Biorecovery of Agricultural Soil Impacted by Waste Motor Oil with Phaseolus vulgaris and Xanthobacter autotrophicus
Abstract
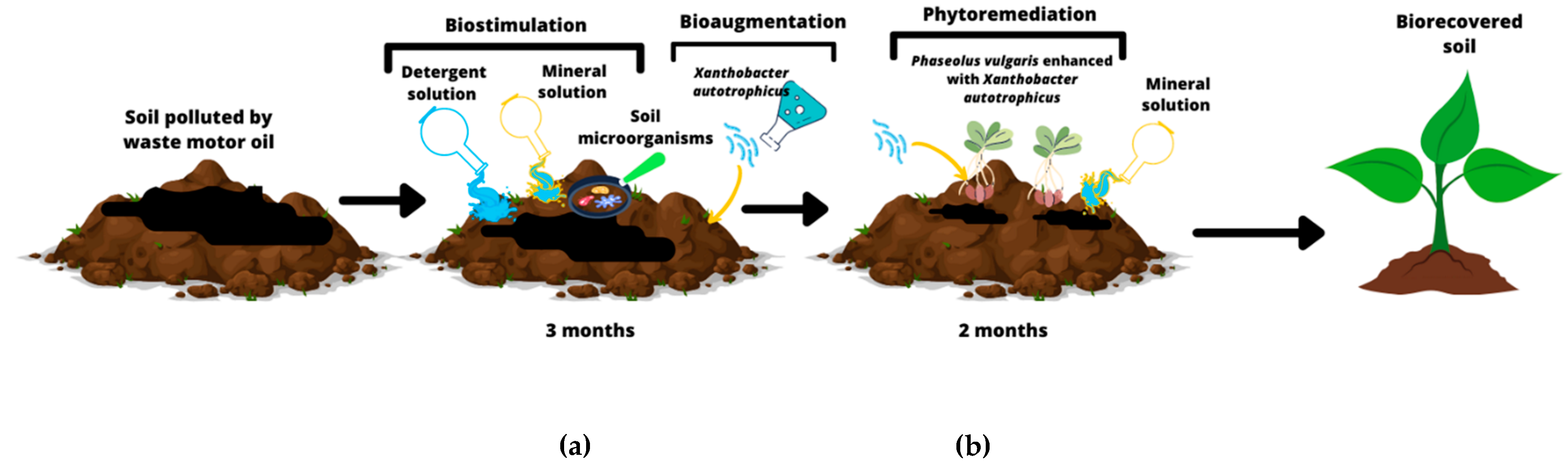
:1. Introduction
2. Results
2.1. Physicochemical Analysis of the Agricultural Soil
2.2. Reduction in Waste Motor Oil after Biostimulation and Soil Bioaugmentation
2.3. Germination of P. vulgaris with X. autotrophicus in Phytoremediation of Soil
2.4. Phenology and Biomass of P. vulgaris Enhanced with X. autotrophicus in Soil Phytoremediation at the Seedling Stage
2.5. Phenology and Biomass of P. vulgaris Enhanced with X. autotrophicus in Soil Phytoremediation at the Physiological Maturity Stage
2.6. Final WMO Concentration in Agricultural Soil after Biostimulation, Bioaugmentation, and Phytoremediation
2.7. Residual Hydrocarbons in WMO after Biostimulation, Bioaugmentation, and Phytoremediation
3. Discussion
4. Materials and Methods
4.1. Agricultural Soil Sample Collection and Preparation
4.2. Biostimulation and Bioaugmentation of Soil Impacted by WMO with X. autotrophicus
4.3. Phytoremediation of Soil Impacted by WMO with P. vulgaris Enhanced with X. autotrophicus
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, G.O. Chemical-biological stabilization of hydrocarbon contaminated soil and drilling cuttings in tropical Mexico. Land Contam. Reclam. 2004, 12, 349–361. [Google Scholar] [CrossRef]
- Adams, R.H.; Zavala-Cruz, J.; Morales-García, F.A. Concentración residual de hidrocarburos en suelo del trópico. II: Afectación a la fertilidad y su recuperación. Interciencia 2008, 33, 483–489. [Google Scholar]
- Islam, M.N.; Jo, Y.T.; Chung, S.Y.; Park, J.H. Assessment of polycyclic aromatic hydrocarbons in school playground soils in urban Gwangju, South Korea. Arch. Environ. Contam. Toxicol. 2018, 74, 431–441. [Google Scholar] [CrossRef]
- NOM-138-SEMARNAT/SSA1-2012. Límites máximos permisibles de hidrocarburos en suelos y lineamientos para el muestreo en la caracterización y especificación para la remediación. Secretaría de Medio Ambiente y Recursos Naturales. Diario Oficial de la Federación. 10 de septiembre de 2010. NORMA OFICIAL MEXICANA NOM-138-SEMARNAT/SSA1-2012|Procuraduria Federal de Proteccion al Ambiente|Gobierno|gob.mx. Available online: www.gob.mx (accessed on 20 February 2022).
