The Macrophyte Indices for Rivers to Assess the Ecological Conditions in the Klina River in the Republic of Kosovo
Abstract
:1. Introduction
- ○
- To determine how aquatic nutrients affect macrophyte species diversity, cover, and density in the Klina River.
- ○
- To use the macrophyte-based indices to classify water quality and measure the ecological status of the river.
- ○
- To determine water quality based on physico-chemical parameters according to the GD161 standard.
2. Materials and Methods
2.1. Study Area
2.2. Macrophyte Sampling
2.3. River Macrophytes Nutrient Index (RMNI)
2.4. River Macrophyte Hydraulic Index (RMHI)
2.5. How Do We Decide the Biological Status?
2.6. Statistical Analysis
3. Results and Discussion
3.1. Water Quality Parameters and Ecological Status
3.2. Richness, Diversity and Cover of Macrophytes
3.3. Macrophytes Indices and Ecological Status
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, D.; Resh, V. Freshwater Biomonitoring and Benthic Macroinvertebrates; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Crutzen, P.J.; Stoermer, E.F. The ‘Anthropocene’. Global Change Newsletter. PeerJ 2000, 9, 17–18. [Google Scholar]
- Kupiec, J.; Staniszewski, R.; Jusik, S. Assessment of the impact of land use in an agricultural catchment area on water quality of lowland rivers. PeerJ 2021, 1, 2–28. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, D.; Retnaningdyah, C.; Arisoesilaningsih, E. Application of water quality and ecology indices of benthic macroinvertebrates to evaluate the water quality of tertiary irrigation in Malang district. J. Trop. Life Sci. 2013, 3, 193–201. [Google Scholar] [CrossRef]
- Selemani, J.R.; Zhang, J.; Muzuka, A.N.N.; Njau, K.N.; Zhang, G.; Mzuza, M.K.; Maggid, A. Nutrients’ distribution and their impact on Pangani River Basin’s ecosystem—Tanzania. Environ. Technol. 2018, 39, 702–716. [Google Scholar] [CrossRef]
- Alavaisha, E.; Lyon, S.W.; Lindborg, R. Assessment of Water Quality Across Irrigation Schemes: A Case Study of Wetland Agriculture Impacts in Kilombero Valley, Tanzania. Water 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Żelazo, J. River and valley restoration. Infrastructure and ecology of rural areas. Polska Akademia Nauk, Oddział w Krakowie, Komisja Technicznej Infrastruktury Wsi 2006, 4, 11–31. (In Polish) [Google Scholar]
- Gebler, D.; Kayzer, D.; Budka, A.; Szoszkiewicz, K. Modelling values of river macrophyte metrics using artificial neural networks. Polska Akademia Nauk, Oddział w Krakowie 2012, 1, 61–70. [Google Scholar]
- Mcgill, B.J.; Dornelas, M.; Gotelli, N.J.; Magurran, A.E. Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 2015, 30, 104–113. [Google Scholar] [CrossRef]
- Alahuhta, J.; Rosbakh, S.; Chepinoga, V. Environmental determinants of lake macrophyte communities in Baikal Siberia. Aquat. Sci. 2020, 82, 39. [Google Scholar] [CrossRef] [Green Version]
- Szoszkiewicz, K.; Jusik, S.; Pietruczuk, K.; Gebler, D. The Macrophyte Index for Rivers (MIR) as an Advantageous Approach to Running Water Assessment in Local Geographical Conditions. Water 2020, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Birk, S.; Bonne, W.; Borja, A. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Umetsu, C.A.; Aguiar, F.C.; Ferreira, M.T.; Cancian, L.F.; Camargo, M. Addressing bioassessment of tropical rivers using macrophytes: The case of Itanhaém Basin, São Paulo, Brazil. Aquat. Bot. 2018, 150, 53–63. [Google Scholar] [CrossRef]
- Muratov, R.; Szoszkiewicz, K.; Zhamangara, A. An attempt to prepare Macrophyte Index for Rivers for assessment watercourses in Kazakhstan. Meteorol. Hydrol. Water Manag. 2015, 3, 27–32. [Google Scholar] [CrossRef]
- Lyche-Solheim, A.; Feld, C.; Birk, S.; Phillips, G.; Carvalho, L.; Morabito, G.; Poikane, S. Ecological status assessment of European lakes: A comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia 2013, 704, 1. [Google Scholar] [CrossRef] [Green Version]
- Bytyqi, P.; Czikkely, M.; Shala-Abazi, A.; Fetoshi, O.; Ismaili, M.; Hyseni-Spahiu, M.; Millaku, M. Macrophytes as biological indicators of organic pollution in the Lepenci River Basin in Kosovo. J. Freshw. Ecol. 2020, 35, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Ciecierska, H.; Kolada, A. ESMI: A macrophyte index for assessing the ecological status of lakes. Environ. Monit. Assess. 2014, 186, 5501–5517. [Google Scholar] [CrossRef] [Green Version]
- Szoszkiewicz, K.; Karolewicz, K.; Ławniczak, A.; Dawson, F.H. An assessment of the MTR aquatic plant bioindication system for determining the trophic status of Polish Rivers. Pol. J. Environ. Stud. 2002, 11, 421–427. [Google Scholar]
- Cadotte, M.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Lorenz, A.W.; Korte, T.; Sundermann, A.; Januschke, K.; Haase, P. Macrophytes respond to reach-scale river restorations. J. Appl. Ecol. 2011, 49, 202–212. [Google Scholar] [CrossRef]
