Can Dairy Slurry Application to Stubble, without Incorporation into the Soil, Be Sustainable?
Abstract
1. Introduction
2. Results
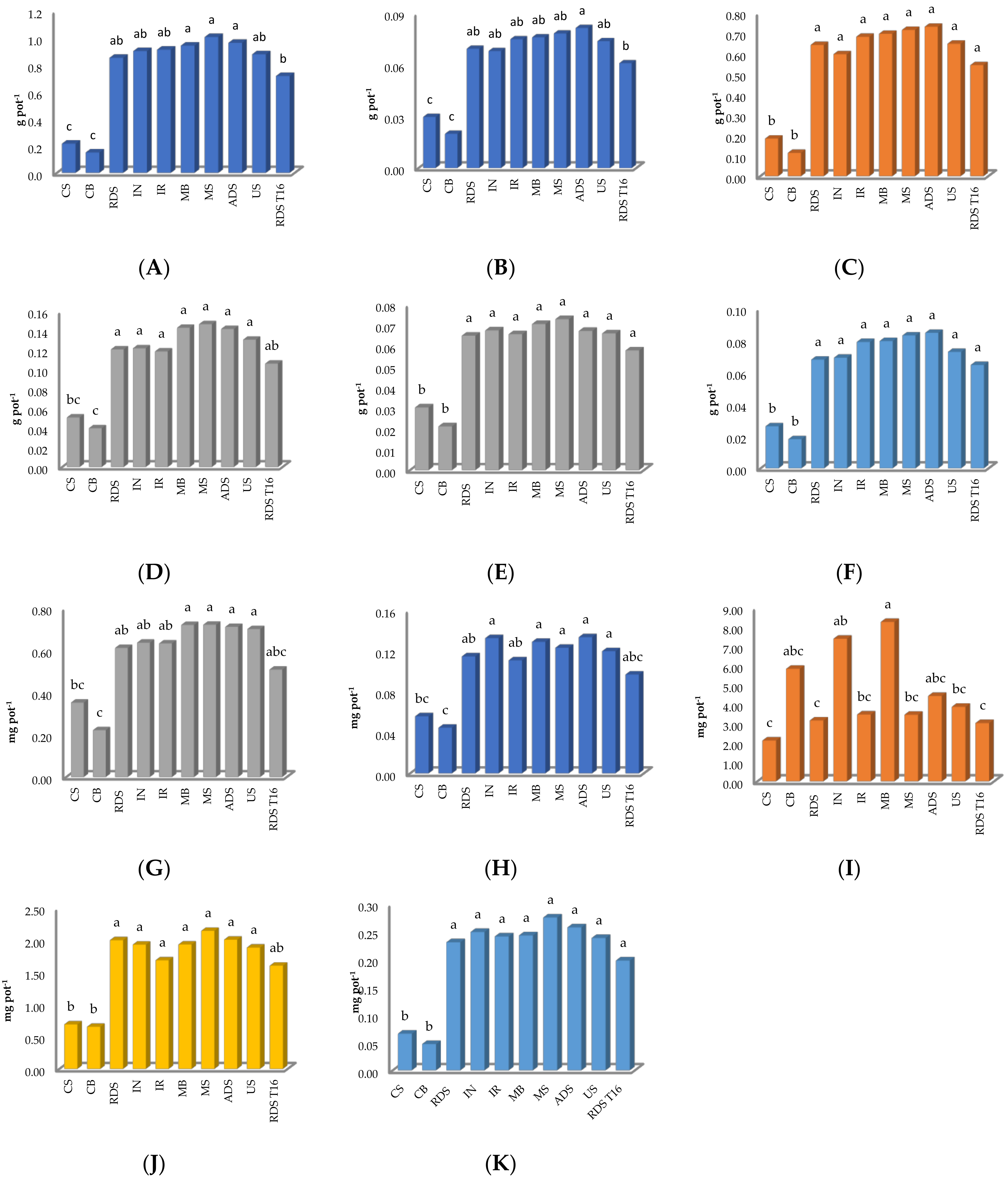
2.1. Ryegrass Aboveground Biomass
2.2. Nutrient Recovery
2.3. Effects on the Soil
3. Discussion
3.1. Ryegrass Yield
3.2. Nutrients Use Efficiency
3.3. Nitrogen-Mineral Fertilizer Equivalence
3.4. Effects on Soil Properties
4. Materials and Methods
4.1. Soil, Slurry and Wheat Stubble
4.2. Experimental Design
- i.
