Comparative Mutagenic Effectiveness and Efficiency of Gamma Rays and Sodium Azide in Inducing Chlorophyll and Morphological Mutants of Cowpea
Abstract
1. Introduction
2. Results
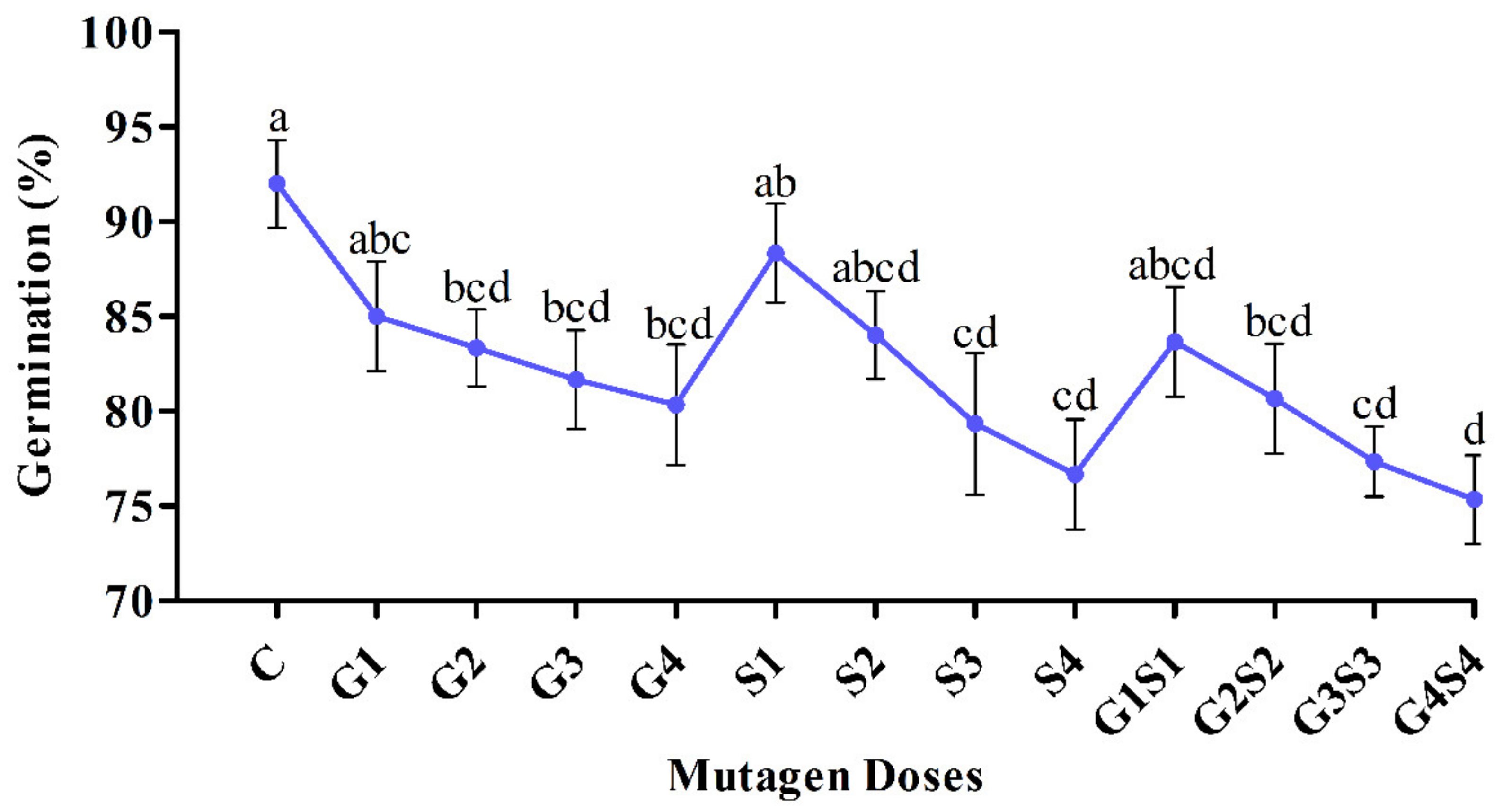
2.1. Seed Germination
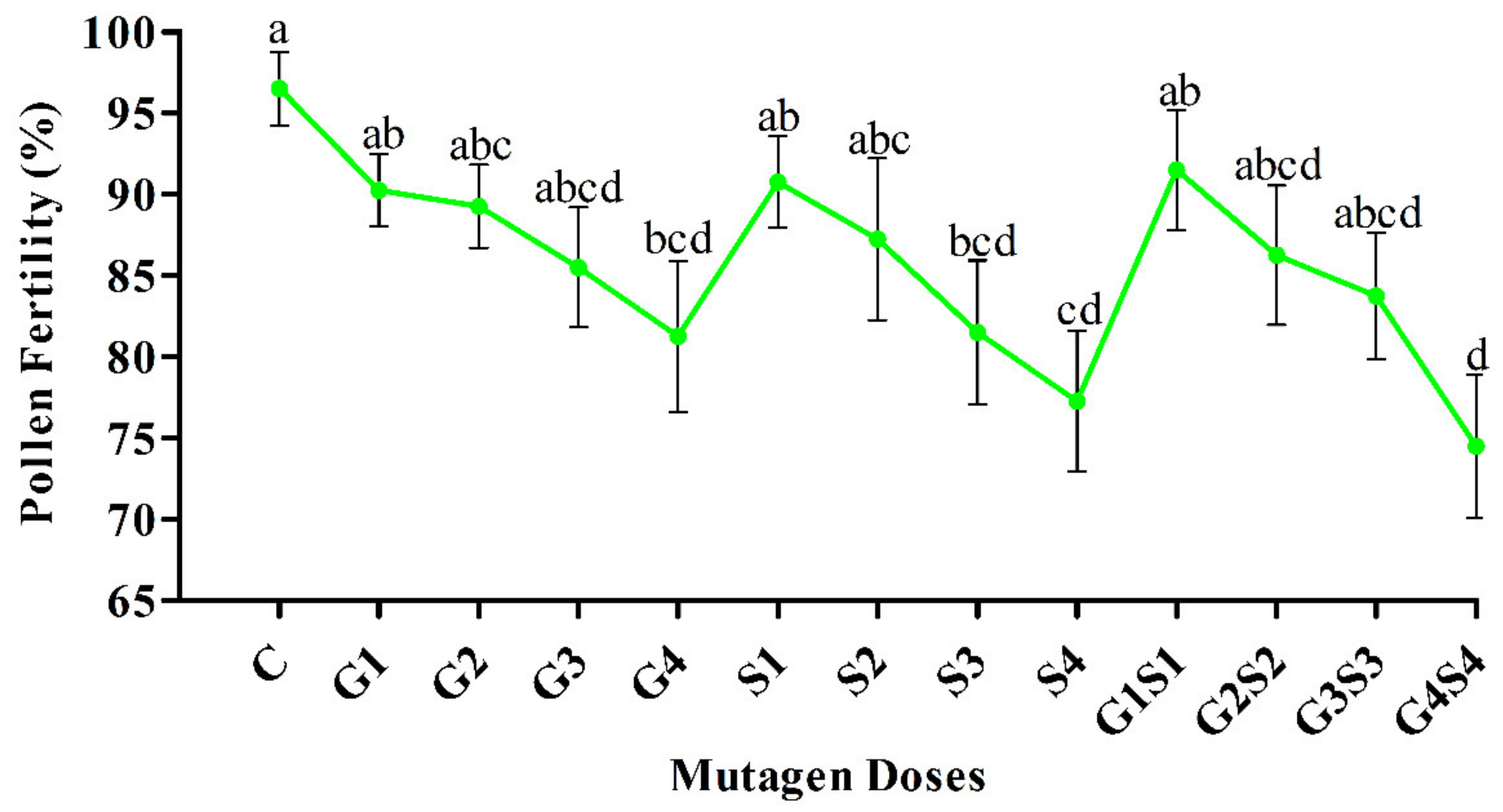
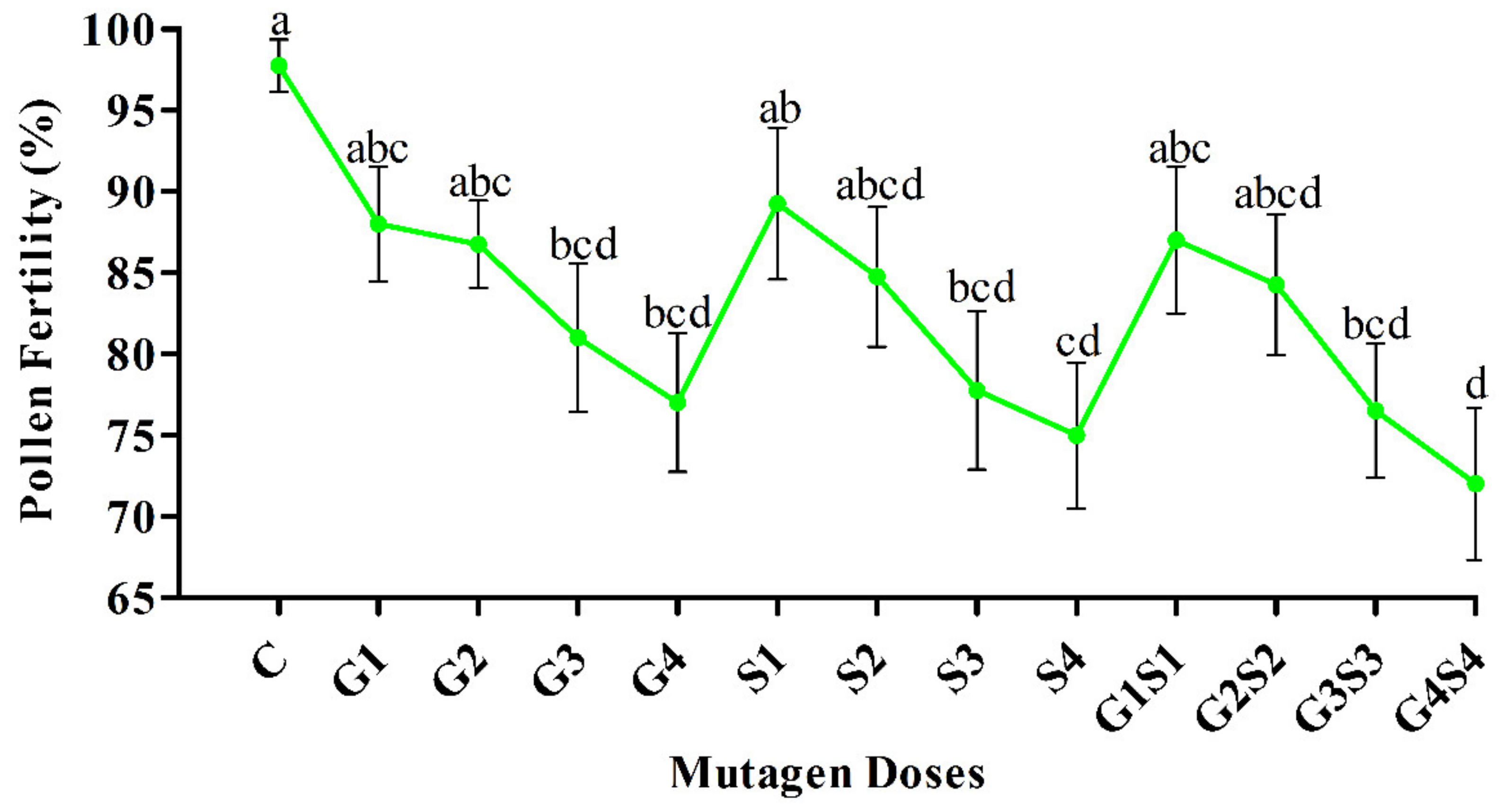
2.2. Pollen Fertility
2.3. Chlorophyll Mutants: Frequency and Spectrum
- Albina: Lethal mutants lacking all photopigments, leaves were white. Seedlings survived for two weeks after germination.
- Chlorina: The first pair of seedling leaves were light green. Plants remained light green throughout the growth period. Seedlings survived to maturity.
- Xantha: Leaves were yellow due to the absence of chlorophyll. Seedlings survived up to three–four leaf stages.
- Tigrina: Leaves showed yellow and green patches. Seedlings survived for 3–4 weeks.
- Viridis: Leaves were initially light/yellow-green (viridine green) but gradually turned green. Seedlings were short in height and slow-growing. Seedlings survived to maturity.
- Xanthaviridis: Leaves were a viridine green color. Seedlings survived to maturity.