- Scheffer, M. The effect of aquatic vegetation on turbidity; how important are the filter feeders? Hydrobiologia 1999, 408, 307–316. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M. Can allelopathically active submerged macrophytes stabilize clear-water states in shallow lakes? Basic Appl. Ecol. 2008, 9, 422–432. [Google Scholar] [CrossRef] [Green Version]
- Van Donk, E.; Van De Bund, W.J. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: Allelopathy versus other mechanisms. Aquat. Bot. 2002, 72, 261–274. [Google Scholar] [CrossRef]
- Dalla Vecchia, A.; Villa, P.; Bolpagni, R. Functional traits in macrophyte studies: Current trends and future research agenda. Aquat. Bot. 2020, 167, 103–290. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Riis, T. Macrophyte diversity and composition in relation to substratum characteristics in regulated and unregulated Danish streams. Freshw. Biol. 1999, 4, 375–385. [Google Scholar] [CrossRef]
- Kuhara, U.; Germ, M.; Gaberscik, A.; Urbanič, G. Development of a River Macrophyte Index (RMI) for assessing river ecological status. Limnologica 2011, 41, 235–243. [Google Scholar] [CrossRef]
- Bornette, G.; Amoros, C.; Chessel, D. Effect of allogenic processes on successional rates in former river channels. J. Veg. Sci. 1944, 5, 237–246. [Google Scholar] [CrossRef]
- Madsen, J.D.; Chambers, P.A.; James, W.F.; Koch, E.W.; Westlake, D.F. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 2001, 444, 71–84. [Google Scholar] [CrossRef]
- Karpati, V.; Pomogyi, P. Accumulation and release of nutrients by aquatic macrophytes. Symp. Biol. Hung. 1979, 19, 33–42. [Google Scholar]
- Desmet, N.J.S.; Van Belleghem, S.; Seuntjens, P.; Bouma, K.; Buis, P.; Meire, P. Quantification of the impact of macrophytes on oxygen dynamics and nitrogen retention in a vegetated lowland river. Phys. Chem. Earth. 2011, 36, 479–489. [Google Scholar] [CrossRef]
- Ambrožič, Š.; Gaberščik, A.; Vrezec, A.; Germ, M. Hydrophyte community structure affects the presence and abundance of the water beetle family Dytiscidae in water bodies along the Drava River. Ecol. Eng. 2018, 120, 397–404. [Google Scholar] [CrossRef]
- Gimmler, H.; Carandang, J.; Boots, A.; Reisberg, E.; Woitke, M. Heavy metal content and distribution within a woody plant during and after seven years. Appl. Bot. 2002, 76, 203–217. [Google Scholar]
- Ogugbue, J.; Tovo, V.; Clinton, I. Assessment of nutrient and heavy metal concentrations in sediments and macrophytes of the Oroma wetland, Nigeria. Maced. J. Ecol. Environ. 2020, 22, 111–126. [Google Scholar]
- MESP. The State of Water Report; MESP: Pristina, Kosovo, 2010. [Google Scholar]
- WDF-UKTAG. Biological Status Methods Rivers—Macrophytes; Water Framework Directive—United Kingdom Advisory Group: London, UK, 2014; ISBN 978-1-906934-06-4. [Google Scholar]
- DiFranco, J.L. Protocols for Sampling Aquatic Macrophytes in Freshwater Wetlands; Maine Department of Environmental Protection: Portland, ME, USA, 2019. [Google Scholar]
- Vangjeli, J.; Ruci, B.; Mullaj, A.; Paparisto, K.X.H.Q. Flora of Albania, 4; Albanian Academy of Sciences and Arts: Tiranë, Albania, 2000. [Google Scholar]
- Paparisto, K.; Demiri, M.; Mitrushi, I.; Qosja, X. Flora of Albania, 1; Albanian Academy of Sciences and Arts: Tiranë, Albania, 1988. [Google Scholar]
- Jordanov, D.; Kitanov, B.; Valjev, S. Flora of RP Bulgaria, 1–2; Bulgarian Academy of Sciences and Arts: Sofia, Bulgaria, 1963. [Google Scholar]
- Jordanov, D.; Kozuharov, B. Flora of RP Bulgaria, 4; Bulgarian Academy of Sciences and Arts: Sofia, Bulgaria, 1970. [Google Scholar]
- Jordanov, D.; Kozuharov, B. Flora of RP Bulgaria, 1; Bulgarian Academy of Sciences and Arts: Sofia, Bulgaria, 1966. [Google Scholar]
- Jordanov, D.; Kozuharov, B. Flora of RP Bulgaria, 7; Bulgarian Academy of Sciences and Arts: Sofia, Bulgaria, 1979. [Google Scholar]
- Gecheva, G.; Yurukova, L. Reference aquatic macrophyte communities at rivers in Southwestern Bulgaria. Comptes Rendus Académie Bulgare Sciences Sciences Mathématiques Naturelles 2013, 66, 223–230. [Google Scholar] [CrossRef]
- Velcev, V.D.; Kozuharov, S. Flora of RP Bulgaria, 8; Bulgarian Academy of Sciences and Arts: Sofia, Bulgaria, 1982. [Google Scholar]
- Josifovic, M. Flora SR Srbije, 1–9; Srpska Akademija Nauka: Belgrade, Serbia, 1970. [Google Scholar]
- Josifovic, M.; Stjepanovic, L.; Jankovic, M.; Gajic, B.; Kojic, M.; Diklic, N. Flora SR Srbije III; SANU: Belgrade, Serbia, 1972. [Google Scholar]
- Josifovic, M.; Stjepanovic, L.; Jankovic, M.; Gajic, B.; Kojic, M.; Diklic, N. Flora SR Srbije I; SANU: Belgrade, Serbia, 1973. [Google Scholar]
- Saric, M.; Diklic, N. Flora SR Srbije X; SANU: Belgrade, Serbia, 1986. [Google Scholar]
- The Plant List, Version 1. 2013. Available online: www.theplantlist.org (accessed on 20 June 2020).
