRef]
- Srivastava, S.; Chandra, D. Pharmacological Potentials of Syzygium cumini: A Review. J. Sci. Food Agric. 2013, 93, 2084–2093. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Eldehna, W.M.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Aly, S.H. GC/MS Analysis of Essential Oil and Enzyme Inhibitory Activities of Syzygium cumini (Pamposia) Grown in Egypt: Chemical Characterization and Molecular Docking Studies. Molecules 2021, 26, 6984. [Google Scholar] [CrossRef]
- Banerjee, S.; Panda, C.K.; Das, S. Clove (Syzygium aromaticum L.), a Potential Chemopreventive Agent for Lung Cancer. Carcinogenesis 2006, 27, 1645–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangmool, S.; Kunpukpong, I.; Kitphati, W.; Anantachoke, N. Antioxidant and Anticholinesterase Activities of Extracts and Phytochemicals of Syzygium antisepticum Leaves. Molecules 2021, 26, 3295. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Mohamed, M.; Sulaiman, S.A.; Wan Ahmad, W.A.N. Autonomic Nervous System Mediates the Hypotensive Effects of Aqueous and Residual Methanolic Extracts of Syzygium polyanthum (Wight) Walp. Var. Polyanthum Leaves in Anaesthetized Rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 716532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, S.; Cock, I.E. Bioactivity of Syzygium jambos Methanolic Extracts: Antibacterial Activity and Toxicity. Pharmacogn. Res. 2010, 2, 4. [Google Scholar]
- Adefeghaa, S.; Oboha, G.; Odubanjoa, T.; Ogunsuyia, O. A Comparative Study on the Antioxidative Activities, Anticholinesterase Properties and Essential Oil Composition of Clove (Syzygium aromaticum) Bud and Ethiopian Pepper (Xylopia Aethiopica). Riv. Ital. Sostanze Grasse 2015, 92, 257–268. [Google Scholar]
- Panahzadeh, F.; Mirnasouri, R.; Rahmati, M. Exercise and Syzygium aromaticum Reverse Memory Deficits, Apoptosis and Mitochondrial Dysfunction of the Hippocampus in Alzheimer’s Disease. J. Ethnopharmacol. 2022, 286, 114871. [Google Scholar] [CrossRef] [PubMed]
- Alikatte, K.L.; Akondi, B.R.; Yerragunta, V.G.; Veerareddy, P.R.; Palle, S. Antiamnesic Activity of Syzygium cumini against Scopolamine Induced Spatial Memory Impairments in Rats. Brain Dev. 2012, 34, 844–851. [Google Scholar] [CrossRef]
- Bonfanti, G.; Bitencourt, P.R.; de Bona, K.S.; da Silva, P.S.; Jantsch, L.B.; Pigatto, A.S.; Boligon, A.; Athayde, M.L.; Gonçalves, T.L.; Moretto, M.B. Syzygium jambos and Solanum guaraniticum Show Similar Antioxidant Properties but Induce Different Enzymatic Activities in the Brain of Rats. Molecules 2013, 18, 9179–9194. [Google Scholar] [CrossRef] [Green Version]
- Devakumar, J.; Keerthana, V.; Sudha, S.S. Identification of Bioactive Compounds by Gas Chromatography-Mass Spectrometry Analysis of Syzygium jambos (L.) Collected from Western Ghats Region Coimbatore, Tamil Nadu. Asian J. Pharm. Clin. Res. 2017, 10, 364–369. [Google Scholar]
- Chagas, V.T.; de Sousa Coelho, R.M.; Gaspar, R.S.; da Silva, S.A.; Mastrogiovanni, M.; de Jesus Mendonca, C.; de Souza Ribeiro, M.N.; de Andrade Paes, A.M.; Trostchansky, A. Protective Effects of a Polyphenol-Rich Extract from Syzygium cumini (L.) Skeels Leaf on Oxidative Stress-Induced Diabetic Rats. Oxid. Med. Cell. Longev. 2018, 2018, 5386079. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, C.C.; Fuchs, F.D.; Blotta, R.M.; Knijnik, J.; Delgado, I.C.; Netto, M.S.; Ferreira, E.; Costa, A.P.; Müssnich, D.G.; Ranquetat, G.G. Effect of Tea Prepared from Leaves of Syzygium jambos on Glucose Tolerance in Nondiabetic Subjects. Diabetes Care 1990, 13, 907–908. [Google Scholar] [CrossRef] [PubMed]