2.4. Mutagenic Effectiveness and Efficiency
2.5. Morphological Mutations
2.5.1. Plant Height Mutants
- Control: The height in untreated plants was 180.32–182.61 cm (Figure 6a).
- Tall mutants: These were tall with broader, dark green foliage, sparse branching, extended internodes, normal seed set, and attained a height of 180–185 cm (Figure 6b). These were induced at a frequency of 0.11 and 0.09% in the G1- and S2-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
- Dwarf mutants: These mutants remained dwarf throughout the growth period, exhibited short internodes, few leaves, reduced pod and seed size, low yield, and were severely stunted, measuring 110–115 cm in height (Figure 6c). These were induced at a frequency of 0.04% in the G2- and S3-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
- Semi-dwarf mutants: These mutants showed a height of 140–150 cm, shorter internodes, decreased branches, pods, and yield (Figure 6d). These were induced at a frequency of 0.01% in the G2S2- and S2-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
2.5.2. Growth Habit Mutants
- Control: Cowpea is an annual herb with a glabrous stem and a robust taproot system with erect or climbing growth habits.
- Semi-dwarf spreading mutants: These mutants reflected Gigas-like characteristics, vigorous growth, longer internodes, broader leaves, and spreading branches with wide branch angles (Figure 6e). These were isolated at a frequency of 0.03 and 0.02% in the G2- and S3-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
- Bushy mutants: These were short with condensed internodes, compact branches, and leaflets (Figure 6f). These mutants appeared at a frequency of 0.08% and 0.07% in the G3- and S2-treated populations of Gomati VU-89 and Pusa-578, respectively.
- One-sided branching mutants: These mutant plants showed branches on one side of the stem, a few pods, and shriveled seeds. These mutants appeared at a frequency of 0.03 and 0.02% in the G2S2- and S3-treated populations of Gomati VU-89 and Pusa-578, respectively.
- Axillary branched mutants: These were profusely branched with reduced internodes and yield (Figure 6g). These were induced at a frequency of 0.03 and 0.02% in the G1- and S2-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
- Prostrate mutants: These were initially straight but showed a trailing habit at the soil surface due to vigorous secondary branching (Figure 6h). These mutants were induced at a frequency of 0.02% and 0.03% in the G1S1- and G3S3-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
2.5.3. Leaf Mutants
- Control: The first pair of true leaves are simple and opposite. The leaves are dark, green, compound, smooth, dull to shiny, and pubescent with three oval leaflets. The two side leaflets are asymmetrical, and one central terminal leaflet is symmetrical (Figure 7a).
- Broad-leaved/Gigas mutants: These mutants were tall, with profuse secondary branching with broader leaflets, extended rachis, and robust growth. The leaflets were larger (two times bigger than the control) with broad lamina. These mutants were induced in moderate γ rays and SA treatments with a frequency of 0.06 and 0.05% in the G1S1- and G3S3-treated populations of varieties Gomati VU-89 and Pusa-578, respectively (Figure 7b).
- Narrow-leaved mutants: These mutants possessed narrow or small needle-like leaflets, pointed leaf tips, small pods, and few seeds. Branching was normal; however, flowering and maturity were delayed (Figure 7c). Such mutants appeared at a frequency of 0.05% and 0.04% in the G2S2- and S3-treated populations of Gomati VU-89 and Pusa-578, respectively.
- Elongated rachis: These mutants revealed increased rachis, narrow leaflets, and pointed leaf tips (Figure 7d). Such mutants appeared at a frequency of 0.04 and 0.03% in the G1- and G3S3-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
- Altered leaf architecture: These mutants exhibited notched leaflets, irregular leaf margins, abnormal leaf tips, and venation (Figure 7e,f). These mutants appeared at a frequency of 0.03 and 0.02% in the G2- and S3-treated populations of Gomati VU-89 and Pusa-578, respectively.
- Abnormal leaflet number: These plants were characterized by an abnormal number of leaflets, including two (bifoliate), four (tetrafoliate), and five (pentafoliate) mutants (Figure 7g–l). These mutants were not stable and showed segregation in the subsequent generations. Hence, these mutants were not included in the frequency calculations.
2.5.4. Flower Mutants
- Control: The flowers were usually in pairs, yellowish in color, with racemose inflorescences born on peduncles in the leaf axils. Peduncles were 2–20 cm long, stout, and grooved. The flowers were 2–3 cm in diameter with a straight keel, diadelphous stamens, a sessile ovary with several ovules, a bearded style, and an oblique stigma (Figure 8a).
- Flower color mutants: These were characterized by flowers that gradually turned white, blue, and red instead of yellow in the parent variety. Few flower mutants exhibited variation even in the color of the petals (Figure 8b). Blue flowers were recorded more frequently than white flowers in Gomati VU-89. However, white mutants appeared more often than blue flowers in Pusa-578 (Figure 8c). The G3 treatment showed a higher frequency of flower mutants in Gomati VU-89 (0.03%) and Pusa-578 (0.04%).
- Multiple flower mutants: In these mutants, each peduncle consisted of three to four normal flowers instead of two flowers in the parent variety. These mutants appeared at a frequency of 0.03% in the G1S1- and S1-treated populations of varieties Gomati VU-89 and Pusa-578, respectively (Figure 8d,e).
- Open flower mutants: These possessed flowers with broad keel and wings, exposed stamens, and stigma. Such mutants appeared at a frequency of 0.06 in the G3- and G4S4-treated populations of varieties Gomati VU-89 and Pusa-578, respectively (Figure 8f).
- Non-flowering mutants: These mutants did not flower at all and remained vegetative throughout the growth period. These appeared at a frequency of 0.01 and 0.02% in the G4- and G4S4-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
- Late flowering mutants: In these mutants, the flowering was delayed by 9 to 10 days compared to untreated plants. Such mutants were commonly observed in populations treated with higher mutagen doses at a frequency of 0.04% and 0.03% in the G4S4- and G4-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
- Early maturing mutants: These mutants matured 3 to 4 days earlier than the untreated population and appeared at a frequency of 0.02% in the G2- and S2-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
2.5.5. Pod Mutants
- Control: Pods were pending and vertical, 23–30 cm long, 5–10 mm wide, containing 9–12 seeds. Pods occurred singly or in pairs (Figure 9a).
- Small/narrow pods: These mutants possessed narrow and small pods that appeared at a frequency of 0.07 and 0.06% in the G2S2- and S3-treated populations of varieties Gomati VU-89 and Pusa-578, respectively (Figure 9b).