- Euro þ Med. Euro þ Med PlantBase—The information Resource for Euro-Mediterranean Plant Diversity. 2006. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 20 June 2020).
- Tutin, H.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1968; Volume 2–5. [Google Scholar]
- Holmes, N.T.H.; Newman, J.R.; Chadd, S.; Rouen, K.J.; Saint, L.; Dawson, F.H. Mean Trophic Rank: A User’s Manual; R&D Technical Report E38; Environment Agency: Bristol, UK, 1999. [Google Scholar]
- Szoszkiewicz, K.; Jusik, S.; Lawniczak, A.E.; Zgola, T. Macrophyte development in unimpacted lowland rivers in Poland. Hydrobiologia 2010, 656, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Szoszkiewicz, K.; Jusik, S.; Lewin, I.; Czerniawska-Kusza, I.; Kupiec, J.M.; Szostak, M. Macrophyte and macroinvertebrate patterns in unimpacted mountain rivers of two European ecoregions. Hydrobiologia 2018, 18, 327–342. [Google Scholar] [CrossRef] [Green Version]
- Willby, N.; Pitt, J.A.; Phillips, G. The Ecological Classification of UK Rivers Using Aquatic Macrophytes; Science Report; Environment Agency: Bristol, UK, 2012; p. 221. [Google Scholar]
- WFD-UKTAG. UKTAG River Assessment Methods Macrophytes and Phytobenthos Macrophytes (River Leaf Pacs); Water Framework Directive—United Kingdom Advisory Group (WFDUKTAG): London, UK, 2008. [Google Scholar]
- Staniszewski, R.; Szoszkiewicz, K.; Zbierska, J.; Lesny, J.; Jusik, S.; Clarke, R.T. Assessment of sources of uncertainty in macrophyte surveys and the consequences for river classification. Hydrobiologia 2006, 566, 235–246. [Google Scholar] [CrossRef]
- Sierka, E.; Tomczak, A. Macrophytes in the assessment of river ecological condition on the example of Pszczynka River (Silesian Upland). Environ. Socio-Econ. Stud. 2013, 1, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Szoszkiewicz, K.; Jusik, S.; Zgola, T.; Czechowska, M.; Hryc, B. Uncertainty of macrophyte-based monitoring for different types of lowland rivers. Belg. J. Bot. 2007, 140, 7–16. [Google Scholar]
- ISO 5567-6; Water Quality Sampling-Part 6. Guidance on Sampling of Rivers and Streams. ISO: Geneva, Switzerland, 2014.
- Zhang, P.; Grutters, B.M.C.; Van Leeuwen, C.J.; Petruzzella, A.; Van Den Berg, F.; Bakker, E.S. Effects of Rising Temperature on the Growth, Stoichiometry, and Palatability of Aquatic Plants. Front. Plant Sci. 2019, 9, 19–47. [Google Scholar] [CrossRef]
- BIS. Standards of Water for Drinking and Other Purposes; Bureau of Indian Standards: New Delhi, India, 2003.
- Middelboe, A.; Markager, S. Depth limits and minimum light requirements of freshwater macrophytes. Freshw. Biol. 1977, 37, 553–568. [Google Scholar] [CrossRef]
- Duarte, C.M.; Kalff, J.; Peters, R.H. Pattern in biomass and cover of aquatic macrophytes in lakes. Can. J. Fish. Aquat. Sci. 1986, 43, 1900–1908. [Google Scholar] [CrossRef]
- Chambers, P.A.; Kalff, J. Depth distribution and biomass of submerged aquatic macrophyte communities in relation to Secchi depth. Can. J. Fish. Aquat. Sci. 1985, 42, 701–709. [Google Scholar] [CrossRef]
- Nurminen, L. Macrophyte species composition reflecting water quality changes in adjacent water bodies of Lake Hiidenvesi, SW Finland. Annales Botanici Fennici 2003, 40, 199–208. [Google Scholar]
- Lv, T.; He, Q.; Hong, Y.; Yo, D. Effects of Water Quality Adjusted by Submerged Macrophytes on the Richness of the Epiphytic Algal Community. Front. Plant Sci. 2019, 9, 1980. [Google Scholar] [CrossRef]
- Rameshkumar, S.; Radhakrishnan, K.; Aanand, S.; Rajaram, R. Influence of physicochemical water quality on aquatic macrophyte diversity in seasonal wetlands. Appl. Water Sci. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Gopalkrushna, M.H. Determination of physico-chemical parameters of surface water samples in and around Akot city. Int. J. Res. Chem. Environ. 2011, 1, 183–187. [Google Scholar]