- unfertilized bare soil, control (CB), injected slurry in bare soil (IN), mineral fertilizer applied on bare soil (MB);
- ii.
- unfertilized stubble-covered soil, control (CS), raw dairy slurry on the stubble (RDS), acidified dairy slurry on the stubble (ADS), irrigation just after RDS application (IR), mineral nitrogen on the stubble (MS), raw slurry applied under the stubble (US), 16-day delayed application of RDS (RDS T16).
4.3. Analytical Methods
4.4. Nutrient Use Efficiency-Related Indicators
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EC. Food, Feed, Fibres, Fuels. Enough Biomass for a Sustainable Bieconomy? The European Comission’s Science and Knowledge Service; EC: Brussels, Belgium, 2019; Available online: https://knowledge4policy.ec.europa.eu/publication/food-feed-fibres-fuels-enough-biomass-sustainable-bioeconomy_en (accessed on 12 December 2021).
- FAO. Global Agriculture Towards 2050. High Level Experts Forum—How to Feed the World in 2050; FAO: Rome, Italy, 2009; Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf (accessed on 8 July 2021).
- Westcott, P.; Trostle, R. Long-Term Prospects for Agriculture Reflect Growing Demand for Food, Fiber and Fuel; USDA: Washington, DC, USA, 2012. Available online: https://www.ers.usda.gov/amber-waves/2012/september/long-term-prospects-for-agriculture (accessed on 4 February 2022).
- Liniger, H.P.; Studer, R.M.; Hauert, C.; Gurtner, M. Gurtner. Sustainable Land Management in Practice—Guidelines and best Practices for Sub-Saharan Africa; TerrAfrica, World Overview of Conservation Approaches and Technologies (WOCAT) and Food and Agriculture Organization of the United Nations (FAO): Bern, Switzerland; Rome, Italy, 2011. [Google Scholar]
- Carvalho, M.D.C.S.; Lourenço, E. Conservation Agriculture—A Portuguese Case Study. J. Agron. Crop Sci. 2014, 200, 317–324. [Google Scholar] [CrossRef]
- Crassweller, R. Orchard Establishment—Row Middle and Tree Row; PennState Extension: State College, PA, USA, 2017; Available online: https://extension.psu.edu/orchard-establishment-row-middle-and-tree-row (accessed on 29 January 2022).
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Michler, J.D.; Baylis, K.; Arends-Kuenning, M.; Mazvimavi, K. Conservation agriculture and climate resilience. J. Environ. Econ. Manag. 2019, 93, 148–169. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; Van Groenigen, K.J.; Lee, J.; Lundy, M.E.; Van Gestel, N.; Six, J.; Venterea, R.T.; Van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Steward, P.R.; Dougill, A.J.; Thierfelder, C.; Pittelkow, C.; Stringer, L.C.; Kudzala, M.; Shackelford, G.E. The adaptive capacity of maize-based conservation agriculture systems to climate stress in tropical and subtropical environments: A meta-regression of yields. Agric. Ecosyst. Environ. 2018, 251, 194–202. [Google Scholar] [CrossRef]
- Barão, L.; Alaoui, A.; Ferreira, C.; Basch, G.; Schwilch, G.; Geissen, V.; Sukkel, W.; Lemesle, J.; Garcia-Orenes, F.; Morugán-Coronado, A.; et al. Assessment of promising agricultural management practices. Sci. Total Environ. 2019, 649, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Corsi, S. Conservation Agriculture: Training Guide for Extension Agents and Farmers in Eastern Europe and Central Asia; FAO: Rome, Italy, 2019; Available online: www.fao.org/emergencies (accessed on 18 July 2021).