- Dhanraj, K.M.; Veerakumari, L. In Vitro Effect of Syzygium Aromaticum on the Motility and Acetylcholinesterase of Cotylophoron Cotylophorum. Ind. J. Vet. Anim. Sci. Res. 2014, 43, 187–194. [Google Scholar]
- De Meneses, A.C.; Marques, E.B.P.; Leimann, F.V.; Gonçalves, O.H.; Ineu, R.P.; de Araújo, P.H.H.; de Oliveira, D.; Sayer, C. Encapsulation of Clove Oil in Nanostructured Lipid Carriers from Natural Waxes: Preparation, Characterization and in Vitro Evaluation of the Cholinesterase Enzymes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123879. [Google Scholar] [CrossRef] [Green Version]
- Borba, L.A.; Wiltenburg, V.D.; Negri, G.; Ibe, M.B.; dos Santos, L.; Mendes, F.R. In Vitro Inhibition of Acetylcholinesterase and Monoamine Oxidase by Syzygium cumini Leaves Extract and Preliminary Assessment in Animal Models. S. Afr. J. Bot. 2022, 146, 553–563. [Google Scholar] [CrossRef]
- Basiru, O.A.; Ojo, O.A.; Akuboh, O.S.; Okesola, M.A.; Idowu, O.T.; Talabi, J.Y. The Protective Effect of Polyphenol-Rich Extract of Syzygium cumini Leaves on Cholinesterase and Brain Antioxidant Status in Alloxan-Induced Diabetic Rats. Jordan J. Biol. Sci. 2017, 11, 163–169. [Google Scholar]
- Darusman, L.K.; Wahyuni, T.W.; Alwi, F. Acetylcholinesterase Inhibition and Antioxidant Activity of Syzygium cumini, S. aromaticum and S. polyanthum from Indonesia. J. Biol. Sci. 2013, 13, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Sharmeen Jugreet, B.; Kouadio Ibrahime, S.; Zengin, G.; Abdallah, H.H.; Fawzi Mahomoodally, M. GC/MS Profiling, In Vitro and In Silico Pharmacological Screening and Principal Component Analysis of Essential Oils from Three Exotic and Two Endemic Plants from Mauritius. Chem. Biodivers. 2021, 18, e2000921. [Google Scholar] [CrossRef]
- Saeedi, M.; Babaie, K.; Karimpour-Razkenari, E.; Vazirian, M.; Akbarzadeh, T.; Khanavi, M.; Hajimahmoodi, M.; Shams Ardekani, M.R. In Vitro Cholinesterase Inhibitory Activity of Some Plants Used in Iranian Traditional Medicine. Nat. Prod. Res. 2017, 31, 2690–2694. [Google Scholar] [CrossRef]
- Rawa, M.S.A.; Hassan, Z.; Murugaiyah, V.; Nogawa, T.; Wahab, H.A. Anti-Cholinesterase Potential of Diverse Botanical Families from Malaysia: Evaluation of Crude Extracts and Fractions from Liquid-Liquid Extraction and Acid-Base Fractionation. J. Ethnopharmacol. 2019, 245, 112160. [Google Scholar] [CrossRef]
- Kadri, Y.; Nciri, R.; Bardaa, S.; Brahmi, N.; Saber, S.; Harrath, A.H.; Aldahmash, W.; Alwasel, S.; Mohany, M.; El Feki, A.; et al. Syzygium aromaticum Alleviates Cerium Chloride-Induced Neurotoxic Effect In The Adult Mice. Toxicol. Mech. Methods 2019, 29, 26–34. [Google Scholar] [CrossRef]
- Kumaran, K.R.; Wahab, H.A.; Hassan, Z. In Vitro Anti-Cholinesterase Activity and in Vivo Screening of Coccoloba Uvifera, Mimusops Elengi and Syzygium aqueum Extracts on Learning and Memory Function of Chronic Cerebral Hypoperfusion Rat. Neurosci. Res. Notes 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Affonso, R.S.; Lima, J.A.; Lessa, B.M.; Caetano, J.V.O.; Obara, M.T.; Nóbrega, A.B.; Nepovimova, E.; Musilek, K.; Kuca, K.; Slana, G.B.C.A. Quantification through TLC-densitometric Analysis, Repellency and Anticholinesterase Activity of the Homemade Extract of Indian Cloves. Biomed. Chromatogr. 2018, 32, e4096. [Google Scholar] [CrossRef] [PubMed]
- Güller, U.; Güller, P.; Çiftci, M. Radical Scavenging and Antiacetylcholinesterase Activities of Ethanolic Extracts of Carob, Clove, and Linden. Altern. Ther. Health Med. 2021, 27, 33–37. [Google Scholar] [PubMed]
- Kassab, R.B.; Bauomy, A.A. The Neuroprotective Efficency of the Aqueous Extract of Clove (Syzygium aromaticum) in Aluniniuminduced Neurotoxicity. Int. J. Pharm. Pharm. Sci. 2014, 6, 503–508. [Google Scholar]