- Kotadiya, N.G.; Acharya, C.A. An assessment of lake water quality index of Manipu lake of district Ahmedabad. Gujarat. Int. J. Sci. Res. 2014, 3, 448–450. [Google Scholar] [CrossRef]
- Shah, M.; Hashmi, H.N.; Ali, A.; Shah, M.; Ghumman, A. Performance assessment of aquatic macrophytes for treatment of municipal wastewater. J. Environ. Health Sci. Eng. 2014, 12, 106. [Google Scholar] [CrossRef] [Green Version]
- Badr El-Din, S.; Abdel-Aziz, R.A. Potential uses of aquatic plants for wastewater treatment. J. Microbiol. Biotechnol. Rep. 2018, 2, 47–48. [Google Scholar]
- Souza, D.C.; Iwakura, L.; Lima, S.B.; De Locastro, J.K. Comparative effect of temperature on the performance of Typha domingensis Pers. and Pontederia parviflora Alexander in phytotreatment. Acta Sci. Technol. 2019, 41, 39038. [Google Scholar] [CrossRef] [Green Version]
- Caselles-Osorio, A.; Garcia, J. Impact of different feeding strategies and plant. Sci. Total Environ. 2007, 378, 253–262. [Google Scholar] [CrossRef]
- Thomas, J.D. The role of dissolved organic matter, particularly free amino acids and humic substances, in freshwater ecosystems. Freshw. Biol. 1997, 38, 1–36. [Google Scholar] [CrossRef]
- Reitsema, R.; Meire, P.; Schoelynck, J. The Future of Freshwater Macrophytes in a Changing World: Dissolved Organic Carbon Quantity and Quality and Its Interactions with Macrophytes. Front. Plant Sci. 2018, 9, 629. [Google Scholar] [CrossRef]
- Veraart, A.J.; De Bruijne, W.J.J.; De Klein, J.J.M.; Peeters, E.T.H.M.; Scheffer, M. Effects of aquatic vegetation type on denitrification. Biogeochemistry 2011, 104, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.F.; Jing, S.R.; Wang, T.W.; Lee, D.Y. Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environ. Pollut. 2002, 119, 413–420. [Google Scholar] [CrossRef]
- Daldorph, P.W.G.; Thomas, J.D. The Effect of Nutrient Enrichment on a Freshwater Community. J. Appl. Ecol. 1991, 28, 685. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Q.; Wei, Y.; Yin, H.; Sun, C.; Liu, G. Uptake Kinetics of NH4+, NO3− and H2PO4− by Typha orientalis, Acorus calamus L., Lythrum salicaria L., Sagittaria trifolia L. and Alisma plantago-aquatica Linn. Sustainability 2021, 13, 434. [Google Scholar] [CrossRef]
- Clarke, E.; Baldwin, A.H. Responses of wetland plants to ammonia and water level. Ecol. Eng. 2002, 18, 257–264. [Google Scholar] [CrossRef]
- Hageman, R.H. Ammonium versus nitrate nutrition of higher plants. In Nitrogen in Crop Production; ASACSSA-SSSA: Madison, WI, USA, 1984; Volume 1, pp. 67–82. [Google Scholar]
- Wang, W. Ammonia toxicity to macrophytes (common duckweed and rice) using static and renewal methods. Environ. Toxicol. Chem. 1991, 10, 1173–1177. [Google Scholar] [CrossRef]
- Dijk, E.; Eck, N. Ammonium toxicity and nitrate response of axenically grown Dactylorhiza incarnata seedlings. New Phytol. 1994, 131, 361–367. [Google Scholar] [CrossRef]
- Magalhaes, J.R.; Machado, A.T.; Huber, D.M. Similarities in response of maize genotypes to waterlogging and ammonium toxicity. J. Plant Nutr. 1995, 18, 2339–2346. [Google Scholar] [CrossRef]
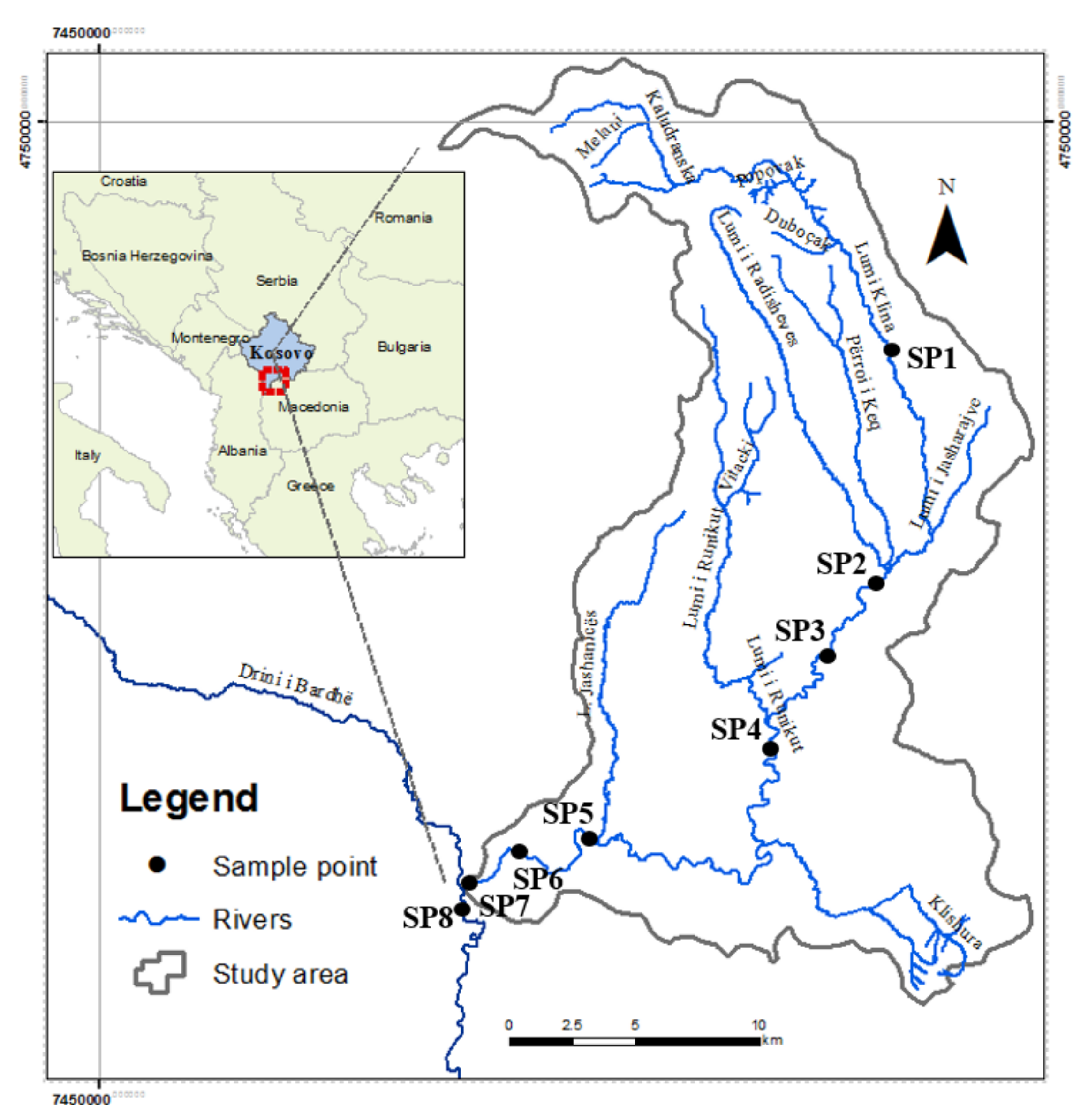

| Sampling Stations | Latitude (N) Longitude(E) | Hydro Morphology | Riparian Vegetation | |
|---|---|---|---|---|
| SP1 | The mountain spring of Klina river in village Kuçicë. | 42°37′8″ N 20°53′49″ E 1200 m | Natural river bed. Flow velocity 2.5 m/s. | Well-developed on both sides of the riverbank, dominated by deciduous trees Salicaceae, Betulaceae, and Fagaceae. |