- Guo, M.; Gong, Z.; Allinson, G.; Tai, P.; Miao, R.; Li, X.; Jia, C.; Zhuang, J. Variations in the bioavailability of polycyclic aromatic hydrocarbons in industrial and agricultural soils after bioremediation. Chemosphere 2016, 144, 1513–1520. [Google Scholar] [CrossRef] [Green Version]
- Nwankwegu, A.S.; Onwosi, C.O.; Azi, F.; Azumini, P.; Anaukwu, C.G. Use of rice husk as bulking agent in bioremediation of automobile gas oil impinged agricultural soil. Soil Sediment Contam. Int. J. 2017, 26, 96–114. [Google Scholar] [CrossRef]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, biostimulation and bioaugmention: A review. Int. J. Environ. Bioremediat. Biodegrad. 2015, 3, 28–39. [Google Scholar] [CrossRef]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef]
- Ite, A.E.; Semple, K.T. Biodegradation of petroleum hydrocarbons in contaminated soils. In Microbial Biotechnology: Energy and Environment; Arora, R., Ed.; CAB International: Wallingford, UK, 2012; Volume 1, pp. 250–278. [Google Scholar]
- Jiang, Y.; Brassington, K.J.; Prpich, G.; Paton, G.I.; Semple, K.T.; Pollard, S.J.T.; Coulon, F. Insights into the biodegradation of weathered hydrocarbons in contaminated soils by bioaugmentation and nutrient stimulation. Chemosphere 2016, 161, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Saez, J.M.; Bigliardo, A.L.; Raimondo, E.E.; Briceno, G.E.; Polti, M.A.; Benimeli, C.S. Lindane dissipation in a biomixture: Effect of soil properties and bioaugmentation. Ecotoxicol. Environ. Saf. 2018, 156, 97–105. [Google Scholar] [CrossRef]
- Ite, A.E.; Ibok, U.J. Role of plants and microbes in bioremediation of petroleum hydrocarbons contaminated soils. Int. J. Environ. Bioremediat. Biodegrad. 2019, 7, 1–19. [Google Scholar] [CrossRef]
- Janssen, D.B.; Stucki, G. Perspectives of genetically engineered microbes for groundwater bioremediation. Environ. Sci. Processes Impacts 2020, 22, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, D.; Kataoka, T.; Sonaka, H.; Hirakata, Y.; Hatamoto, M.; Yamaguchi, T. Evaluation of key factors for residual rubber coagulation in natural rubber processing wastewater. J. Water. Process. Eng. 2020, 33, 101041. [Google Scholar] [CrossRef]
- Akram, M.S.; Rashid, N.; Basheer, S. Physiological and molecular basis of plants tolerance to linear halogenated hydrocarbons. In Handbook of Bioremediation; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 591–602. [Google Scholar] [CrossRef]
- Tikariha, H.; Purohit, H.J. Genomic adaptation and metabolic hierarchy: Microbial community response to oxygen stress in community derived from sludge treating refinery wastewater. J. Clean. Prod. 2021, 320, 128808. [Google Scholar] [CrossRef]