- Zamli, K.M.; Asari, A.; Khaw, K.Y.; Murugaiyah, V.; Al-Rashida, H.M.; Yusoff, H.M.; Hasnah, N.H.A.W. Cholinesterase Inhibition Activity and Molecular Docking Study of Eugenol Derivatives. Sains Malays. 2021, 50, 1037–1045. [Google Scholar] [CrossRef]
- Topal, F. Anticholinergic and Antidiabetic Effects of Isoeugenol from Clove (Eugenia Caryophylata) Oil. Int. J. Food Prop. 2019, 22, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Amor, E.C.; Villaseñor, I.M.; Nawaz, S.A.; Hussain, M.S.; Choudhar, I. A Dihydrochalcone from Syzygium samarangense with Anticholinesterase Activity. Philipp. J. Sci. 2005, 134, 105. [Google Scholar]
- Zulcafli, A.S.; Lim, C.; Ling, A.P.; Chye, S.; Koh, R. Focus: Plant-Based Medicine and Pharmacology: Antidiabetic Potential of Syzygium Sp.: An Overview. Yale J. Biol. Med. 2020, 93, 307. [Google Scholar]
- Sharma, S.B.; Rajpoot, R.; Nasir, A.; Prabhu, K.M.; Murthy, P.S. Ameliorative Effect of Active Principle Isolated from Seeds of Eugenia jambolana on Carbohydrate Metabolism in Experimental Diabetes. Evid.-Based Complement. Altern. Med. 2011, 2011, 789871. [Google Scholar] [CrossRef] [Green Version]
- Sanches, J.R.; França, L.M.; Chagas, V.T.; Gaspar, R.S.; Dos Santos, K.A.; Gonçalves, L.M.; Sloboda, D.M.; Holloway, A.C.; Dutra, R.P.; Carneiro, E.M. Polyphenol-Rich Extract of Syzygium cumini Leaf Dually Improves Peripheral Insulin Sensitivity and Pancreatic Islet Function in Monosodium L-Glutamate-Induced Obese Rats. Front. Pharmacol. 2016, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Sahana, D.A.; Shivaprakash, G.; Baliga, R.; MR, A.P.; Ganesh, J.; Pai, M. Effect of Eugenia jambolana on Plasma Glucose, Insulin Sensitivity and HDL-C Levels: Preliminary Results of a Randomized Clinical Trial. J. Pharm. Res. 2010, 3, 1268–1270. [Google Scholar]
- Sharma, A.K.; Bharti, S.; Kumar, R.; Krishnamurthy, B.; Bhatia, J.; Kumari, S.; Arya, D.S. Syzygium cumini Ameliorates Insulin Resistance and β-Cell Dysfunction via Modulation of PPARγ, Dyslipidemia, Oxidative Stress, and TNF-α in Type 2 Diabetic Rats. J. Pharmacol. Sci. 2012, 119, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.-C.; Chang, W.-C.; Chang, C.-L. Fraction from Wax Apple [Syzygium samarangense (Blume) Merrill and Perry] Fruit Extract Ameliorates Insulin Resistance via Modulating Insulin Signaling and Inflammation Pathway in Tumor Necrosis Factor α-Treated FL83B Mouse Hepatocytes. Int. J. Mol. Sci. 2012, 13, 8562–8577. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Abdelaal, S.; Mohammed, H.O.; El-Shazly, A.M.; Daoud, R.; Abdelfattah, M.A.O.; Sobeh, M. Syzygium Aqueum (Burm.f.) Alston Prevents Streptozotocin-Induced Pancreatic Beta Cells Damage via the TLR-4 Signaling Pathway. Front. Pharmacol. 2021, 12, 769244. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-W.; Chang, W.-C.; Wu, J.S.-B.; Shih, R.-W.; Shen, S.-C. Vescalagin from Pink Wax Apple [Syzygium samarangense (Blume) Merrill and Perry] Alleviates Hepatic Insulin Resistance and Ameliorates Glycemic Metabolism Abnormality in Rats Fed a High-Fructose Diet. J. Agric. Food Chem. 2016, 64, 1122–1129. [Google Scholar] [CrossRef]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Potential Antihyperglycaemic Effect of Myricetin Derivatives from Syzygium malaccense. J. Funct. Foods 2016, 22, 325–336. [Google Scholar] [CrossRef]
- Konda, P.Y.; Chennupati, V.; Dasari, S.; Sharma, N.; Muthulingam, M.; Ramakrishnan, R.; Sade, A.; Jagadheeshkumar, V.; Natesan, V.; Jaiswal, K.K. Ethno-Pharmacological Insulin Signaling Induction of Aqueous Extract of Syzygium paniculatum Fruits in a High-Fat Diet Induced Hepatic Insulin Resistance. J. Ethnopharmacol. 2021, 268, 113576. [Google Scholar] [CrossRef]