| SP2 | Llaushë | 42°43′21″ N 20°45′60″ E | Located 1 km from the city of Skenderaj, where the river receives discharges from municipal wastewaters, agriculture and industry. Water flow l.5 m/s, water color is dark with bad odor. | Well-developed on both sides of the riverbank, dominated by deciduous trees Salicaceae. |
| SP3 | Tushilë | 44°42′43″ N 20°45′39″ E | The river receives discharges from agriculture and industry. Water flow l m/s, water with bad odor. | Well-developed on both sides of the riverbank, dominated by deciduous trees Betulaceae. |
| SP4 | Açarevë | 42°39′6″ N 20°43′15″ E | Riverbed with waste and agricultural runoff due to the land use. Flow velocity 2 m/s. | Deciduous trees and ground vegetation on both sides of the riverbank, dominated by Salicaceae and Betulaceae trees. |
| SP5 | Pogragjë | 42°38′1″ N 20°37′26″ E | The river receives discharges from village houses, agriculture, and other sources. | Deciduous trees and ground vegetation on both sides of the riverbank dominated by Salicaceae and Betulaceae. |
| SP6 | Klinë–Center | 42°61′52.56″ N 20°57′57.35″ E | Inside the city of Klina, concrete river channel on both sides. Municipal wastewaters and agricultural, industrial runoff discharge in the river. | The riverbank is concreted on both sides. |
| SP7 | Klinë–lower part of the city | 42°35′48″ N 20°34′0″ E | Lower part of the city of Klina. Municipal wastewaters and agricultural, industrial runoff discharge in the river. | The riverbank is dominated by Salicaceae trees. |
| SP8 | Klinë | 42°36′41″ N20°34′05″ E | Municipal wastewaters and agricultural, industrial runoff discharge in the river. | The riverbank is dominated by Salicaceae and Betulaceae. |
| No. | Ecological Status Class | Lowland River Sandy and Organic | Quality Class of Water |
|---|---|---|---|
| 1 | Very good | ≥44.5 | I |
| 2 | Good | 44.5–35.0> | II |
| 3 | Moderate | 35.0–25.4> | III |
| 4 | Poor | 25.4–15.8> | IV |
| 5 | Bad | ≤15.8 | V |
| Status | EQR Values |
|---|---|
| High | 0.8 |
| Good | 0.6 |
| Moderate | 0.4 |
| Poor | 0.2 |
| Bad | <0.2 |
| Variable | Units | SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | SP7 | SP8 | Minimum and Maximum | M ± SD | Variance | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water temperature | °C | 12.1 | 15.6 | 17.0 | 19.1 | 18.5 | 17.1 | 18.1 | 12.7 | 12.1–19.1 | 16.2 ± 2.6 | 6.8 | ||
| Air temperature | 16.8 | 17.4 | 17.9 | 20.9 | 21.3 | 23 | 21.1 | 21.1 | 16.8–23 | 19.9 ± 2.24 | 5.04 | |||
| Turbidity | NTU | 2.8 | 23.5 | 28.4 | 30.6 | 26.9 | 25.5 | 18.6 | 6.8 | 2.8–30.6 | 20.3 ± 10.3 | 106.2 | ||
| Electrical conductivity Ec | µS/cm | 459 | 856 | 829 | 766 | 797 | 762 | 753 | 376 | 376–856 | 699.7 ± 179.0 | 32,064 | ||
| Total dissolved solids (TDS) | mg/L | 229 | 428 | 415 | 333 | 398 | 377 | 373 | 186 | 186–428 | 342.3 ± 88.8 | 7890.2 | ||
| Total suspended solids (TSS) | mg/L | 14 | 46 | 85 | 39 | 28 | 3.9 | 3.6 | 1.1 | 1.1–85 | 27.5 ± 28.7 | 826.9 | ||
| pH | 0–14 | 7.93 | 7.46 | 7.60 | 8.26 | 7.70 | 7.08 | 7.62 | 8.11 | 7.08–8.26 | 7.72 ± 0.37 | 0.141 | ||
| Dissolved oxygen (DO) | mg/L | 5.33 | 3.15 | 0.03 | 5.97 | 4.73 | 7.2 | 4.5 | 10.9 | 0.03–10.9 | 5.22 ± 3.13 | 9.8 | ||
| Quality class according to GD161 standard | III | V | V | III | IV | II | IV | I | III | |||||
| Dissolved oxygen saturation (DOS) | % | 70.5 | 44.9 | 0.3 | 89.4 | 70 | 82 | 53 | 117 | 0.3–117 | 65.8 ± 34.5 | 1195.9 | ||
| Biochemical oxygen demand (BOD) | mg/L | 1.5 | 22.6 | 42.2 | 33.5 | 25.8 | 9.0 | 9.5 | 3.9 | 1.5–42.2 | 18.5 ± 14.7 | 218.6 | ||
| Quality class according to GD161 standard | I | V | V | V | V | III | III | III | III | |||||
| Chemical oxygen demand (COD) | mg/L | 4.2 | 49.6 | 87.1 | 63.5 | 58.0 | 18 | 19 | 6.8 | 4.2–87.1 | 38.2 ± 30.4 | 924.5 | ||
