A New Approach to the Study of Plastidial Stress Granules: The Integrated Use of Arabidopsis thaliana and Chlamydomonas reinhardtii as Model Organisms
Abstract
:1. Introduction
2. Plastidial Stress Granules
3. cpSGs and Chlamydomonas reinhardtii
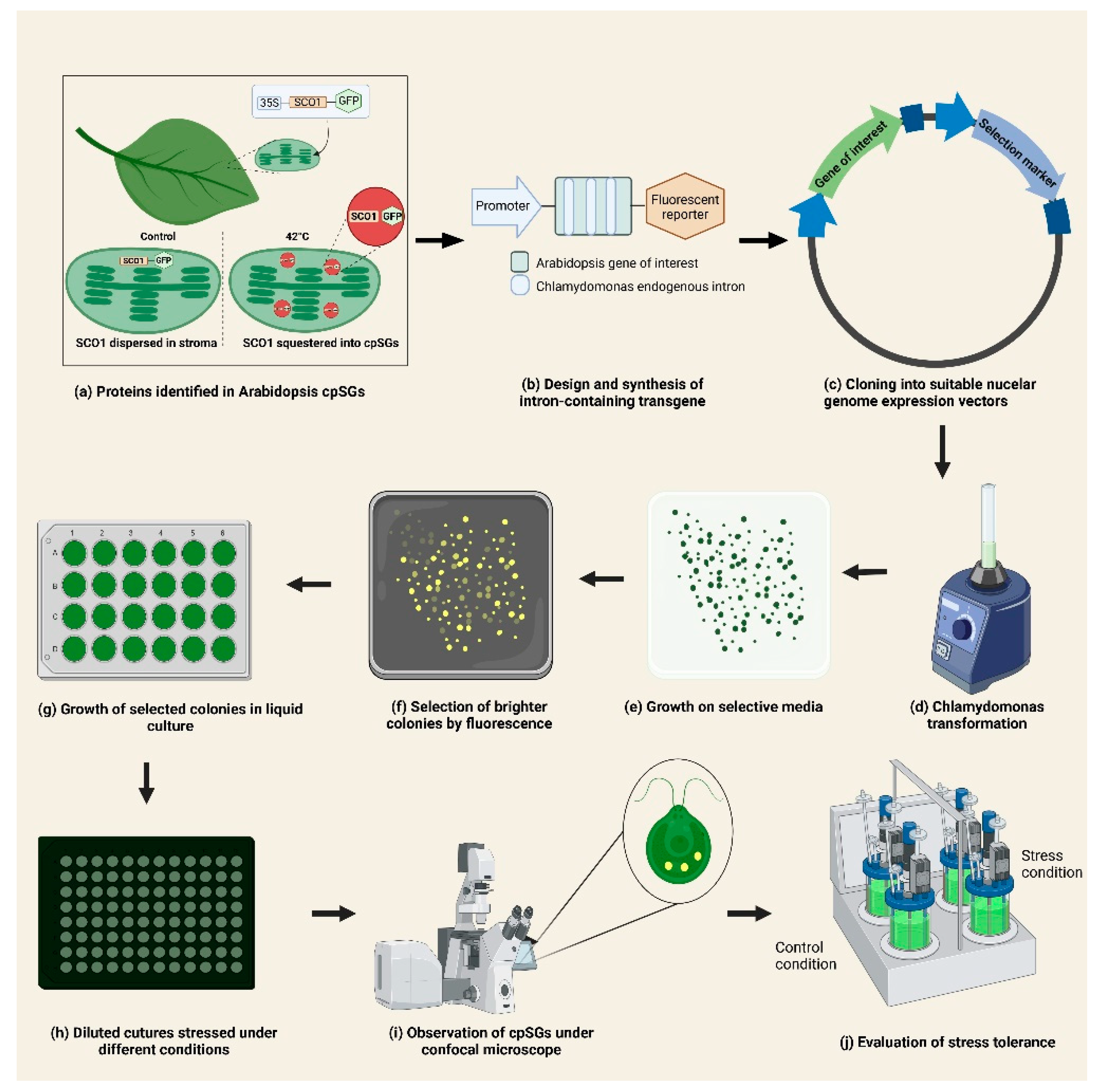
4. Combined Use of A. thaliana and C. reinhardtii as Complementary Models for the Study of cpSGs and Chloroplast Stress Response
5. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leister, D. Chloroplast research in the genomic age. Trends Genet. 2003, 19, 47–56. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, N.L.; Tan, Y.F.; Jacoby, R.P.; Millar, A.H. Abiotic environmental stress induced changes in the Arabidopsis thaliana chloroplast, mitochondria and peroxisome proteomes. J. Proteom. 2009, 72, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.J.; Sowden, R.G.; Jarvis, P. Abiotic stress-induced chloroplast proteome remodelling: A mechanistic overview. J. Exp. Bot. 2018, 69, 2773–2781. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Joshi, P.N.; Raval, M.K.; Biswal, U.C. Photosynthesis, a global sensor of environmental stress in green plants: Stress signalling and adaptation. Curr. Sci. 2011, 101, 47–56. [Google Scholar]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Hemme, D.; Veyel, D.; Mühlhaus, T.; Sommer, F.; Jüppner, J.; Unger, A.K.; Sandmann, M.; Fehrle, I.; Schönfelder, S.; Steup, M.; et al. Systems-wide analysis of acclimation responses to long-term heat stress and recovery in the photosynthetic model organism Chlamydomonas reinhardtii. Plant Cell 2014, 26, 4270–4297. [Google Scholar] [CrossRef] [Green Version]
- Maruri-López, I.; Figueroa, N.E.; Hernández-Sánchez, I.E.; Chodasiewicz, M. Plant stress granules: Trends and beyond. Front. Plant Sci. 2021, 1538. [Google Scholar] [CrossRef]
- Chodasiewicz, M.; Sokolowska, E.M.; Nelson-Dittrich, A.C.; Masiuk, A.; Beltran, J.C.; Nelson, A.D.; Skirycz, A. Identification and characterization of the heat-induced plastidial stress granules reveal new insight into Arabidopsis stress response. Front. Plant Sci. 2020, 1674. [Google Scholar] [CrossRef]