- Sampath, S.; Narasimhan, A.; Chinta, R.; Nair, K.R.J.; Khurana, A.; Nayak, D.; Kumar, A.; Karundevi, B. Effect of Homeopathic Preparations of Syzygium jambolanum and Cephalandra indica on Gastrocnemius Muscle of High Fat and High Fructose-Induced Type-2 Diabetic Rats. Homeopathy 2013, 102, 160–171. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Abdelaal, S.; Mohammed, H.O.; El-Shazly, A.M.; Daoud, R.; El Raey, M.A.; Sobeh, M. Syzygium jambos Extract Mitigates Pancreatic Oxidative Stress, Inflammation and Apoptosis and Modulates Hepatic IRS-2/AKT/GLUT4 Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 2021, 142, 112085. [Google Scholar] [CrossRef]
- Hossain, S.; Islam, J.; Bhowmick, S.; Haque, M.; Rahaman, A. Effects of Syzygium cumini Seed Extract on the Memory Loss of Alzheimer’s Disease Model Rats. Adv. Alzheimer’s Dis. 2017, 6, 53–73. [Google Scholar] [CrossRef] [Green Version]
- Mendes, R.F.; Bellozi, P.M.Q.; Conegundes, J.L.; Fernandes, M.F.; Pinto, N.C.C.; Silva, J.M.; Costa, J.C.; Chedier, L.M.; Dias, A.C.P.; Scio, E. In Vivo Anti-Inflammatory and Antinociceptive Effects, and in Vitro Antioxidant, Antiglycant and Anti-Neuroinflammatory Actions of Syzygium malaccense. An. Acad. Bras. Ciências 2021, 93, e20210457. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.; Akhtar, S.; Ismail, T.; Yuan, Y.; Ahmad, N.; Tawab, A.; Ismail, A.; Barnard, R.T.; Cooper, M.A.; Blaskovich, M.A.T. Syzygium cumini (L.), Skeels Fruit Extracts: In Vitro and in Vivo Anti-Inflammatory Properties. J. Ethnopharmacol. 2021, 271, 113805. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, B.; Taneja, N.; Saraf, M. Betulinic Acid, Isolated from the Leaves of Syzygium cumini (L.) Skeels, Ameliorates the Proteinuria in Experimental Membranous Nephropathy through Regulating Nrf2/NF-ΚB Pathways. Chem. Biol. Interact. 2017, 274, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Abdin, M.; Hamed, Y.S.; Akhtar, H.M.S.; Chen, D.; Chen, G.; Wan, P.; Zeng, X. Antioxidant and Anti-Inflammatory Activities of Target Anthocyanins Di-Glucosides Isolated from Syzygium cumini Pulp by High Speed Counter-Current Chromatography. J. Food Biochem. 2020, 44, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pathak, S.; Gupta, G.; Sharma, S.K.; Singh, L.; Sharma, R.K.; Mishra, A.; Dua, K. Pharmacological Evaluation of Aqueous Extract of Syzygium cumini for Its Antihyperglycemic and Antidyslipidemic Properties in Diabetic Rats Fed a High Cholesterol Diet-Role of PPARγ and PPARα. Biomed. Pharmacother. 2017, 89, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Atale, N.; Mishra, C.B.; Kohli, S.; Mongre, R.K.; Prakash, A.; Kumari, S.; Yadav, U.C.S.; Jeon, R.; Rani, V. Anti-Inflammatory Effects of S. cumini Seed Extract on Gelatinase-B (MMP-9) Regulation against Hyperglycemic Cardiomyocyte Stress. Oxidative Med. Cell. Longev. 2021, 2021, 8839479. [Google Scholar] [CrossRef]
- Heendeniya, S.; Ratnasooriya, W.D.; Pathirana, R.N. In Vitro Investigation of Anti-Inflammatory Activity and Evaluation of Phytochemical Profile of Syzygium caryophyllatum. J. Pharmacogn. Phytochem. 2018, 7, 1759–1763. [Google Scholar]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A Polyphenol-Rich Leaf Extract Exhibits Antioxidant, Hepatoprotective, Pain-Killing and Anti-Inflammatory Activities in Animal Models. Front. Pharmacol. 2018, 9, 566. [Google Scholar] [CrossRef] [Green Version]
- Chandran, R.; George, B.P.; Abrahamse, H. Anti-Proliferative, Analgesic and Anti-Inflammatory Properties of Syzygium mundagam Bark Methanol Extract. Molecules 2020, 25, 2900. [Google Scholar] [CrossRef]