| Quality class according to GD161 standard | I | III | V | V | V | III | III | II | III | |||||
| Total organic carbon (TOC) | mg/L | 1.1 | 17.0 | 28.8 | 19.7 | 18.3 | 6.3 | 6.5 | 1.9 | 1.1–28.8 | 12.4 ± 9.9 | 98.3 | ||
| Nitrates (NO3−) | mg/L | 2.5 | 8.1 | 0.7 | 26.2 | 17.2 | 6.9 | 5.6 | 4.8 | 0.7–26.2 | 9.0 ± 8.5 | 72.6 | ||
| Quality class according to GD161 standard | I | III | I | V | V | III | III | II | III | |||||
| n,n-diethyltryptamine (DET) | mg/L | <0.1 | <0.1 | 0.8 | 0.2 | 0.1 | 0.2 | 0.4 | <0.1 | |||||
| Orthophosphates (PO43−) | mg/L | 0.01 | 0.04 | 0.32 | 0.18 | 0.14 | 0.10 | 0.18 | 0.06 | 0.01–0.32 | 0.13 ± 0.09 | 0.010 | ||
| Quality class according to GD161 standard | I | I | V | V | V | V | V | I | V | |||||
| Total phosphorus (PT) | mg/L | 0.04 | 0.64 | 1.28 | 0.99 | 0.77 | 0.28 | 0.32 | 0.13 | 0.04–1.28 | 0.56 ± 0.43 | 0.193 | ||
| Quality class according to GD161 standard | I | II | V | III | III | I | I | I | II | |||||
| Ammonia (NH4+) | mg/L | 0.03 | 0.32 | 1.65 | 0.88 | 0.77 | 0.84 | 1.02 | 0.041 | 0.03–1.65 | 0.69 ± 0.54 | 0.299 | ||
| Quality class according to GD161 standard | I | I | III | II | I | II | II | I | I | |||||
| Nitrites (NO2−) | mg/L | 0.02 | 0.22 | 1.10 | 0.84 | 0.63 | 1.14 | 3.75 | 0.26 | 0.02–3.75 | 0.99 ± 1.18 | 1.4 | ||
| Quality class according to GD161 standard | I | II | V | V | V | V | V | V | V | |||||
| Sulphate (SO42−) | mg/L | 19.5 | 28.9 | 16.5 | 38.3 | 18.3 | 15.9 | 14.8 | 7.9 | 7.9–38.3 | 20.01 ± 9.4 | 88.5 | ||
| Quality class according to GD161 standard | I | I | I | I | I | I | I | I | I | |||||
| Calcium (Ca2+) | mg/L | 70.87 | 130.1 | 134.1 | 112.1 | 121.3 | 123.3 | 127.3 | 70.8 | 70.87–134.1 | 111.2 ± 25.7 | 663.9 | ||
| Quality class according to GD161 standard | I | II | II | II | II | II | II | I | II | |||||
| Magnesium (Mg2+) | mg/L | 15.1 | 26.7 | 18.7 | 27.2 | 20.7 | 22.4 | 28.2 | 15.1 | 15.1–28.2 | 21.76 ± 5.2 | 27.8 | ||
| Quality class according to GD161 standard | I | I | I | I | I | I | I | I | I | |||||
| Sodium (Na+) | mg/L | 6.55 | 8.20 | 10.5 | 7.58 | 8.01 | 8.64 | 8.89 | 6.55 | 6.55–10.5 | 8.12 ± 1.30 | 1.7 | ||
| Quality class according to GD161 standard | I | I | I | I | I | I | I | I | I | |||||
| Potassium (K+) | mg/L | 2.21 | 2.66 | 2.68 | 3.00 | 3.22 | 3.11 | 3.51 | 2.21 | 2.21–3.51 | 2.83 ± 0.46 | 0.220 | ||
| Chloride (Cl−) | mg/L | 7.1 | 35.5 | 65.3 | 54.6 | 46.1 | 19.8 | 23.4 | 4.26 | 4.26–65.3 | 32.0 ± 22.1 | 492.3 | ||
| Taxon | Family | SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | SP7 | SP8 |
|---|---|---|---|---|---|---|---|---|---|
| Adoxa moschatellina L. | Adoxaceae | + | |||||||
| Agrostis stolonifera L. | Poaceae | + | |||||||
| Amblystegium riparium (Hedw.) Schimp. | Amblystegiaceae | + | |||||||
| Berula erecta Huds. | Apiaceae | + | + | ||||||
| Bidens tripartita Bigelow, 1824 | Asteraceae | + | + | ||||||
| Callitriche cophocarpa Sendtn. | Plantaginaceae | + | |||||||
| Callitriche stagnalis Scop. | Plantaginaceae | + | |||||||
| Cardamine amara L. | Brassicaceae | + | |||||||
| Cardamine flexuosa With. | Brassicaceae | + | |||||||
| Ceratophyllum demersum L. | Ceratophyllaceae | + | |||||||
| Cinclidotus aquaticus (Hedw.) B. & S. | Pottiaceae | + | |||||||
| Cinclidotus aquaticus Bruch & W.P.Schimper, 1842 | Cinclidotaceae | + | |||||||
| Cinclidotus fontinaloides Palisot de Beauvois | Cinclidotaceae | + | |||||||
| Cratoneuron filicinum Spruce | Amblystegiaceae | + | |||||||
| Epilobium hirsutum L. | Onagraceae | + | + | + | |||||
| Epilobium sp. | + | ||||||||
| Epilobium tetragonum Lour. | Onagraceae | + | + | ||||||
| Galium palustre L. | Rubiaceae | + | |||||||
| Glyceria maxima (Hartm.) Holmb | Poaceae | + | + | + | + | ||||
| Helosciadium repens (Jacq.) Koch | Apiaceae | + | |||||||