- Baoune, H.; Aparicio, J.D.; Acuña, A.; El Hadj-khelil, A.O.; Sanchez, L.; Polti, M.A.; Alvarez, A. Effectiveness of the Zea mays-Streptomyces association for the phytoremediation of petroleum hydrocarbons impacted soils. Ecotoxicol. Environ. Saf. 2019, 184, 109591. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Mudhoo, A.; Gunaseelan, D. Biosurfactants: Synthesis, Properties and Applications in Environmental Bioremediation. In Bioremediation and Sustainability: Research and Applications; Mohee, R., Mudhoo, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 137–211. [Google Scholar] [CrossRef]
- Bosco, F.; Casale, A.; Chiampo, F.; Godio, A. Removal of diesel oil in soil microcosms and implication for geophysical monitoring. Water 2019, 11, 1661. [Google Scholar] [CrossRef] [Green Version]
- Sarma, H.; Nava, A.R.; Prasad, M.N.V. Mechanistic understanding and future prospect of microbe-enhanced phytoremediation of polycyclic aromatic hydrocarbons in soil. Environ. Technol. Innov. 2019, 13, 318–330. [Google Scholar] [CrossRef]
- Ebadi, A.; Sima, N.A.K.; Olamaee, M.; Hashemi, M.; Nasrabadi, R.G. Remediation of saline soils contaminated with crude oil using the halophyte Salicornia persica in conjunction with hydrocarbon-degrading bacteria. J. Environ. Manag. 2018, 219, 260–268. [Google Scholar] [CrossRef]
- Ionescu Topa, S.; Mihailescu, S.; Strat, D.; Gheorghe, I. Effects of oil pollution on seed germination and seedling emergence toxicity. Rom. Biotechnol. Lett. 2020, 25, 1194–1201. [Google Scholar] [CrossRef]
- Essabri, A.M.; Aydinlik, N.P.; Williams, N.E. Bioaugmentation and biostimulation of total petroleum hydrocarbon degradation in a petroleum-contaminated soil with fungi isolated from olive oil effluent. Water Air Soil Pollut. 2019, 230, 1–16. [Google Scholar] [CrossRef]
- Sáez, F.; Pozo, C.; Gómez, M.A.; Martínez-Toledo, M.V.; Rodelas, B.; Gónzalez-López, J. Growth and denitrifying activity of Xanthobacter autotrophicus CECT 7064 in the presence of selected pesticides. Appl. Microbiol. Biotechnol. 2006, 71, 563–567. [Google Scholar] [CrossRef]
- Liu, C.; Sakimoto, K.K.; Colón, B.C.; Silver, P.A.; Nocera, D.G. Ambient nitrogen reduction cycle using a hybrid inorganic–biological system. Proc. Natl. Acad. Sci. USA 2017, 114, 6450–6455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrbacher, F.; St-Arnaud, M. Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Płociniczak, T.; Fic, E.; Pacwa-Płociniczak, M.; Pawlik, M.; Piotrowska-Seget, Z. Improvement of phytoremediation of an aged petroleum hydrocarbon-contaminated soil by Rhodococcus erythropolis CD 106 strain. Int. J. Phytoremediat. 2017, 19, 614–620. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Selberg, A.; Budashova, J.; Tenno, T. Column study of the leaching and degradation of anionic surfactants in oil-polluted soil. Proc. Estonian Acad. Sci. Chem. 2007, 56, 87–97. [Google Scholar] [CrossRef]
- Adams, R.H.; Morales-García, F. Concentración Residual de Hidrocarburos en Suelo del Trópico. I: Consideraciones Para la Salud Pública y Protección al Ganado. Interciencia 2008, 33, 476–482. [Google Scholar]
- Singer, A.C.; Crowley, D.E.; Thompson, I.P. Secondary plant metabolites in phytoremediation and biotransformation. Trends Biotechnol. 2003, 21, 123–130. [Google Scholar] [CrossRef]