- Bold-seeded pods: These mutants showed robust growth, profuse branching, broad leaflets, large-sized flowers, and longer pods containing bold seeds. Such mutants were induced at lower and intermediate doses with 0.06 and 0.05% frequency in the G1- and S2-treated populations of Gomati VU-89 and Pusa-578, respectively (Figure 9c).
2.5.6. Seed Mutants
- Control: Seeds were brown and white with smooth seed coats in Gomati VU-89 and Pusa-578, respectively (Figure 10).
- Seed coat color mutants: These were upright and straight, with light green leaves, compared to the parent variety’s dark green leaves. Such mutants were characterized by red or black smooth seed coats. Such mutants were induced at an equal frequency of 0.06% in the G2S2- and G3S3-treated populations of Gomati VU-89 and Pusa-578, respectively.
- Seed coat pattern mutants: These mutants revealed streaked, speckled, and stippled rough seed coats. Such mutants appeared at a frequency of 0.07% and 0.06% in the G4- and S3-treated populations of Gomati VU-89 and Pusa-578, respectively.
- Seed shape and surface mutants: These mutants revealed alterations in seed attributes and appeared at a frequency of 0.09% and 0.08% in the G2S2- and S3-treated populations of varieties Gomati VU-89 and Pusa-578, respectively.
3. Discussion
3.1. Seed Germination
3.2. Pollen Fertility
3.3. Chlorophyll Mutations
3.4. Mutagenic Effectiveness and Efficiency
3.5. Morphological Mutations
4. Materials and Methods
4.1. Experimental Materials and Seed Irradiation
4.2. Experimental Site and Crop Cultivation
Details of the Field Trials
4.3. Field Analysis
4.3.1. Seed Germination
4.3.2. Chlorophyll Mutants
4.4. Mutagenic Effectiveness and Efficiency
4.5. Morphological Mutants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raina, A.; Khan, S.; Wani, M.R.; Laskar, R.A.; Mushtaq, W. Chickpea (Cicer arietinum L.) cytogenetics, genetic diversity and breeding. In Advances in Plant Breeding Strategies: Legumes; Al-Khayri, J.M., Jain, M., Johnson, D.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 7, pp. 53–112. [Google Scholar]
- Food and Agriculture Organization. Definition and Classification of Commodities: Pulses and Derived Products; Food and Agriculture Organization: Roma, Italia, 1994. [Google Scholar]
- Raina, A.; Laskar, R.A.; Tantray, Y.R.; Khursheed, S.; Wani, M.R.; Khan, S. Characterization of Induced High Yielding Cowpea Mutant Lines Using Physiological, Biochemical and Molecular Markers. Sci. Rep. 2020, 10, 3687. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.N.; Ghebrehiwot, H.M.; Shimelis, H.A. Selection of novel cowpea genotypes derived through gamma irradiation. Front. Plant Sci. 2016, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.D.; Hall, A.E. 1997. Cowpea (Vigna unguiculata L. walp.). Field Crops Res. 1997, 53, 187–204. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Production Statistics; Food and Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- Goyal, S.; Wani, M.R.; Laskar, R.A.; Raina, A.; Khan, S. Assessment on cytotoxic and mutagenic potency of Gamma rays and EMS in Vigna mungo L. Hepper. Biotecnol. Vegetal. 2019, 19, 193–204. [Google Scholar]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Al-Qurainy, F.; Anwar, F. Sodium azide: A chemical mutagen for enhancement of agronomic traits of crop plants. Environ. Int. J. Sci. Technol. 2009, 4, 1–21. [Google Scholar]
- Gruszka, D.; Szarejko, I.; Maluszynski, M. Sodium azide as a mutagen. In Plant Mutation Breeding and Biotechnology; Shu, Q.Y., Forster, B.P., Nakagawa, H., Eds.; CABI: Wallingford, UK, 2012; pp. 159–166. [Google Scholar]
- Goyal, S.; Wani, M.R.; Khan, S. Frequency and Spectrum of Chlorophyll Mutations Induced by Single and Combination Treatments of Gamma Rays and EMS in Urdbean. Asian J. Biol. Sci. 2019, 12, 156–163. [Google Scholar] [CrossRef][Green Version]
- Goyal, S.; Wani, M.R.; Laskar, R.A.; Raina, A.; Khan, S. Mutagenic effectiveness and efficiency of individual and combination treatments of gamma rays and Ethyl Methanesulfonate in black gram [Vigna mungo (L.) Hepper]. Adv. Zool. Bot. 2020, 8, 163–168. [Google Scholar] [CrossRef]
- Raina, A.; Laskar, R.A.; Khursheed, S.; Amin, R.; Parveen, K.; Khan, S. Role of mutation breeding in crop improvement-past, present and future. Asian Res. J. Agr. 2016, 2, 1–13. [Google Scholar] [CrossRef]
- Mutant Variety Database. Available online: https://mvd.iaea.org/ (accessed on 30 March 2021).