- Chandran, R.; Abrahamse, H.; Parimelazhagan, T. Cytotoxic, Analgesic and Anti-Inflammatory Properties of Syzygium calophyllifolium Bark. Biomed. Pharmacother. 2018, 103, 1079–1085. [Google Scholar] [CrossRef]
- Fatima, H.; Shahid, M.; Jamil, A.; Naveed, M. Therapeutic Potential of Selected Medicinal Plants Against Carrageenan Induced Inflammation in Rats. Dose Response 2021, 19, 15593258211058028. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Beltrán-Villalobos, K.L.; Aguilar-Mariscal, H.; González-Trujano, M.E.; Ángeles-López, G.E.; Ventura-Martínez, R. Synergistic Herb-Herb Interaction of the Antinociceptive and Anti-Inflammatory Effects of Syzygium aromaticum and Rosmarinus officinalis Combination. Evid.-Based Complement. Altern. Med. 2021, 2021, 8916618. [Google Scholar] [CrossRef] [PubMed]
- Chniguir, A.; Zioud, F.; Marzaioli, V.; El-Benna, J.; Bachoual, R. Syzygium aromaticum Aqueous Extract Inhibits Human Neutrophils Myeloperoxidase and Protects Mice from LPS-Induced Lung Inflammation. Pharm. Biol. 2019, 57, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendi, A.; Yağci, B.G.; Kiziloğlu, M.; Saraç, N.; Yilmaz, D.; Uğur, A.; Uçkan, D. Effects of Syzygium aromaticum, Cinnamomum zeylanicum, and Salvia triloba Extracts on Proliferation and Differentiation of Dental Pulp Stem Cells. J. Appl. Oral Sci. 2017, 25, 515–522. [Google Scholar] [CrossRef]
- Chniguir, A.; Pintard, C.; Liu, D.; Dang, P.M.-C.; El-Benna, J.; Bachoual, R. Eugenol Prevents FMLF-Induced Superoxide Anion Production in Human Neutrophils by Inhibiting ERK1/2 Signaling Pathway and P47phox Phosphorylation. Sci. Rep. 2019, 9, 18540. [Google Scholar] [CrossRef]
- Hobani, Y.H.; Mohan, S.; Shaheen, E.; Abdelhaleem, A.; Faruque Ahmad, M.; Bhatia, S.; Abou-Elhamd, A.S. Gastroprotective Effect of Low Dose Eugenol in Experimental Rats against Ethanol Induced Toxicity: Involvement of Antiinflammatory and Antioxidant Mechanism. J. Ethnopharmacol. 2022, 289, 115055. [Google Scholar] [CrossRef]
- Beltrán-Villalobos, K.L.; Déciga-Campos, M.; Aguilar-Mariscal, H.; González-Trujano, M.E.; Martínez-Salazar, M.F.; de Ramírez-Cisneros, M.L.Á.; Rios, M.Y.; López-Muñoz, F.J. Synergistic Antinociceptive Interaction of Syzygium aromaticum or Rosmarinus officinalis Coadministered with Ketorolac in Rats. Biomed. Pharmacother. 2017, 94, 858–864. [Google Scholar] [CrossRef]
- Chang, W.-C.; Wu, J.S.; Shen, S.-C. Vescalagin from Pink Wax Apple (Syzygium samarangense (Blume) Merrill and Perry) Protects Pancreatic β-Cells against Methylglyoxal-Induced Inflammation in Rats. Plants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Khamchan, A.; Paseephol, T.; Hanchang, W. Protective Effect of Wax Apple (Syzygium samarangense (Blume) Merr. & L.M. Perry) against Streptozotocin-Induced Pancreatic ß-Cell Damage in Diabetic Rats. Biomed. Pharmacother. 2018, 108, 634–645. [Google Scholar] [CrossRef]
- Hasan, R.; Lindarto, D.; Siregar, G.A.; Mukhtar, Z. The Effect of Bay Leaf Extract Syzygium polyanthum (Wight) Walp. on C-Reactive Protein (CRP) and Myeloperoxidase (MPO) Level in the Heart of Rat Model of Myocardial Infarction. Med. Glas. Off. Publ. Med. Assoc. Zenica-Doboj Canton Bosnia Herzeg. 2020, 17, 41–45. [Google Scholar] [CrossRef]
- Go, Y.-M.; Jones, D.P. Redox Control Systems in the Nucleus: Mechanisms and Functions. Antioxid. Redox Signal. 2010, 13, 489–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, A.B.M.N.; Hossain, F.; Reza, A.S.M.A.; Nasrin, M.S.; Alam, A.H.M.K. Traditional Uses, Pharmacological Activities, and Phytochemical Constituents of the Genus Syzygium: A Review. Food Sci. Nutr. 2022, 1–31. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, B.; Wang, Y. Clove Essential Oil Confers Antioxidant Activity and Lifespan Extension in C. Elegans via the DAF-16/FOXO Transcription Factor. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 242, 108938. [Google Scholar] [CrossRef] [PubMed]