- Vastola, A.; Zdruli, P.; D’Amico, M.; Pappalardo, G.; Viccaro, M.; Di Napoli, F.; Cozzi, M.; Romano, S. A comparative multidimensional evaluation of conservation agriculture systems: A case study from a Mediterranean area of Southern Italy. Land Use Policy 2017, 68, 326–333. [Google Scholar] [CrossRef]
- Phillips, R.E.; Thomas, G.W.; Blevins, R.L.; Frye, W.W.; Phillips, S.H. No-Tillage Agriculture. Science 1980, 208, 1108–1113. [Google Scholar] [CrossRef]
- FAO. Nutrient Flows an Environmental Impacts in Livestock Supply Chain: Guidelines for Assessment; FAO: Rome, Italy, 2018; Available online: https://www.fao.org/publications/card/en/c/CA1328EN (accessed on 12 December 2021).
- Soane, B.B.; Ball, B.C.; Arvidsson, J.; Basch, G.L.; Moreno, F.; Roger-Estrade, J. No-till in northern, western and south western Europe: A review of problems and opportunities for crop production and the environment to cite this version: HAL Id: Hal-00956463. Soil Tillage Res. 2012, 118, 66–87. [Google Scholar] [CrossRef]
- Cameira, M.D.R.; Valente, F.; Li, R.; Surgy, S.; Abreu, F.G.; Coutinho, J.; Fangueiro, D. Band application of acidified slurry as an alternative to slurry injection in Mediterranean winter conditions: Impact on nitrate leaching. Soil Tillage Res. 2019, 187, 172–181. [Google Scholar] [CrossRef]
- EUROSTAT. Agri-Environmental Indicator—Soil Erosion. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_soil_erosion#Introduction (accessed on 2 December 2021).
- Kumar, R.R.; Park, B.J.; Cho, J.Y. Application and environmental risks of livestock manure. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 497–503. [Google Scholar] [CrossRef]
- Ozlu, E.; Sandhu, S.S.; Kumar, S.; Arriaga, F.J. Soil health indicators impacted by long-term cattle manure and inorganic fertilizer application in a corn-soybean rotation of South Dakota. Sci. Rep. 2019, 9, 11776. [Google Scholar] [CrossRef]
- Fangueiro, D.; Pereira, J.L.D.S.; Bichana, A.; Surgy, S.; Cabral, F.; Coutinho, J. Effects of cattle-slurry treatment by acidification and separation on nitrogen dynamics and global warming potential after surface application to an acidic soil. J. Environ. Manag. 2015, 162, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, D.; Pereira, J.L.D.S.; Macedo, S.; Trindade, H.; Vasconcelos, E.; Coutinho, J. Surface application of acidified cattle slurry compared to slurry injection: Impact on NH3, N2O, CO2 and CH4 emissions and crop uptake. Geoderma 2017, 306, 160–166. [Google Scholar] [CrossRef]
- Morino, C.C. A Aplicação de Dejetos de Suínos no Solo Como Insumo. Escola Superior da CETESB. 2021. Available online: https://cetesb.sp.gov.br/escolasuperior/wp-content/uploads/sites/30/2021/08/Camila-Canesi-Morino_TCC-T2-2021-versao-final.pdf (accessed on 8 December 2021).
- UNECE. Guidance Document on Preventing and Abating Ammonia Emissions from Agricultural Sources; UNECE: Geneva, Switzerland, 2014. [Google Scholar]
- Sommer, S.; Génermont, S.; Cellier, P.; Hutchings, N.; Olesen, J.E.; Morvan, T. Processes controlling ammonia emission from livestock slurry in the field. Eur. J. Agron. 2003, 19, 465–486. [Google Scholar] [CrossRef]
- Koelsch, R. Extending the Manure Application Window: Post Plant Experiences. 2020. Available online: https://water.unl.edu/article/animal-manure-management/extending-manure-application-window-post-plant-experiences (accessed on 17 June 2021).