| Juncus effusus L. | Juncaceae | + | + | + | |||||
| Juncus inflexus L. | Juncaceae | + | |||||||
| Leersia oryzoides Michx. | Poaceae | + | |||||||
| Lemna minor L. | Araceae | + | + | + | + | + | + | ||
| Lycopus europaeus L. | Lamiaceae | + | + | ||||||
| Lysimachia nummularia L. | Primulaceae | + | |||||||
| Lysimachia vulgaris L. | Primulaceae | + | + | ||||||
| Lythrum salicaria L. | Lythraceae | + | |||||||
| Marchantia polymorpha L. var aquatic | Marchantiaceae | + | |||||||
| Mentha aquatica L. | Lamiaceae | + | + | + | + | + | |||
| Mentha longifolia (L.) Huds. | Lamiaceae | + | |||||||
| Myriophyllum spicatum L. | Haloragaceae | + | |||||||
| Nasturtium microphyllum (Boenn. ex Rchb.) Rchb. | Brassicaceae | + | |||||||
| Nasturtium officinale R.Br. | Brassicaceae | + | + | + | |||||
| Persicaria amphibia (L.) Delarbre | Polygonaceae | + | |||||||
| Petasites japonicus subsp. Japonicus | Asteraceae | + | |||||||
| Phalaris arundinacea L. | Poaceae | + | + | ||||||
| Plantago media L. | Plantaginaceae | + | |||||||
| Polygonum lapathifolium L. | Polygonaceae | + | + | + | |||||
| Polygonum latifolium Giesecke | Polygonaceae | ||||||||
| Polygonum mite Schrank | Polygonaceae | + | + | + | |||||
| Persicaria glabra (Willd.) M.Gómez | Polygonaceae | + | |||||||
| Potamogeton crispus L. | Potamogetonaceae | + | + | + | + | ||||
| Potamogeton fluitans Sm. | Potamogetonaceae | + | |||||||
| Potamogeton gramineus L. | Potamogetonaceae | + | |||||||
| Potamogeton natans Sturm | Potamogetonaceae | + | + | + | |||||
| Potamogeton nodosus Poir. | Potamogetonaceae | + | |||||||
| Ranunculus repens S.Watson | Ranunculaceae | + | + | + | + | ||||
| Ranunculus aquatilis (Dumort.) Bab. | Ranunculaceaea | + | |||||||
| Rorippa palustris (L.) Besser | Brassicaceae | + | + | + | |||||
| Rorippa sylvestris (L.) Besser | Brassicaceae | + | + | ||||||
| Rumex aquaticus Campd. | Polygonaceae | + | + | ||||||
| Rumex hydrolapathum Campd. | Polygonaceae | + | + | + | |||||
| Rumex longifolius DC. | Polygonaceae | + | |||||||
| Rumex palustris Sm. | Polygonaceae | + | |||||||
| Schoenoplectus lacustris (L.) Palla | Cyperaceae | + | + | ||||||
| Scrophularia auriculata L. | Scrophulariaceae | + | + | ||||||
| Scrophularia nodosa L. | Scrophulariaceae | + | |||||||
| Scrophularia umbrosa Salzm. ex Benth | Scrophulariaceae | + | |||||||
| Sparaganium erectum L | Typhaceae | + | + | + | + | ||||
| Stratiotes aloides L. | Hydrocharitaceae | + | |||||||
| Stuckenia pectinata (L.) Börner | Potamogetonaceae | + | |||||||
| Trichophorum cespitosum (L.) Schur, 1853 | Cyperaceae | + | + | ||||||
| Typha angustifolia Eckl. & Zeyh. ex Rohrb., 1869 | Typhaceae | + | + | + | + | + | |||
| Typha latifolia G.Mey. | Typhaceae | + | + | + | |||||
| Veronica anagallis-aquatica L. | Plantaginaceae | + | + | + | |||||
| Veronica beccabunga L. | Plantaginaceae | + | + | + | |||||
| MIR (Macrophyte Index for Rivers) | 45 | 44 | 25 | 40 | 76 | 57 | 20 | 23 | |
| Quality class of water | I | II | IV | II | I | I | IV | IV | |
| RMNI (River Macrophyte Nutrient Index) | 3.44 | 5.95 | 8.56 | 4.77 | 5.81 | 5.62 | 7.49 | 5.85 | |
| The ecological quality ratio for the parameter RMNI | 1 | 0.61 | 0.21 | 0.80 | 0.63 | 0.68 | 0.38 | 0.63 | |
| RMHI (River Macrophyte Hydraulic Index) | 3.57 | 6.27 | 8.42 | 5.96 | 5.77 | 5.61 | 8.5 | 5.20 | |
| The ecological quality ratio for the parameter RMHI | 1 | 0.57 | 0.24 | 0.92 | 0.65 | 0.68 | 0.23 | 0.74 | |
| SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | SP7 | SP8 | |
|---|---|---|---|---|---|---|---|---|
| Water depth | 20–40 cm | 60–100 cm | 50–100 cm | 40–60 cm | 40–60 cm | 60 cm | 1.5 m | 25 cm |