- Alfikri, F.N.; Pujiarti, R.; Wibisono, M.G.; Hardiyanto, E.B. Yield, Quality, and Antioxidant Activity of Clove (Syzygium aromaticum L.) Bud Oil at the Different Phenological Stages in Young and Mature Trees. Scientifica 2020, 2020, 9701701. [Google Scholar] [CrossRef]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; da Calabrese, K.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid.-Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef]
- Simas Frauches, N.; Montenegro, J.; Amaral, T.; Abreu, J.P.; Laiber, G.; Junior, J.; Borguini, R.; Santiago, M.; Pacheco, S.; Nakajima, V.M.; et al. Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules 2021, 26, 564. [Google Scholar] [CrossRef]
- Franco, R.R.; Ribeiro Zabisky, L.F.; Pires de Lima Júnior, J.; Mota Alves, V.H.; Justino, A.B.; Saraiva, A.L.; Goulart, L.R.; Espindola, F.S. Antidiabetic Effects of Syzygium cumini Leaves: A Non-Hemolytic Plant with Potential against Process of Oxidation, Glycation, Inflammation and Digestive Enzymes Catalysis. J. Ethnopharmacol. 2020, 261, 113132. [Google Scholar] [CrossRef]
- Koop, B.L.; Knapp, M.A.; Di Luccio, M.; Pinto, V.Z.; Tormen, L.; Valencia, G.A.; Monteiro, A.R. Bioactive Compounds from Jambolan (Syzygium cumini (L.)) Extract Concentrated by Ultra- and Nanofiltration: A Potential Natural Antioxidant for Food. Plant Foods Hum. Nutr. 2021, 76, 90–97. [Google Scholar] [CrossRef]
- Siddika, A.; Das, P.K.; Asha, S.Y.; Aktar, S.; Tareq, A.R.M.; Siddika, A.; Rakib, A.; Islam, F.; Khanam, J.A. Antiproliferative Activity and Apoptotic Efficiency of Syzygium cumini Bark Methanolic Extract against EAC Cells In Vivo. Anticancer Agents Med. Chem. 2021, 21, 782–792. [Google Scholar] [CrossRef]
- De Morais Sousa, M.; de Lima, R.M.T.; de Lima, A.; Reis, A.C.; Cavalcante, A.A.D.C.M.; Sattler, J.A.G.; de Almeida-Muradian, L.B.; de Lima Neto, J.S.; Moreira-Araujo, R.S.D.R.; do Nogueira, N. Antioxidant Action and Enzyme Activity Modulation by Bioaccessible Polyphenols from Jambolan (Syzygium cumini (L.) Skeels). Food Chem. 2021, 363, 130353. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Ojo, O.A.; Oyinloye, B.E.; Akuboh, O.; Okesola, M.A.; Idowu, O.; Kappo, A.P. In Vitro Antioxidant and Inhibitory Activities of Polyphenolic-Rich Extracts of Syzygium cumini (Linn) Skeels Leaf on Two Important Enzymes Relevant to Type II Diabetes Mellitus. Pak. J. Pharm. Sci. 2020, 33, 523–529. [Google Scholar] [PubMed]
- Mahindrakar, K.V.; Rathod, V.K. Valorization of Waste Syzygium cumini Seed Kernels by Three-Phase Partitioning Extraction and Evaluation of in Vitro Antioxidant and Hypoglycemic Potential. Prep. Biochem. Biotechnol. 2021, 51, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Yadav, Y.; Singh, A.P.; Pradhan, R.; Desai, G.R.; Dey, A.B.; Dey, S. Neuroprotection by Ethanolic Extract of Syzygium aromaticum in Alzheimer’s Disease like Pathology via Maintaining Oxidative Balance through SIRT1 Pathway. Exp. Gerontol. 2018, 110, 277–283. [Google Scholar] [CrossRef]
- Jawed, H.; Shamim, M.; Sohail, S.; Firdous, U.; Iqbal Khan, N. Antioxidative Activity of Clove (Syzygium aromaticum) Oil Administration in Middle Cerebral Artery Occlusion (Mcao) Models of Acute Focal Cerebral Ischemia. Pak. J. Neurol. Sci. 2019, 14, 10–15. [Google Scholar]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and Antioxidant Activity of Unencapsulated and Encapsulated Clove (Syzygium aromaticum, L.) Essential Oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef]