- Jensen, L.S. Animal Manure Fertilizer Value, Crop Utilisation and Soil Quality Impacts. In Animal Manure: Recycling, Treatment and Management, 1st ed.; Sommer, S.G., Christensen, M.L., Schmidt, T., Jensen, L.S., Eds.; Wiley: West Sussex, UK, 2013; pp. 295–328. [Google Scholar]
- Bittman, S.; Dedina, M.; Howard, C.M.; Oenema, O.; Sutton, M.A. Options for Ammonia Mitigation: Guidance from the UNECE Task Force on Reactive Nitrogen; TFRN-CLRTAP, Centre of Ecology and Hydrology: Lancaster, UK, 2014. [Google Scholar]
- Bell, M. A Review of Nitrogen use Efficiency in Sugarcane. Research Report of Sugarcane; Sugarcane Research Australia: Brisbane, Australia, 2015; p. 315. [Google Scholar]
- Chien, S.H.; Gearhart, M.M.; Villagarcía, S. Comparison of ammonium sulfate with other nitrogen and sulfur fertilizers in increasing crop production and minimizing environmental impact: A review. Soil Sci. 2011, 176, 327–335. [Google Scholar] [CrossRef]
- Vieira, R.F. Ciclo do Nitrogênio em Sistemas Agrícolas; Embrapa: Brasília, Brazil, 2017; p. 163. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/175460/1/2017LV04.pdf (accessed on 12 October 2021).
- Donaghy, D.; Fulkerson, W. Principles for Developing an Effective Grazing Management System for Ryegrass-Based Pastures; Tasmanian Institute of Agricultural Research: Hobart, TAS, Australia, 2001; pp. 1–10. Available online: http://www.heritageseeds.com.au/ASSETS/latestpressreleases/Managementprincipal.pdf (accessed on 20 September 2021).
- Hunt, W.F.; Easton, H.S. Fifty years of ryegrass research in New Zealand. Proc. N. Z. Grassl. Assoc. 1989, 50, 1–23. [Google Scholar] [CrossRef]
- Ayanz, A.S.M. Gramíneas de Interés Para Implantación de Praderas y la Revegetación de Zonas Degradadas; Ecología y Pautas Básicas de Utilización; Universidad Politécnica de Madrid: Madrid, Spain, 2008; p. 25. [Google Scholar]
- Hart, J.; Mellybye, M.E.; Young, W.C., III; Silberstein, T.B. Annual Ryegrass Grown for Seed (Western Oregon). Nutrient Management Guide; Extension Service, Oregon State University: Corvallis, OR, USA, 2011; Available online: http://ir.library.oregonstate.edu/xmlui/handle/1957/20032 (accessed on 22 January 2022).
- Dominico, C.D.F.T.; Lustosa, S.B.C.; De Ávila, F.W. Acúmulo de matéria seca e absorção de nitrogênio, fósforo e potássio por azevém (Lolium multiflorum Lam.) cultivar BARjumbo. Res. Soc. Dev. 2020, 9, 1–23. [Google Scholar] [CrossRef]
- Miller, J.O. pH Affects Nutrient Availability; University of Maryland Extension: College Park, MD, USA, 2016; p. 5. Available online: https://extension.umd.edu/resource/soil-ph-affects-nutrient-availability (accessed on 4 November 2021).
- AWI; MLA. Healthy Soils. In Making More from Sheep; Australian Wool Innovation Limited; Meet & Livestock Australia Limited: Sydney, Australia, 2008; Available online: http://www.makingmorefromsheep.com.au/healthy-soils/tool_6.5.htm (accessed on 12 June 2020).