- Raina, A.; Laskar, R.A.; Khursheed, S.; Khan, S.; Parveen, K.; Amin, R.; Khan, S. Induce physical and chemical mutagenesis for improvement of yield attributing traits and their correlation analysis in chickpea. Int. Lett. Nat. Sci. 2017, 61, 14–22. [Google Scholar] [CrossRef]
- Muthusamy, A.; Jayabalan, N. Effect of mutagens on pollen fertility of cotton (Gossypium hirsutum L.). Indian J. Genet. 2002, 62, 187. [Google Scholar]
- Pawar, N.; Pai, S.; Nimbalkar, M.; Kolar, F.; Dixit, G. Induction of chlorophyll mutants in Zingiber officinale Roscoe by gamma rays and EMS. Emirates J. Food Agric. 2010, 22, 406–411. [Google Scholar] [CrossRef]
- Monica, S.; Seetharaman, N. Physical and chemical mutagenic effect on pollen fertility in M1 generation of Garden Bean [Lablab purpureus (L.) Sweet var. typicus cv. CO (Gb) 14]. Eur. J. Exp. Biol. 2015, 5, 6–9. [Google Scholar]
- Taziun, T.; Laskar, R.A.; Amin, R.; Parveen, S.K. Effects of dosage and durations of different mutagenic treatment in lentil (Lens culinaris Medik.) cultivars Pant L 406 and DPL 62. Legume Res. 2017, 41, 500–509. [Google Scholar] [CrossRef]
- Wani, M.R.; Khan, S. Chlorophyll mutations in lentil. Trop. Agric. 2003, 154, 21–26. [Google Scholar]
- Ganesh, B.K. Effect of mutagen on pollen fertility and other parameter in horse gram [Macrotyloma uniflorum (Lam) Verdcourt]. Biosci. Discov. J. 2011, 2, 146–150. [Google Scholar]
- Sangle, S.M.; Mahamune, S.E.; Kharat, S.N.; Kothekar, V.S. Effect of mutagenesis on germination and pollen sterility in pigeonpea. Biosci. Discov. J. 2011, 2, 127–130. [Google Scholar]
- Neto, T.A.; Ando, A.; Figueira, A. Genetic Improvement of Crops by Mutation Techniques in Brazil. Plant Mutat. Rep. 2011, 2, 3. [Google Scholar]
- Girija, M.; Dhanavel, D. Mutagenic effectiveness and efficiency of gamma rays, ethyl methylmethane sulphonate and their combined treatment in cowpea (Vigna unguiculata L. Walp.). Glob. J. Mol. Sci. 2009, 4, 68–75. [Google Scholar]
- Maluszynski, M.I.; Szarejko, C.R.; Bhatia, K.; Nichterlein and Lagoda, P.J.L. Plant breeding and farmer participation. In Methodologies for Generating Variability. Part 4: Mutation Techniques; Ceccarelli, S., Weltzien, E., Eds.; Food and Agriculture Organization: Rome, Italy, 2009; pp. 159–194. [Google Scholar]
- Gustafsson, A. The mutation system of the chlorophyll apparatus. Lunds Univ. Arsskr. 1940, 51, 11. [Google Scholar]
- Mackay, J. Neutron and X-ray experiment in Barley. Hereditas 1951, 32, 421–464. [Google Scholar]
- Singh, D.P.; Sharma, S.P.; Lal, M.; Ranwah, B.R.; Sharma, V. Induction of Genetic variability for polygentraits through physical and chemical mutagens in cowpea (Vigna unguiculata). Legume Res. 2013, 36, 10–14. [Google Scholar]
- Goyal, S.; Khan, S. Induced mutagenesis in urdbean (Vigna mungo L. Hepper): A review. Int. J. Bot. 2010, 6, 194–206. [Google Scholar] [CrossRef]
- Kozgar, M.I. Induced Mutagenesis: Biophysiological Damages and Cytological Abberrations. In Mutation Breeding in Chickpea; Kozgar, M.I., Ed.; De Gruyter Open: Warszawa, Poland, 2014; pp. 13–30. [Google Scholar]
- Bhat, I.A.; Pandit, U.J.; Sheikh, I.S.; Hassan, Z.U. Physical and chemical mutagenesis in Linum usitatissimum L. to induce variability in seed germination, survival and growth rate traits. Curr. Bot. 2016, 7, 28–32. [Google Scholar] [CrossRef]
- Sharma, R.P. Increased mutation frequency and wider mutation spectrum in barley induced by combining gamma rays with ethyl methanesulphonate. Indian J. Genet. 1970, 30, 180–186. [Google Scholar]
- Konzak, C.F.; Nilan, R.A.; Wagner, J.; Foster, R.J. Efficient chemical mutagenesis. Rad. Bot. 1965, 5, 49–70. [Google Scholar]
- Bhosale, S.S.; Kothekar, V.S. Mutagenic efficiency and effectiveness in Cluster Bean (Cyamopsis tetragonoloba (L.) Taub.). J. Phytol. 2010, 2, 21–27. [Google Scholar]
- Nair, R.; Mehta, A.K. Induced mutagenesis in cowpea [Vigna unguiculata (L.) Walp] var. Arka Garima. Indian J. Agric. Res. 2014, 48, 247–257. [Google Scholar] [CrossRef]
- Shah, T.M.; Mirza, J.I.; Ahsanul, M.H.; Atta, B.M. Induced genetic variability in Chickpea (Cicer arietinum L.). II. Comparative mutagenic effectiveness and efficiency of physical and chemical mutagens. Pak. J. Bot. 2008, 40, 605–613. [Google Scholar]
- Laskar, R.A.; Khan, S. Mutagenic Effectiveness and Efficiency of Gamma Rays and HZ with Phenotyping of Induced Mutations in Lentil Cultivars. Int. Lett. Nat. Sci. 2017, 64, 17–31. [Google Scholar] [CrossRef]
- Dhanavel, D.; Pavadai, P.; Mullainathan, L.; Mohana, D.; Raju, G.; Girija, M.; Thilagavathi, C. Effectiveness and efficiency of chemical mutagens in Cowpea [Vigna unguiculata (L.) Walp.]. Afr. J. Biotech. 2008, 7, 4116–4117. [Google Scholar]