- De Santos, M.V.O.; da Silva, A.M.; Praxedes, É.A.; Borges, A.A.; de Teles Filho, A.C.A.; Souza-Junior, J.B.F.; Bertini, L.M.; Silva, A.R.; Pereira, A.F. Antioxidant Effects of the Essential Oil of Syzygium aromaticum on Bovine Epididymal Spermatozoa. Andrologia 2019, 51, e13448. [Google Scholar] [CrossRef]
- Chang, H.-J.; Kim, Y.-H.; Kang, Y.-H.; Choi, M.-H.; Lee, J.-H. Antioxidant and Antibacterial Effects of Medicinal Plants and Their Stick-Type Medicinal Concentrated Beverages. Food Sci. Biotechnol. 2020, 29, 1413–1423. [Google Scholar] [CrossRef]
- Wathsara, H.P.T.; Weeratunge, H.D.; Mubarak, M.N.A.; Godakumbura, P.I.; Ranasinghe, P. In Vitro Antioxidant and Antidiabetic Potentials of Syzygium caryophyllatum L. Alston. Evid. Based. Complement. Alternat. Med. 2020, 2020, 9529042. [Google Scholar] [CrossRef]
- Kumari, G.U.W.U.P.; Gunathilake, K.D.P.P. In Vitro Bioaccessibility and Antioxidant Activity of Black Plum (Syzygium caryophyllatum). J. Food Biochem. 2020, 44, e13499. [Google Scholar] [CrossRef]
- Kasunmala, I.G.G.; Bandara Navarathne, S.; Wickramasinghe, I. Preparation of Liquid-Core Hydrogel Beads Using Antioxidant-Rich Syzygium caryophyllatum Fruit Pulp as a Healthy Snack. J. Texture Stud. 2020, 51, 937–947. [Google Scholar] [CrossRef]
- Kim, S.; Semple, S.J.; Simpson, B.S.; Deo, P. Antioxidant and Antiglycation Activities of Syzygium paniculatum Gaertn and Inhibition of Digestive Enzymes Relevant to Type 2 Diabetes Mellitus. Plant Foods Hum. Nutr. 2020, 75, 621–627. [Google Scholar] [CrossRef]
- Konda, P.Y.; Dasari, S.; Konanki, S.; Nagarajan, P. In Vivo Antihyperglycemic, Antihyperlipidemic, Antioxidative Stress and Antioxidant Potential Activities of Syzygium paniculatum Gaertn. in Streptozotocin-Induced Diabetic Rats. Heliyon 2019, 5, e01373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoh, S.O.; Okoh, O.O.; Okoh, A.I. Seasonal Variation of Volatile Oil Composition and Antioxidant Property of Aerial Parts of Syzygium paniculatum Gaertn. Grown in the Eastern Cape, South Africa. Nat. Prod. Res. 2019, 33, 2276–2280. [Google Scholar] [CrossRef] [PubMed]
- Itam, A.; Anna, L. Antioxidant Activities, Cytotoxic Properties and Total Phenolic Content of Syzygium malaccense (L.) Merr. & L.M. Perry Leaves Extracts: A West Sumatera Indonesian Plant. Pak. J. Pharm. Sci. 2020, 33, 175–181. [Google Scholar] [PubMed]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Protective Effect of Myricetin Derivatives from Syzygium malaccense against Hydrogen Peroxide-Induced Stress in ARPE-19 Cells. Mol. Vis. 2019, 25, 47–59. [Google Scholar] [PubMed]
- Itam, A.; Wati, M.S.; Agustin, V.; Sabri, N.; Jumanah, R.A.; Efdi, M. Comparative Study of Phytochemical, Antioxidant, and Cytotoxic Activities and Phenolic Content of Syzygium aqueum (Burm. f. Alston f.) Extracts Growing in West Sumatera Indonesia. Sci. World J. 2021, 2021, 5537597. [Google Scholar] [CrossRef] [PubMed]
- Hartanti, L.; Yonas, S.M.K.; Mustamu, J.J.; Wijaya, S.; Setiawan, H.K.; Soegianto, L. Influence of Extraction Methods of Bay Leaves (Syzygium polyanthum) on Antioxidant and HMG-CoA Reductase Inhibitory Activity. Heliyon 2019, 5, e01485. [Google Scholar] [CrossRef] [Green Version]
- Mohamed Yunus, S.N.; Abas, F.; Jaafar, A.H.; Azizan, A.; Zolkeflee, N.K.Z.; Abd Ghafar, S.Z. Antioxidant and α-Glucosidase Inhibitory Activities of Eight Neglected Fruit Extracts and UHPLC-MS/MS Profile of the Active Extracts. Food Sci. Biotechnol. 2021, 30, 195–208. [Google Scholar] [CrossRef]