- Plaza-Bonilla, D.; Cantero-Martínez, C.; Bareche, J.; Arrúe, J.L.; Lampurlanés, J.; Álvaro-Fuentes, J. Do no-till and pig slurry application improve barley yield and water and nitrogen use efficiencies in rainfed Mediterranean conditions? Field Crop. Res. 2017, 203, 74–85. [Google Scholar] [CrossRef]
- Abreu, C.A.; Lopes, A.S.; Santos, G.C.G. Micronutrientes. In Fertilidade do Solo; Novais, R.F., Alvarez, V.V.H., Barros, N.F., Fontes, R.L., Cantarutti, R.B., Neves, J.C.L., Eds.; SBCS: Viçosa, Brazil, 2007; pp. 645–736. [Google Scholar]
- IPNI. Ferro. In Nutri-Fatos: Informação Agronômica Sobre Nutrientes Para as Plantas; IPNI: Piracicaba, Brazil, 2009; pp. 12–13. [Google Scholar] [CrossRef]
- Kovaleski, S.; Heldwein, A.B.; Dalmago, A.G.; Cunha, G.R.; Fochesatto, E.; Gouvêa, J.A.; Liska, B. Temperatura e Fluxo de Calor no Solo em Dossel de Canola em Função da Distribuição da Palha na Superfície, em Noites de Ocorrência de Geada. In Proceedings of the XIX Congresso Brasileiro de Agrometeorologia, Lavras, Brazil, 23–28 August 2015. [Google Scholar]
- Imran; Amanullah. Phosphorus and Boron Application Optimizing Biofortification of P and Productivity of French Bean (Phaseolus vulgaris L.). Commun. in Soil Sci. Plant Anal. 2021, 52, 2876–2883. [Google Scholar] [CrossRef]
- The, S.V.; Snyder, R.; Tegeder, M. Targeting Nitrogen Metabolism and Transport Processes to Improve Plant Nitrogen Use Efficiency. Front. Plant Sci. 2021, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dobermann, A. Nutrient use efficiency–measurement and management. In Proceedings of the IFA International Workshop on Fertilizer Best Management Practices, Brussels, Belgium, 7–9 March 2007; Available online: http://sustainablecropnutrition.net/ifacontent/download/7163/113016/version/1/file/2007_IFA_FBMP+Workshop_Brussels.pdf#page=8 (accessed on 30 July 2021).
- Ferreira, P.A.A.; Ceretta, C.A.; Lourenzi, C.R.; De Conti, L.; Marchezan, C.; Girotto, E.; Tiecher, T.L.; Palermo, N.M.; Parent, L.; Brunetto, G. Long-Term Effects of Animal Manures on Nutrient Recovery and Soil Quality in Acid Typic Hapludalf under No-Till Conditions. Agronomy 2022, 12, 243. [Google Scholar] [CrossRef]
- Fangueiro, D.; Surgy, S.; Fraga, I.; Monteiro, F.; Cabral, F.; Coutinho, J. Acidification of animal slurry affects the nitrogen dynamics after soil application. Geoderma 2016, 281, 30–38. [Google Scholar] [CrossRef]
- Forrestal, P.J.; Harty, M.; Carolan, R.; Lanigan, G.J.; Watson, C.J.; Laughlin, R.J.; McNeill, G.; Chambers, B.J.; Richards, K. Ammonia emissions from urea, stabilized urea and calcium ammonium nitrate: Insights into loss abatement in temperate grassland. Soil Use Manag. 2016, 32, 92–100. [Google Scholar] [CrossRef]
- Silva, A.A.; Fangueiro, D.; Carvalho, M. Slurry Acidification as a Solution to Minimize Ammonia Emissions from the Combined Application of Animal Manure and Synthetic Fertilizer in No-Tillage. Agronomy 2022, 12, 265. [Google Scholar] [CrossRef]
- Buck, G.B.; De Castro, G.F.; Mattiello, E.M.; Zotarelli, L. Applications of Gypsum and Ammonium Sulfate Change Soil Chemical Properties of a Salt-Affected Agricultural Soil. J. Agric. Sci. 2020, 12, 1. [Google Scholar] [CrossRef]
- Fageria, N.K.; Dos Santos, A.B.; Moraes, M.F. Influence of Urea and Ammonium Sulfate on Soil Acidity Indices in Lowland Rice Production. Commun. Soil Sci. Plant Anal. 2010, 41, 1565–1575. [Google Scholar] [CrossRef]
- Pedersen, I.F.; Rubæk, G.H.; Sørensen, P. Cattle slurry acidification and application method can improve initial phosphorus availability for maize. Plant Soil 2017, 414, 143–158. [Google Scholar] [CrossRef]