- Wani, A. Mutagenic effectiveness and efficiency of gamma rays, Ethyl Methane Sulphonate and their combination treatments in Chickpea (Cicer arietinum L.). Asian J. Plant Sci. 2009, 8, 318–321. [Google Scholar] [CrossRef]
- Van Harten, A.M. Mutation Breeding, Theory and Practical Applications; Cambridge University Press: Cambridge, UK, 1998; pp. 127–140. [Google Scholar]
- Tyagi, B.S.; Gupta, P.K. Induced mutations for fasciation in lentil (Lens culinaris Med.). Indian J. Genet. 1991, 51, 326–331. [Google Scholar]
- Wi, S.G.; Chung, B.Y.; Kim, J.H.; Baek, M.H.; Yang, D.H.; Lee, J.W.; Kim, J.S. Ultrastructural changes of cell organelles in Arabidopsis stems after gamma irradation. J. Plant Biol. 2005, 48, 195–200. [Google Scholar] [CrossRef]
- Waghmare, V.N.; Mehra, R.B. Induced chlorophyll mutations, mutagenic effectiveness and efficiency in Lathyrus sativus L. Indian J. Genet. 2001, 61, 53–56. [Google Scholar]
- Dhole, V.J.; Maheswari, J.J.; Patil, S. Studies on mutations induced by EMS in Soybean (Glycine max L. Merrill.). Agric. Sci. Digest. 2003, 23, 226–228. [Google Scholar]
- Gnanamurthy, S.; Mariyammal, S.; Dhanavel, D.; Bharathi, T. Effect of gamma rays on yield and yield components characters R3 generation in cowpea (Vigna unguiculata (L.). Walp.). Int. Res. J. Plant Sci. 2012, 2, 39–42. [Google Scholar]
- Wani, M.R.; Khan, S.; Kozgar, M.I.; Goyal, S. Induction of morphological mutants in mungbean (Vigna radiata (L.) Wilczek) through chemical mutagens. Nucleus 2011, 48, 243–247. [Google Scholar]
- Sharma, S.K.; Sharma, B. Pattern of induced mutability in different genotypes of lentil (Lens culinaris Medik.). Zeitscrift fur Pflanzenzuchttung 1979, 83, 315–320. [Google Scholar]
- Gunckel, J.E.; Sparrow, A.H. Ionizing Radiation: Biochemical, Physiological and Morphological Aspects of Their Effects on Plants; Ruhland, W., Ed.; Encycl Plant Physiol XVI; Springer: Berlin/Heidelberg, Germany, 1961; pp. 555–611. [Google Scholar]
- Henikoff, S.; Comai, L. Single nucleotide mutations for plant functional genomics. Ann. Rev. Plant Biol. 2003, 54, 375–401. [Google Scholar] [CrossRef]
- Saikat, K.B.; Surya, N.A.; James, E.T. Genetic improvement of fenugreek (Trigonella foenum-graecum L.) through EMS induced mutation breeding for higher seed yield under western Canada prairie conditions. Euphytica 2008, 160, 249–258. [Google Scholar]
- Khursheed, S.; Raina, A.; Parveen, K.; Khan, S. Induced phenotypic diversity in the mutagenized populations of faba bean using physical and chemical mutagenesis. J. Saudi Soc. Agric. Sci. 2019, 18, 113–119. [Google Scholar] [CrossRef]
- Badigannavar, A.M.; Mondal, S. Induction of mutations for plant height and inheritance of dwarf mutant in groundnut (Arachis hypogaea L.) through gamma ray irradiation. Electron. J. Plant Breed. 2010, 1, 156–161. [Google Scholar]
- Anjana, G.; Thimmaiah, S.K. Evaluation of dwarf mutant of cowpea (Vigna unguiculata L. Walp.) developed through gamma irradiation for nitrogen fixation characters. J. Nucl. Agric. Biol. 2002, 31, 94–98. [Google Scholar]
- Cheng, Q.; Dong, L.; Su, T.; Li, T.; Gan, Z.; Nan, H.; Kong, F. CRISPR/Cas9-mediated targeted mutagenesis of GmLHY genes alters plant height and internode length in soybean. BMC Plant Biol. 2019, 19, 562. [Google Scholar] [CrossRef]
- Naik, B.S.; Singh, B.; Kole, C. A promising mungbean (Vigna radiata (L.) Wilczek) genotype with high protein content and seed yield. Indian J. Genet. 2002, 62, 342–344. [Google Scholar]
- Hall, A.E. Breeding for adaptation to drought and heat in cowpea. Eur. J. Agron. 2004, 21, 447–454. [Google Scholar] [CrossRef]
- Martins, C.M.; Lawlor, D.W.; Quilambo, O.A.; Kunert, K.J. Evaluation of four Mozambican cowpea landraces for drought tolerance. S. Afr. J. Plant Soil 2014, 31, 87–91. [Google Scholar] [CrossRef]
- Khan, S.; Parveen, K.; Goyal, S. Induced mutations in chickpea-morphological mutants. Front. Agric. China 2011, 5, 35–39. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Mori, H. Control of outgrowth and dormancy in axillary buds. Plant Physiol. 2001, 127, 1405–1413. [Google Scholar] [CrossRef]
- Singh, B.B.; Ajeigbe, H.A.; Tarawali, S.A.; Fernandez-Rivera, S.; Abubakar, M. Improving the production and utilization of cowpea as food and fodder. Field Crop Res. 2003, 84, 169–177. [Google Scholar] [CrossRef]
- Adekola, O.F.; Oluleye, F. Induction of genetic variation in Cowpea (Vigna unguiculata L. Walp.) by gamma irradiation. Asian J. Plant Sci. 2007, 6, 869–873. [Google Scholar] [CrossRef][Green Version]
- Jackai, L.E. A field screening technique for resistance of cowpea (Vigna unguiculata) to the pod-borer Maruca testulalis (Geyer) (Lepidoptera: Pyralidae). Bull. Entomol. Res. 1982, 72, 145–156. [Google Scholar] [CrossRef]
- Thombre, M.V.; Mehetre, S.S. A pistillate mutant in cotton. Indian J. Genet. 1980, 40, 388–390. [Google Scholar]