- Ite, A.E.; Adebisi, O.O.; Hanney, N.F.; Semple, K.T. The Effect of Rhizosphere Soil and Root Tissues Amendment on Microbial Mineralisation of Target 14C Hydrocarbons in Contaminated Soil. Int. J. Environ. Bioremediat. Biodegrad. 2016, 4, 21–34. [Google Scholar] [CrossRef]
- Verâne, J.; Dos Santos, N.C.; da Silva, V.L.; de Almeida, M.; de Oliveira, O.M.; Moreira, Í.T. Phytoremediation of polycyclic aromatic hydrocarbons (PAHs) in mangrove sediments using Rhizophora mangle. Mar. Pollut. Bull. 2020, 160, 111687. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-021-SEMARNAT-2000, que Establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos, Estudio, Muestreo y Análisis. Mexico. DOF Secretaria de Gobernación [en linea]. 2013. Available online: https://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/libros2009/DO2280n.pdf (accessed on 2 August 2021).
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]
- Cecotti, M.; Coppotelli, B.M.; Mora, V.C.; Viera, M.; Morelli, I.S. Efficiency of surfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbon-contaminated soil: Link with bioavailability and the dynamics of the bacterial community. Sci. Total Environ. 2018, 634, 224–234. [Google Scholar] [CrossRef]
- Ouriache, H.; Moumed, I.; Arrar, J.; Namane, A.; Lounici, H. Influence of C/N/P ratio evolution on biodegradation of petroleum hydrocarbons-contaminated soil. Alger. J. Environ. Sci. Technol. 2020, 6, 1604–1611. [Google Scholar]
- Interiano-López, M.L.; Ramírez-Coutiño, V.A.; Godinez-Tovar, L.A.; Zamudio-Pérez, E.; Rodríguez-Valadez, F.J. Bioremediation methods assisted with humic acid for the treatment of oil-contaminated drill cuttings. Rev. Mex. Ing. Quim. 2019, 18, 929–937. [Google Scholar] [CrossRef]
- Ayeni, A.O.; Oyekunle, D.T.; Adegbite, O.; Alagbe, E.; Ejekwu, O. Physico-chemical remediation of polycyclic aromatic hydrocarbons contaminated soil. J. Phys. Conf. Ser. 2019, 1299, 012121. [Google Scholar] [CrossRef]
- Shahzad, A.; Siddiqui, S.; Bano, A.; Sattar, S.; Hashmi, M.Z.; Qin, M.; Shakoor, A. Hydrocarbon degradation in oily sludge by bacterial consortium assisted with alfalfa (Medicago sativa L.) and maize (Zea mays L.). Arab. J. Geosci. 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, Q.; Cai, Z.; Zhang, Z. Phytoremediation of petroleum contaminated soils by Mirabilis Jalapa L. in a greenhouse plot experiment. J. Hazard. Mat. 2009, 168, 1490–1496. [Google Scholar] [CrossRef]
- Košnář, Z.; Částková, T.; Wiesnerová, L.; Praus, L.; Jablonský, I.; Koudela, M.; Tlustoš, P. Comparing the removal of polycyclic aromatic hydrocarbons in soil after different bioremediation approaches in relationto the extracellular enzyme activities. J. Environ. Sci. 2019, 76, 249–258. [Google Scholar] [CrossRef]
- Walpole, E.R.; Myers, R.H.; Myers, S.L. Experimentos con un solo factor: Generales. In Probabilidad y Estadística Para Ingeniería y Ciencias, 9th ed.; López, B.G., Hernández, C.F., Eds.; PEARSON: Naucalpan de Juárez, Estado de Mexico, Mexico, 2007; Volume 1, pp. 507–560. [Google Scholar]