- Roboredo, M.; Fangueiro, D.; Lage, S.; Coutinho, J. Phosphorus dynamics in soils amended with acidified pig slurry and derived solid fraction. Geoderma 2012, 189–190, 328–333. [Google Scholar] [CrossRef]
- Feilberg, A.; Sommer, S.G. Ammonia and Malodorous Gases: Sources and Abatement Technologies. In Animal Manure Recycling: Treatment and Management; Sommer, S.G., Christensen, M.L., Schmidt, T., Jensen, L.S., Eds.; John Wiley and Sons Ltd: Chinchester, UK, 2013; pp. 153–176. [Google Scholar]
- Sigurdarson, J.J.; Svane, S.; Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Bio/Technol. 2018, 17, 241–258. [Google Scholar] [CrossRef]
- Seidel, A.; Pacholski, A.; Nyord, T.; Vestergaard, A.; Pahlmann, I.; Herrmann, A.; Kage, H. Effects of acidification and injection of pasture applied cattle slurry on ammonia losses, N2O emissions and crop N uptake. Agric. Ecosyst. Environ. 2017, 247, 23–32. [Google Scholar] [CrossRef]
- Pantelopoulos, A.; Magid, J.; Jensen, L.S.; Fangueiro, D. Nutrient uptake efficiency in ryegrass fertilized with dried digestate solids as affected by acidification and drying temperature. Plant Soil 2017, 421, 401–416. [Google Scholar] [CrossRef]
- Reetz, H.F. Fertilizantes e o Seu uso Eficiente; Associação Nacional para Difusão de Adubos: São Paulo, Brazil, 2017; Available online: http://www.ufla.br/dcom/wp-content/uploads/2018/03/Fertilizantes-e-seu-uso-eficiente-WEBWord-Ouubro-2017x-1 (accessed on 29 November 2021).
- Gautam, A.; Guzman, J.; Kovacs, P.; Kumar, S. Manure and inorganic fertilization impacts on soil nutrients, aggregate stability, and organic carbon and nitrogen in different aggregate fractions. Arch. Agron. Soil Sci. 2021, 1–13. [Google Scholar] [CrossRef]
- Carmo, D.L.D.; De Lima, L.B.; Silva, C.A. Soil Fertility and Electrical Conductivity Affected by Organic Waste Rates and Nutrient Inputs. Rev. Bras. De Ciência Do Solo 2016, 40. [Google Scholar] [CrossRef]
- Nemali, K. Details of Electrical Conductivity Measurements in Greenhouse Production; Purdue Extension: Lafayette, LA, USA, 2018; Available online: https://www.extension.purdue.edu/extmedia/HO/HO-286-w.pdf (accessed on 10 February 2022).
- Haynes, R.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Gross, A.; Glaser, B. Meta-analysis on how manure application changes soil organic carbon storage. Sci. Rep. 2021, 11, 5516. [Google Scholar] [CrossRef]
- Corsi, S.; Friedrich, T.; Kassam, A.; Pisante, M.; Sà, J.D.M. Soil Organic Carbon Accumulation and Greenhouse Gas Emission Reductions from Conservation Agriculture: A Literature Review; FAO: Rome, Italy, 2012; Available online: https://www.fao.org/3/i2672e/i2672e.pdf (accessed on 12 September 2021).
- Luce, M.S.; Ziadi, N.; Chantigny, M.H.; Braun, J. Long-term effects of tillage and nitrogen fertilization on soil C and N fractions in a corn–soybean rotation. Can. J. Soil Sci. 2021, 1–16. [Google Scholar] [CrossRef]
- Abagandura, G.O.; Mahal, N.K.; Butail, N.P.; Dhaliwal, J.K.; Gautam, A.; Bawa, A.; Kovács, P.; Kumar, S. Soil labile carbon and nitrogen fractions after eleven years of manure and mineral fertilizer applications. Arch. Agron. Soil Sci. 2022, 1–16. [Google Scholar] [CrossRef]
- Whitney, T. Building Soil Organic Matter Takes Time; UNL Water: Lincoln, NB, USA, 2018; Available online: https://water.unl.edu/article/animal-manure-management/building-soil-organic-matter-takes-time (accessed on 18 February 2022).