| Submersed plant density (0–5) | 1 | 4 | 2 | 3 | 4 | 5 | 3 | 1 |
| Plant density (0–5) | 1 | 3 | 1 | 3 | 4 | 5 | 3 | 1 |
| Cover (0–5) | 1 | 4 | 2 | 2 | 4 | 4 | 2 | 1 |
| Substrate | Gravel and rock | Silt and clay | Gravel and rock | Gravel and rock | Gravel and rock | Silt and clay | Silt and clay | Gravel and rock |
| Detritus | Present | Present | Present | Present | Present | Present | Present | Absent |
| Habitate | Terrestrial | Aquatic | Terrestrial | Terrestrial | Aquatic | Aquatic | Aquatic | Aquatic |
| S | + | + | + | + | + | + | ||
| F | + | + | + | + | + | + | ||
| E | + | + | + | + | + | + | + | + |
| Plant Density | RMNI | RMHI | EQR-RMNI | EQR-RMHI | MIR | |
|---|---|---|---|---|---|---|
| Water temperature | 0.714 * | 0.406 | 0.606 | −0.400 | −0.389 | 0.241 |
| Turbidity | 0.726 * | 0.436 | 0.555 | −0.431 | −0.358 | 0.286 |
| Electrical conductivity Ec | 0.780 * | 0.483 | 0.626 | −0.482 | −0.516 | 0.272 |
| Total dissolved solids (TDS) | 0.765 * | 0.545 | 0.639 | −0.545 | −0.600 | 0.281 |
| Total suspended solids (TSS) | 0.003 | 0.459 | 0.446 | −0.469 | −0.349 | −0.082 |
| pH | −0.652 | −0.355 | −0.279 | 0.344 | 0.470 | −0.288 |
| Dissolved oxygen (DO) | −0.202 | −0.496 | −0.569 | 0.505 | 0.554 | 0.009 |
| Dissolved oxygen saturation (DOS) | −0.112 | −0.640 | −0.649 | 0.646 | 0.692 | 0.160 |
| Biochemical oxygen demand (BOD) | 0.245 | 0.474 | 0.534 | −0.481 | −0.336 | 0.054 |
| Chemical oxygen demand (COD) | 0.268 | 0.484 | 0.527 | −0.492 | −0.358 | 0.106 |
| Total organic carbon (CTO) | 0.283 | 0.515 | 0.552 | −0.523 | −0.396 | 0.091 |
| Nitrates (NO3−) | 0.424 | −0.332 | −0.117 | 0.332 | 0.408 | 0.430 |
| Orthophosphates (PO43−) | 0.098 | 0.762 * | 0.805 * | −0.760 * | −0.649 | −0.268 |
| Total phosphorous (PT) | 0.238 | 0.503 | 0.562 | −0.509 | −0.364 | 0.031 |
| Ammonia (NH4+) | 0.330 | 0.746 * | 0.812 * | −0.740 * | −0.686 | −0.127 |
| Nitrites (NO2−) | 0.222 | 0.557 | 0.730 * | −0.548 | −0.678 | −0.398 |
| Suphates (SO42−) | 0.340 | −0.338 | −0.064 | 0.335 | 0.350 | 0.211 |
| Calcium (Ca2+) | 0.741 * | 0.652 | 0.760 * | −0.649 | −0.688 | 0.135 |
| Magnezium (Mg2+) | 0.670 | 0.223 | 0.518 | −0.217 | −0.317 | −0.015 |
| Sodium (Na+) | 0.368 | 0.847 ** | 0.850 ** | −0.842 ** | −0.834 * | −0.160 |
| Potassium (K+) | 0.745 * | 0.374 | 0.574 | −0.365 | −0.465 | 0.227 |
| Chloride (Cl−) | 0.333 | 0.472 | 0.564 | −0.478 | −0.362 | 0.114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bytyçi, P.; Shala-Abazi, A.; Zhushi-Etemi, F.; Bonifazi, G.; Hyseni-Spahiu, M.; Fetoshi, O.; Çadraku, H.; Feka, F.; Millaku, F. The Macrophyte Indices for Rivers to Assess the Ecological Conditions in the Klina River in the Republic of Kosovo. Plants 2022, 11, 1469. https://doi.org/10.3390/plants11111469
Bytyçi P, Shala-Abazi A, Zhushi-Etemi F, Bonifazi G, Hyseni-Spahiu M, Fetoshi O, Çadraku H, Feka F, Millaku F. The Macrophyte Indices for Rivers to Assess the Ecological Conditions in the Klina River in the Republic of Kosovo. Plants. 2022; 11(11):1469. https://doi.org/10.3390/plants11111469
Chicago/Turabian StyleBytyçi, Pajtim, Albona Shala-Abazi, Ferdije Zhushi-Etemi, Giuseppe Bonifazi, Mimoza Hyseni-Spahiu, Osman Fetoshi, Hazir Çadraku, Fidan Feka, and Fadil Millaku. 2022. "The Macrophyte Indices for Rivers to Assess the Ecological Conditions in the Klina River in the Republic of Kosovo" Plants 11, no. 11: 1469. https://doi.org/10.3390/plants11111469
APA StyleBytyçi, P., Shala-Abazi, A., Zhushi-Etemi, F., Bonifazi, G., Hyseni-Spahiu, M., Fetoshi, O., Çadraku, H., Feka, F., & Millaku, F. (2022). The Macrophyte Indices for Rivers to Assess the Ecological Conditions in the Klina River in the Republic of Kosovo. Plants, 11(11), 1469. https://doi.org/10.3390/plants11111469