| Parameter | Value | Interpretation |
|---|---|---|
| pH | 5.67 | Moderately acidic (5.1–6.5) |
| Organic matter (%) | 10.44 | Very high (>6.0) |
| Texture (%) | 31.8 (clay), 26.92 (sand), 42.0 (silt) | Clayey silt |
| Total nitrogen (ppm) | 3200 | Very high (>0.25) |
| Phosphorus (ppm) | 219.34 | Very high |
| Sodium (Na+) (ppm) | 153.38 | High |
| Potassium (K+) (ppm) | 168.61 | High |
| Microelements (ppm): | ||
| Iron (Fe2+) | 13.91 | Appropriate (>4.5) |
| Zinc (Zn2+) | 0.37 | Deficient (<0.5) |
| Copper (Cu2+) | 0.54 | Appropriate (>0.2) |
| Manganese (Mn2+) | 4.62 | Appropriate 1 (>1) |
| Soil | WMO Concentration after 3 Months (ppm) | Mineralization Percentage (%) |
|---|---|---|
| Negative control soil impacted by WMO * | 39,215 ± 0.06 c,** | 34.65 ± 0.01 c |
| T1: Soil impacted by WMO biostimulated with Tween® 80 + Triton® X-100 and 50% MISO | 23,088 ± 0.06 b | 61.62 ± 0.03 b |
| T2: Soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO and bioaugmented with X. autotrophicus | 17,599 ± 0.12 a | 70.67 ± 0.24 a |
| Phaseolus vulgaris | Emergency Days | Germination Percentage (%) |
|---|---|---|
| Absolute control P. vulgaris without WMO * soil irrigated only water | 6 ± 0.13 b,** | 75 ± 0.15 c |
| Relative control P. vulgaris without WMO fed 100% MISO | 6 ± 0.02 b | 80 ± 0.10 b |
| T1: P. vulgaris in soil impacted by WMO + biostimulated with Tween® 80/Triton® X-100 and 50% MISO | 5 ± 0.03 a | 75 ± 0.01 c |
| T2: P. vulgaris in soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO and bioaugmented with X. autotrophicus | 5 ± 0.08 a | 83 ± 0.03 b |
| T3: P. vulgaris enhanced with X. autotrophicus in soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO and bioaugmented with X. autotrophicus | 5 ± 0.16 a | 92 ± 0.13 a |
| Phaseolus vulgaris | Plant Height (cm) | Root Length (cm) | Aerial Fresh Weight (g) | Root Fresh Weight (g) | Aerial Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|
| Absolute control P. vulgaris without WMO * soil irrigated only water | 27.25 ± 0.02 c,** | 7.16 ± 0.06 a | 1.46 ± 0.18 d | 0.1 ± 0.14 a | 0.161 ± 0.14 c | 0.016 ± 0.10 a |
| Relative control P. vulgaris without WMO fed 100% MISO | 31.27 ± 0.10 b | 6.5 ± 0.23 b | 1.82 ± 0.27 b | 0.09 ± 0.05 b | 0.16 ± 0.05 c | 0.012 ± 0.18 c |
| T1: P. vulgaris in soil impacted by WMO + biostimulated with Tween® 80/Triton® X-100 and 50% MISO | 30 ± 0.15 b | 4.14 ± 0.27 d | 1.39 ± 0.18 d | 0.04 ± 0.30 d | 0.169 ± 0.30 c | 0.009 ± 0.15 e |
| T2: P. vulgaris in soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO and bioaugmented with X. autotrophicus | 36.42 ± 0.16 a | 5.85 ± 0.05 c | 2.13 ± 0.15 a | 0.04 ± 0.18 d | 0.219 ± 0.23 a | 0.011 ± 0.06 d |
| T3: P. vulgaris enhanced with X. autotrophicus in soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO and bioaugmented with X. autotrophicus | 27.25 ± 0.18 c | 6.58 ± 0.20 b | 1.62 ± 0.10 c | 0.06 ± 0.22 c | 0.184 ± 0.10 b | 0.014 ± 0.16 b |