- Leister, D. Piecing the puzzle together: The central role of reactive oxygen species and redox hubs in chloroplast retrograde signaling. Antioxid. Redox Signal. 2019, 30, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Leister, D.; Wang, L.; Kleine, T. Organellar gene expression and acclimation of plants to environmental stress. Front. Plant Sci. 2017, 8, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, C.; Nover, L.; Fauth, M. Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J. 2008, 56, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Chantarachot, T.; Bailey-Serres, J. Polysomes, stress granules, and processing bodies: A dynamic triumvirate controlling cytoplasmic mRNA fate and function. Plant Physiol. 2018, 176, 254–269. [Google Scholar] [CrossRef] [Green Version]
- Kosmacz, M.; Gorka, M.; Schmidt, S.; Luzarowski, M.; Moreno, J.C.; Szlachetko, J.; Leniak, E.; Sokolowska, E.M.; Sofroni, K.; Schnittger, A.; et al. Protein and metabolite composition of Arabidopsis stress granules. New Phytol. 2019, 222, 1420–1433. [Google Scholar] [CrossRef] [Green Version]
- Protter, D.S.; Parker, R. Principles and properties of stress granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Uniacke, J.; Zerges, W. Stress induces the assembly of RNA granules in the chloroplast of Chlamydomonas reinhardtii. J. Cell Biol. 2008, 182, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, F.; Berry, C.; Prestigiacomo, L.; Van Hoewyk, D. Proteasome inhibition rapidly exacerbates photoinhibition and impedes recovery during high light stress in Chlamydomonas reinhardtii. BMC Plant Biol. 2020, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Gutman, B.L.; Niyogi, K.K. Chlamydomonas and Arabidopsis. A dynamic duo. Plant Physiol. 2004, 135, 607–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohr, M.; Im, C.S.; Grossman, A.R. Genome-based examination of chlorophyll and carotenoid biosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 2005, 138, 490–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bremer, K. Summary of green plant phylogeny and classification. Cladistics 1985, 1, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Schroda, M.; Hemme, D.; Mühlhaus, T. The Chlamydomonas heat stress response. Plant J. 2015, 82, 466–480. [Google Scholar] [CrossRef]
- Hedges, S.B. The origin and evolution of model organisms. Nat. Rev. Genet. 2002, 3, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, D.; Baier, T.; Lauersen, K.J. Intronserter, an advanced online tool for design of intron containing transgenes. Algal Res. 2019, 42, 101588. [Google Scholar] [CrossRef]
- Schroda, M. Good news for nuclear transgene expression in Chlamydomonas. Cells 2019, 8, 1534. [Google Scholar] [CrossRef] [Green Version]