- Ntonifor, N.N.; Jackai, L.E.N.; Ewete, F.K. Influence of host plant abundance and insect diet on the host selection behaviour of M. Testulalis Geyer (Lepidotera: Pyralidae) and Riptortus dentipes Fab (Hemiptera: Alydidae). Agric. Ecosyst. Environ. 1996, 60, 71–78. [Google Scholar] [CrossRef]
- Wani, A.A. Spectrum and frequency of macromutations induced in chickpea (Cicer arietinum L.). Turk. J. Biol. 2011, 35, 221–231. [Google Scholar]
- IITA. Biotechnology. Annual Report for 1988/1989; IITA: Ibadan, Nigeria, 1989; pp. 23–26. [Google Scholar]
- Singh, B.B.; Raj, M.D.R.; Dashiell, K.E.; Jackai, L.E.N. (Eds.) Advances in Cowpea Research, co Publication of International Institute of Tropical Agriculture (IITA) and Japan International Research Center for Agricultural Sciences (JIRCAS), IITA, Ibadan, Nigeria; Sayce Publishing: Devon, UK, 1997. [Google Scholar]
- Singh, R.K. Gamma rays induced bold seeded mutant in Vigna mungo (L.) Hepper. Indian J. Genet. 1996, 56, 104–108. [Google Scholar]
- Gumber, R.K.; Singh, S.; Singh, K. Frequency and spectrum of mutations induced by gamma rays in Desi and Kabuli Chickpea. Int. Chickpea Newsl. 1995, 2, 8. [Google Scholar]
- Badre, R.S.; Choudary, A.D. Induced mutations in Linseed (Linum usitatissimum L.). Indian J. Genet. 2004, 64, 159–160. [Google Scholar]
- Joshi, P.; Verma, R.C. Radiation induced pod and seed mutant in faba bean (Vicia faba L.). Indian J. Genet. 2004, 64, 155–156. [Google Scholar]
- Alghamdi, S.S.; Migdadi, H.M. Morphological diversity of faba bean (Vicia faba L.) M2 mutant populations induced by gamma radiation and diethyl sulfate. J. King Saud Univ. Sci. 2020, 32, 1647–1658. [Google Scholar]
- Shen, S.; Tang, Y.; Zhang, C.; Yin, N.; Mao, Y.; Sun, F.; Qu, C. Metabolite Profiling and Transcriptome Analysis Provide Insight into Seed Coat Color in Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7215. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.K.; Smith, A.M.; Ellis, T.N.; Hedley, C.; Martin, C. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme. Cell 1990, 60, 115–122. [Google Scholar] [CrossRef]
- Kolar, F.R.; Ghatge, S.R.; Nimbalkar, N.S.; Dixit, G.B. Mutational changes in Delphinium malabaricum (Huth.) Munz.: A potential ornamental. Plant J. Hort. Res. 2015, 23, 5–15. [Google Scholar] [CrossRef]
- Toker, C. A note on the evolution of kabuli chickpeas as shown by induced mutations in (Cicer reticulatum Ladizinsky). Genet. Resour. Crop Evol. 2009, 56, 7–12. [Google Scholar] [CrossRef]
- Shah, T.M.; Mirza, J.I.; Haq, M.A.; Atta, B.M. Induced genetic variability in chickpea (Cicer arietinum L.). I. Frequency and spectrum of chlorophyll mutations. Pak. J. Bot. 2006, 38, 1217. [Google Scholar]
- Laskar, R.A.; Khan, S.; Khursheed, S.; Raina, A.; Amin, R. Quantitative analysis of induced phenotypic diversity in chickpea using physical and chemical mutagenesis. J. Agron. 2015, 14, 102–111. [Google Scholar] [CrossRef]
- Goyal, S.; Wani, M.R.; Raina, A.; Laskar, R.A.; Khan, S. Quantitative assessments on induced high yielding mutant lines in urdbean [Vigna mungo (L.) hepper]. Legume Sci. 2021, e125. [Google Scholar] [CrossRef]
- Laskar, R.A.; Wani, M.R.; Raina, A.; Amin, R.; Khan, S. Morphological characterization of gamma rays induced multipodding mutant (mp) in lentil cultivar Pant L 406. Int. J. Rad. Biol. 2018, 94, 1049–1053. [Google Scholar] [CrossRef]
- Goyal, S.; Wani, M.R.; Raina, A.; Laskar, R.A.; Khan, S. Phenotypic diversity in mutagenized population of urdbean (Vigna mungo (L.) Hepper). Heliyon 2021, 7, e06356. [Google Scholar] [CrossRef]
- Laskar, R.A.; Laskar, A.A.; Raina, A.; Khan, S.; Younus, H. Induced mutation analysis with biochemical and molecular characterization of high yielding lentil mutant lines. Int. J. Biol. Macromol. 2018, 109, 167–179. [Google Scholar] [CrossRef]
- Khursheed, S.; Raina, A.; Laskar, R.A.; Khan, S. Effect of gamma radiation and EMS on mutation rate: Their effectiveness and efficiency in faba bean (Vicia faba L). Caryologia Int. J. Cytol. Cytosyst. Cytogenet. 2018, 71, 397–404. [Google Scholar] [CrossRef]
- Goyal, S.; Wani, M.R.; Laskar, R.A.; Raina, A.; Amin, R.; Khan, S. Induction of morphological mutations and mutant phenotyping in black gram [Vigna mungo (L.) Hepper] using gamma rays and EMS. Vegetos 2019, 32, 464–472. [Google Scholar] [CrossRef]
- Wani, M.R.; Dar, A.R.; Tak, A.; Amin, I.; Shah, N.H.; Rehman, R.; Baba, M.Y.; Raina, A.; Laskar, R.; Kozgar, M.I.; et al. Chemo-induced pod and seed mutants in mungbean (Vigna radiata L. Wilczek). SAARC J. Agric. 2017, 15, 57–67. [Google Scholar] [CrossRef][Green Version]
- Khursheed, S.; Raina, A.; Wani, M.R.; Amin, R.; Khan, S. Quantitative analysis of genetic parameters in the mutagenized population of faba bean (Vicia faba L.). Res. Crops 2018, 19, 276–284. [Google Scholar] [CrossRef]