- Gonzatto, R.; Miola, E.C.C.; Doneda, A.; Pujol, S.B.; Aita, C.; Giacomini, S.J. Volatilização de amônia e emissão de óxido nitroso após aplicação de dejetos líquidos de suínos em solo cultivado com milho. Ciência Rural 2013, 43, 1590–1596. [Google Scholar] [CrossRef][Green Version]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Fangueiro, D.; Surgy, S.; Fraga, I.; Cabral, F.; Coutinho, J. Band application of treated cattle slurry as an alternative to slurry injection: Implications for gaseous emissions, soil quality, and plant growth. Agric. Ecosyst. Environ. 2015, 211, 102–111. [Google Scholar] [CrossRef]


| Treatment | Harvest | Total Yield | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||
| DM g Pot−1 | % | DM g Pot−1 | % | DM g Pot−1 | % | DM g Pot−1 | % | DM g Pot−1 | |
| CB | 0.92 ab | 16.77 | 1.10 c | 20.18 | 1.30 d | 23.84 | 2.14 c | 39.21 | 5.47 d |
| CS | 1.04 ab | 12.64 | 1.87 bc | 22.72 | 1.51 d | 18.31 | 3.81 bc | 46.33 | 8.23 c |
| IN | 0.92 ab | 4.69 | 2.63 ab | 13.37 | 9.55 ab | 48.52 | 6.58 ab | 33.42 | 19.69 ab |
| MB | 0.92 ab | 4,22 | 3.03 ab | 13,90 | 9.15 abc | 41.93 | 8.72 a | 39.95 | 21.82 ab |
| MS | 1.30 a | 5.54 | 3.67 a | 15.67 | 9.48 ab | 40.49 | 8.97 a | 38.30 | 23.42 ab |
| RDS | 0.91 ab | 4.60 | 1.95 bc | 9.82 | 8.65 abc | 43.53 | 8.35 a | 42.05 | 19.86 ab |
| ADS | 1.21 ab | 4.94 | 3.19 ab | 13.01 | 10.65 a | 43.45 | 9.46 a | 38.60 | 24.52 a |
| IR | 0.83 ab | 3.80 | 2.52 ab | 11.61 | 9.40 ab | 43.26 | 8.98 a | 41.33 | 21.74 ab |
| US | 1.15 ab | 5.52 | 3.04 ab | 14.61 | 7.69 bc | 36.91 | 8.95 a | 42.96 | 20.83 ab |
| RDS T16 | 0.69 b | 3.96 | 1.92 bc | 11.05 | 6.41 c | 36.83 | 8.38 a | 48.15 | 17.40 b |
| Treatment | N (g g−1 Applied N) | P (g g−1 Applied P) | K (g g−1 Applied K) |
|---|---|---|---|
| MB | 0.53 a | 0.26 a | 0.90 |
| MS | 0.53 a | 0.22 ab | 0.82 |
| ADS | 0.50 a | 0.24 ab | 0.84 |
| IN | 0.50 a | 0.22 ab | 0.74 |
| IR | 0.46 ab | 0.21 ab | 0.77 |
| US | 0.44 ab | 0.20 ab | 0.72 |
| RDS | 0.42 ab | 0.18 ab | 0.71 |
| RDS T16 | 0.33 b | 0.15 b | 0.55 |
| Treatments | pH | EC | OM | P | K | Ca++ | Mg++ | K+ | Na+ |
|---|---|---|---|---|---|---|---|---|---|
| H2O | μS cm−1 | g Kg−1 | mg Kg−1 | cmolc Kg−1 | |||||
| CB | 7.44 a | 142.5 c | 25.8 | 153.41 | 193.75 | 34.54 | 10.09 | 0.24 | 0.47 |
| CS | 7.28 ab | 158.1 bc | 28.5 | 158.47 | 211.67 | 32.96 | 9.63 | 0.25 | 0.52 |
| IN | 7.02 abc | 552.3 ab | 23.8 | 155.78 | 172.90 | 35.82 | 10.34 | 0.24 | 0.64 |
| MB | 6.64 c | 758.3 a | 28.3 | 148.71 | 192.64 | 34.61 | 10.73 | 0.27 | 0.66 |