- Baier, T.; Jacobebbinghaus, N.; Einhaus, A.; Lauersen, K.J.; Kruse, O. Introns mediate post-transcriptional enhancement of nuclear gene expression in the green microalga Chlamydomonas reinhardtii. PLoS Genet. 2020, 16, e1008944. [Google Scholar] [CrossRef]
- Crozet, P.; Navarro, F.J.; Willmund, F.; Mehrshahi, P.; Bakowski, K.; Lauersen, K.J.; Pérez-Pérez, M.E.; Auroy, P.; Gorchs-Rovira, A.; Sauret-Gueto, S.; et al. Birth of a photosynthetic chassis: A MoClo toolkit enabling synthetic biology in the microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 2018, 7, 2074–2086. [Google Scholar] [CrossRef] [Green Version]
- Lauersen, K.J.; Kruse, O.; Mussgnug, J.H. Targeted expression of nuclear transgenes in Chlamydomonas reinhardtii with a versatile, modular vector toolkit. Appl. Microbiol. Biotechnol. 2015, 99, 3491–3503. [Google Scholar] [CrossRef]
- Abdallah, M.N.; Wellman, G.B.; Overmans, S.; Lauersen, K.J. Combinatorial engineering for photoautotrophic production of recombinant products from green microalga Chlamydomonas reinhardtii. bioRxiv 2022. [Google Scholar] [CrossRef]
- Mackinder, L.C.M.; Chen, C.; Leib, R.D.; Patena, W.; Blum, S.R.; Rodman, M.; Ramundo, S.; Adams, C.M.; Jonikas, M.C. A spatial interactome reveals the protein organization of the algal CO2-concentrating mechanism. Cell 2017, 171, 133–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafique, F.; Lauersen, K.J.; Chodasiewicz, M.; Figueroa, N.E. A New Approach to the Study of Plastidial Stress Granules: The Integrated Use of Arabidopsis thaliana and Chlamydomonas reinhardtii as Model Organisms. Plants 2022, 11, 1467. https://doi.org/10.3390/plants11111467
Rafique F, Lauersen KJ, Chodasiewicz M, Figueroa NE. A New Approach to the Study of Plastidial Stress Granules: The Integrated Use of Arabidopsis thaliana and Chlamydomonas reinhardtii as Model Organisms. Plants. 2022; 11(11):1467. https://doi.org/10.3390/plants11111467
Chicago/Turabian StyleRafique, Fareena, Kyle J. Lauersen, Monika Chodasiewicz, and Nicolás E. Figueroa. 2022. "A New Approach to the Study of Plastidial Stress Granules: The Integrated Use of Arabidopsis thaliana and Chlamydomonas reinhardtii as Model Organisms" Plants 11, no. 11: 1467. https://doi.org/10.3390/plants11111467
APA StyleRafique, F., Lauersen, K. J., Chodasiewicz, M., & Figueroa, N. E. (2022). A New Approach to the Study of Plastidial Stress Granules: The Integrated Use of Arabidopsis thaliana and Chlamydomonas reinhardtii as Model Organisms. Plants, 11(11), 1467. https://doi.org/10.3390/plants11111467