| Phaseolus vulgaris | Plant Height (cm) | Root Length (cm) | Aerial Fresh Weight (g) | Root Fresh Weight (g) | Aerial Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|
| Absolute control P. vulgaris without WMO * soil irrigated only with water | 76.42 ± 0.07 d,** | 20.42 ± 0.02 a | 2.4 ± 0.15 e | 1.38 ± 0.23 d | 0.71 ± 0.03 c | 0.14 ± 0.15 b |
| Relative control P. vulgaris without WMO fed 100% MISO | 70.56 ± 0.12 e | 18.25 ± 0.18 b | 3.78 ± 0.23 d | 1.24 ± 0.11 e | 0.72 ± 0.13 c | 0.08 ± 0.22 d |
| T1: P. vulgaris in soil impacted by WMO + biostimulated with Tween® 80/Triton® X-100 and 50% MISO | 84.85 ± 0.1 c | 17.28 ± 0.12 c | 4.18 ± 0.15 c | 1.79 ± 0.08 d | 0.91 ± 0.24 b | 0.12 ± 0.14 c |
| T2: P. vulgaris in soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO and bioaugmented with X. autotrophicus | 108.57 ± 0.13 a | 20.28 ± 0.35 a | 8.37 ± 0.06 a | 3.61 ± 0.15 a | 1.12 ± 0.31 a | 0.21 ± 0.04 a |
| T3: P. vulgaris enhanced with X. autotrophicus in soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO and bioaugmented with X. autotrophicus | 93.38 ± 0.12 b | 17.33 ± 0.40 c | 4.58 ± 0.01 b | 1.51 ± 0.02 c | 0.63 ± 0.10 d | 0.1 ± 0.14 c |
| Soil | WMO Final Concentration (ppm) |
|---|---|
| Negative control soil impacted by WMO * | 35,210 ± 0.12 d |
| T1: Soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO and phytoremediated with P. vulgaris | 3040 ± 0.01 c |
| T2: Soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO, bioaugmented with X. autotrophicus and phytoremediated with P. vulgaris | 190 ± 0.03 a,** |
| T3: Soil impacted by WMO biostimulated with Tween® 80/Triton® X-100, 50% MISO, bioaugmented with X. autotrophicus, and phytoremediated with P. vulgaris inoculated with X. autotrophicus | 680 ± 0.09 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, B.C.S.; Benavides, L.M.; Santoyo, G.; Sánchez-Yáñez, J.M. Biorecovery of Agricultural Soil Impacted by Waste Motor Oil with Phaseolus vulgaris and Xanthobacter autotrophicus. Plants 2022, 11, 1419. https://doi.org/10.3390/plants11111419
Martínez BCS, Benavides LM, Santoyo G, Sánchez-Yáñez JM. Biorecovery of Agricultural Soil Impacted by Waste Motor Oil with Phaseolus vulgaris and Xanthobacter autotrophicus. Plants. 2022; 11(11):1419. https://doi.org/10.3390/plants11111419
Chicago/Turabian StyleMartínez, Blanca Celeste Saucedo, Liliana Márquez Benavides, Gustavo Santoyo, and Juan Manuel Sánchez-Yáñez. 2022. "Biorecovery of Agricultural Soil Impacted by Waste Motor Oil with Phaseolus vulgaris and Xanthobacter autotrophicus" Plants 11, no. 11: 1419. https://doi.org/10.3390/plants11111419
APA StyleMartínez, B. C. S., Benavides, L. M., Santoyo, G., & Sánchez-Yáñez, J. M. (2022). Biorecovery of Agricultural Soil Impacted by Waste Motor Oil with Phaseolus vulgaris and Xanthobacter autotrophicus. Plants, 11(11), 1419. https://doi.org/10.3390/plants11111419