| MS | 6.82 bc | 842.3 a | 27.5 | 136.21 | 183.58 | 33.94 | 10.40 | 0.28 | 0.53 |
| RDS | 7.05 abc | 618.3 a | 27.6 | 154.40 | 208.86 | 34.46 | 9.93 | 0.26 | 0.58 |
| ADS | 6.86 bc | 588.3 a | 32.3 | 142.66 | 182.28 | 34.96 | 10.71 | 0.25 | 0.67 |
| IR | 6.84 bc | 603.6 a | 28.3 | 156.95 | 188.61 | 34.35 | 10.62 | 0.26 | 0.70 |
| US | 7.11 abc | 611.6 a | 25.4 | 151.99 | 207.54 | 34.19 | 10.46 | 0.26 | 0.54 |
| RDS T16 | 7.01 abc | 543.8 ab | 28.2 | 142.49 | 182.07 | 33.81 | 10.48 | 0.25 | 0.63 |
| Parameters | 0–5 cm Layer | 5–20 cm Layer |
|---|---|---|
| Organic Matter (g kg−1) | 35.4 | 34.5 |
| pH (H2O) | 7.08 | 7.13 |
| EC (μS cm−1) | 281.65 | 264.40 |
| Extractable P (mg kg−1) | 214.24 | 238.77 |
| Mg (cmolc kg−1) | 14.70 | 11.30 |
| K (cmolc kg−1) | 1.07 | 0.64 |
| Ca (cmolc kg−1) | 44.7 | 63.85 |
| Na (cmolc kg−1) | 1.19 | 0.35 |
| Cu (mg kg−1) | 0.81 | 1.15 |
| Zn (mg kg−1) | 0.36 | 0.36 |
| Fe (mg kg−1) | 452.87 | 450.50 |
| Mn (mg kg−1) | 889.33 | 882.73 |
| CEC (cmolc kg−1) | 61.94 | 77.08 |
| Total-N (g kg−1) | 1.67 | 1.76 |
| Organic N (g kg−1) | 1.65 | 1.74 |
| NH4-N (mg kg−1) | 8.72 | 9.60 |
| NO3-N (mg kg−1) | 12.07 | 10.73 |
| Parameters | Dairy Slurry | Wheat Stubble |
|---|---|---|
| Dry matter (g kg−1) | 113.0 | 921.6 |
| pH | 7.5 | - |
| EC (mS cm−1) | 16.5 | - |
| Total Organic C (g kg−1) * | 304.6 | 416.4 |
| Total N (g kg−1) | 3.2 | 5.2 |
| NH4-N (g kg−1) | 1.2 | - |
| Total P (g kg−1) | 0.9 | 0.1 |
| Total K (g kg−1) | 4.2 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.A.; Carvalho, M.; Coutinho, J.; Vasconcelos, E.; Fangueiro, D. Can Dairy Slurry Application to Stubble, without Incorporation into the Soil, Be Sustainable? Plants 2022, 11, 1473. https://doi.org/10.3390/plants11111473
Silva AA, Carvalho M, Coutinho J, Vasconcelos E, Fangueiro D. Can Dairy Slurry Application to Stubble, without Incorporation into the Soil, Be Sustainable? Plants. 2022; 11(11):1473. https://doi.org/10.3390/plants11111473
Chicago/Turabian StyleSilva, Arejacy A., Mario Carvalho, João Coutinho, Ernesto Vasconcelos, and David Fangueiro. 2022. "Can Dairy Slurry Application to Stubble, without Incorporation into the Soil, Be Sustainable?" Plants 11, no. 11: 1473. https://doi.org/10.3390/plants11111473
APA StyleSilva, A. A., Carvalho, M., Coutinho, J., Vasconcelos, E., & Fangueiro, D. (2022). Can Dairy Slurry Application to Stubble, without Incorporation into the Soil, Be Sustainable? Plants, 11(11), 1473. https://doi.org/10.3390/plants11111473