- Wahed, T.B.; Mondal, M.; Rahman, M.A.; Hossen, M.S.; Bhoumik, N.C.; Saha, S.; Tanvir, E.M.; Khalil, M.I.; Kundu, S.K.; Islam, M.T.; et al. Protective Role of Syzygium cymosum Leaf Extract Against Carbofuran-Induced Hematological and Hepatic Toxicities. Chem. Res. Toxicol. 2019, 32, 1619–1629. [Google Scholar] [CrossRef]
- Rahaman, A.; Hossain, S.; Rahman, M.; Hossain, I.; Nahar, T.; Uddin, B.; Khalil, I. Syzygium cumini (L.) Seed Extract Improves Memory Related Learning Ability of Old Rats in Eight Arm Radial Maze. J. Pharmacogn. Phytochem. 2013, 1, 85–94. [Google Scholar]
- Ledreux, A.; Wang, X.; Schultzberg, M.; Granholm, A.-C.; Freeman, L.R. Detrimental Effects of a High Fat/High Cholesterol Diet on Memory and Hippocampal Markers in Aged Rats. Behav. Brain Res. 2016, 312, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Batista, Â.G.; Mendonça, M.C.P.; Soares, E.S.; da Silva-Maia, J.K.; Dionísio, A.P.; Sartori, C.R.; da Cruz-Höfling, M.A.; Maróstica Júnior, M.R. Syzygium malaccense Fruit Supplementation Protects Mice Brain against High-Fat Diet Impairment and Improves Cognitive Functions. J. Funct. Foods 2020, 65, 103745. [Google Scholar] [CrossRef]
- Amir Rawa, M.S.; Nurul Azman, N.A.; Mohamad, S.; Nogawa, T.; Wahab, H.A. In Vitro and In Silico Anti-Acetylcholinesterase Activity from Macaranga tanarius and Syzygium jambos. Molecules 2022, 27, 2648. [Google Scholar] [CrossRef] [PubMed]
- Jessica Elizabeth, D.L.T.; Gassara, F.; Kouassi, A.P.; Brar, S.K.; Belkacemi, K. Spice Use in Food: Properties and Benefits. Crit. Rev. Food Sci. Nutr. 2017, 57, 1078–1088. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Soh, W.-K.; Parnell, J. A Revision of Syzygium Gaertn.(Myrtaceae) in Indochina (Cambodia, Laos and Vietnam). Adansonia 2015, 37, 179–275. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical Composition and Antioxidant Properties of Clove Leaf Essential Oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Amber, S.; Shah, S.A.A.; Ahmed, T.; Zahid, S. Syzygium aromaticum Ethanol Extract Reduces AlCl3-Induced Neurotoxicity in Mice Brain through Regulation of Amyloid Precursor Protein and Oxidative Stress Gene Expression. Asian Pac. J. Trop. Med. 2018, 11, 123. [Google Scholar]
- Kumar, A.; Aggrawal, A.; Pottabathini, R.; Singh, A. Possible Neuroprotective Mechanisms of Clove Oil against Icv-Colchicine Induced Cognitive Dysfunction. Pharmacol. Rep. 2016, 68, 764–772. [Google Scholar] [CrossRef]
- Milind, P.; Deepa, K. Pro-Cholinergi, Hypo-Cholesterolemic and Memory Improving Effects of Clove. Int. Res. J. Pharm. 2011, 2, 119–126. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amir Rawa, M.S.; Mazlan, M.K.N.; Ahmad, R.; Nogawa, T.; Wahab, H.A. Roles of Syzygium in Anti-Cholinesterase, Anti-Diabetic, Anti-Inflammatory, and Antioxidant: From Alzheimer’s Perspective. Plants 2022, 11, 1476. https://doi.org/10.3390/plants11111476
Amir Rawa MS, Mazlan MKN, Ahmad R, Nogawa T, Wahab HA. Roles of Syzygium in Anti-Cholinesterase, Anti-Diabetic, Anti-Inflammatory, and Antioxidant: From Alzheimer’s Perspective. Plants. 2022; 11(11):1476. https://doi.org/10.3390/plants11111476
Chicago/Turabian StyleAmir Rawa, Mira Syahfriena, Mohd Khairul Nizam Mazlan, Rosliza Ahmad, Toshihiko Nogawa, and Habibah A. Wahab. 2022. "Roles of Syzygium in Anti-Cholinesterase, Anti-Diabetic, Anti-Inflammatory, and Antioxidant: From Alzheimer’s Perspective" Plants 11, no. 11: 1476. https://doi.org/10.3390/plants11111476
APA StyleAmir Rawa, M. S., Mazlan, M. K. N., Ahmad, R., Nogawa, T., & Wahab, H. A. (2022). Roles of Syzygium in Anti-Cholinesterase, Anti-Diabetic, Anti-Inflammatory, and Antioxidant: From Alzheimer’s Perspective. Plants, 11(11), 1476. https://doi.org/10.3390/plants11111476