- Tantray, A.; Raina, A.; Khursheed, S.; Amin, R.; Khan, S. Chemical Mutagen affects Pollination and Locule Formation in Capsules of Black Cumin (Nigella sativa L.). Int. J. Agric. Sci. 2017, 8, 108–118. [Google Scholar]
- Raina, A.; Khursheed, S.; Khan, S. Optimisation of mutagen doses for gamma rays and sodium azide in cowpea genotypes. Trends Biosci. 2018, 11, 2386–2389. [Google Scholar]
- UPOV. Cowpea (Vigna unguiculata L. Walp. Subsp. sesquipedalis (L.) Verdc.); International Union for The Protection of New Varieties of Plants Nairobi: Nairobi, Kenya, 2007; pp. 1–20. [Google Scholar]
- IBPGR. Cowpea Descriptors; IBPGR Secretariat: Rome, Italy, 1983; pp. 1–30. [Google Scholar]







| Treatment | Var. Gomati VU-89 | Var. Pusa-578 | ||||
|---|---|---|---|---|---|---|
| No. of M1 Plant Progenies | No. of Plant Progenies Segregating in M2 | Mutated Plant (%) | No. of M1 Plant Progenies | No. of Plant Progenies Segregating in M2 | Mutated Plant (%) | |
| C | 270 | 0 | 0.00 f | 260 | 0 | 0.00 i |
| G1 | 245 | 2 | 0.82 h | 235 | 1 | 0.43 h |
| G2 | 223 | 11 | 4.93 b | 213 | 5 | 2.35 f |
| G3 | 208 | 7 | 3.37 e | 200 | 6 | 3.00 de |
| G4 | 195 | 9 | 4.62 b | 185 | 6 | 3.24 cd |
| S1 | 235 | 2 | 0.85 h | 225 | 1 | 0.44 h |
| S2 | 218 | 5 | 2.29 g | 208 | 5 | 2.40 f |
| S3 | 203 | 5 | 2.46 g | 193 | 4 | 2.07 g |
| S4 | 183 | 6 | 3.28 ef | 173 | 6 | 3.47 bc |
| G1S1 | 230 | 7 | 3.04 f | 220 | 6 | 2.73 e |
| G2S2 | 210 | 8 | 3.81d | 200 | 6 | 3.00 de |
| G3S3 | 192 | 8 | 4.17 c | 182 | 7 | 3.85 a |
| G4S4 | 178 | 10 | 5.62 a | 168 | 6 | 3.57 b |
| Doses | N | Var. Gomati VU-89 | F (%) | k | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Albina | Chlorina | Xantha | Tigrina | Viridis | Xanthviridis | CMS | ||||
| C | 3255 | - | - | - | - | - | - | - | 0.00 e | - |
| G1 | 3205 | 12 | 8 | 5 | 1 | 3 | - | 29 | 0.90 bc | - |
| G2 | 3146 | 13 | 7 | 5 | - | 4 | - | 29 | 0.92 bc | - |
| G3 | 3102 | 10 | 8 | 4 | 2 | 4 | 5 | 33 | 1.06 b | - |
| G4 | 3050 | 9 | 8 | 2 | - | 1 | 2 | 22 | 0.72 cd | - |
| S1 | 3185 | 13 | 10 | 1 | 3 | 1 | - | 28 | 0.88 bc | - |
| S2 | 3102 | 14 | 8 | 1 | - | 3 | 2 | 28 | 0.90 bc | - |
| S3 | 3052 | 10 | 7 | 5 | 4 | - | 2 | 28 | 0.92 bc | - |
| S4 | 2925 | 9 | 10 | 4 | - | 2 | 1 | 26 | 0.89 bc | - |
| G1S1 | 3025 | 11 | - | - | 1 | - | 4 | 16 | 0.53 d | 0.30 d |
| G2S2 | 2956 | 13 | 12 | - | 1 | - | 3 | 29 | 0.98 b | 0.54 c |
| G3S3 | 2854 | 15 | 15 | 6 | 4 | 6 | 2 | 48 | 1.68 a | 0.85 b |
| G4S4 | 2705 | 15 | 13 | 7 | 1 | 7 | 1 | 44 | 1.63 a | 1.02 a |
| C | 3147 | Var. Pusa-578 | 0.00 g | - | ||||||
| - | - | - | - | - | - | - | ||||
| G1 | 3097 | 10 | 6 | 0 | 1 | 1 | - | 18 | 0.58 ef | - |
| G2 | 3038 | 11 | 5 | 3 | - | 2 | - | 21 | 0.69 de | - |
| G3 | 2994 | 9 | 5 | 3 | 2 | 2 | 2 | 23 | 0.77 cd | - |
| G4 | 2942 | 7 | 7 | 0 | - | 2 | 1 | 17 | 0.58 ef | - |
| S1 | 3077 | 11 | 10 | 1 | 4 | 3 | - | 29 | 0.94 bc | - |
| S2 | 2994 | 13 | 10 | 2 | - | 2 | 2 | 29 | 0.97 b | - |
| S3 | 2944 | 13 | 7 | 3 | 3 | 2 | 2 | 30 | 1.02 b | - |
| S4 | 2817 | 11 | 9 | 2 | - | 2 | 1 | 25 | 0.89 bc | - |
| G1S1 | 2917 | 10 | - | - | 1 | - | 3 | 14 | 0.48 f | 0.32 c |
| G2S2 | 2848 | 14 | 9 | - | 2 | - | 2 | 27 | 0.95 bc | 0.57 b |
| G3S3 | 2746 | 16 | 14 | 6 | 5 | 6 | 2 | 49 | 1.78 a | 0.99 a |
| G4S4 | 2597 | 14 | 14 | 6 | 3 | 5 | 2 | 44 | 1.69 a | 1.15 a |
| Mutagen | Var. Gomati VU-89 | Frequency | |||||
|---|---|---|---|---|---|---|---|
| Albina | Chlorina | Xantha | Tigrina | Viridis | Xanthviridis | ||
| γ rays | 0.35 b | 0.25 a | 0.13 a | 0.02 b | 0.10 a | 0.06 a | 0.90 |
| SA | 0.38 ab | 0.29 a | 0.09 a | 0.06 a | 0.05 a | 0.04 a | 0.90 |
| γ rays + SA | 0.47 a | 0.35 a | 0.11 a | 0.06 a | 0.11 a | 0.09 a | 1.19 |
| Average Frequency | 0.40 | 0.30 | 0.11 | 0.04 | 0.09 | 0.06 | 1.00 |
| γ rays | Var. Pusa-578 | 0.65 | |||||
| 0.31 a | 0.19 a | 0.05 a | 0.03 b | 0.06 a | 0.02 b | ||
| SA | 0.41 a | 0.30 a | 0.07 a | 0.06 ab | 0.08 a | 0.04 b | 0.96 |
| γ rays + SA | 0.49 a | 0.33 a | 0.11 a | 0.10 a | 0.10 a | 0.08 a | 1.21 |
| Average Frequency | 0.40 | 0.27 | 0.07 | 0.06 | 0.09 | 0.04 | 0.94 |
| Mutagen Doses | Var. Gomati VU-89 | Mp/I | Mp/S | Mp/Me | ||||
|---|---|---|---|---|---|---|---|---|
| Seedling Injury (I) | Pollen Sterility (S) | Meiotic Aberrations (Me) | Mutated Plant (Mp) | Effectiveness | ||||
| C | -- | -- | -- | -- | -- | -- | -- | -- |
| G1 | 10.91 g | 6.41 g | 3.19 f | 2 f | 0.02 e | 0.18 cde | 0.31 cd | 0.63 f |
| G2 | 16.37 f | 7.69 fg | 5.14 de | 11 a | 0.06 e | 0.67 a | 1.43 a | 2.14 a |
| G3 | 18.88 ef | 11.54 de | 5.47 de | 7 cde | 0.02 e | 0.37 bc | 0.61 bc | 1.28 bc |
| G4 | 25.61 b | 15.90 c | 8.51 ab | 9 abc | 0.02 e | 0.35 bc | 0.57 bcd | 1.06 bcde |
| S1 | 17.40 ef | 5.90 g | 1.63 fg | 2 f | 33.33 b | 0.11 de | 0.34 cd | 1.23 bcd |
| S2 | 19.64 def | 9.74 ef | 3.50 ef | 5 e | 41.66 a | 0.25 bcd | 0.51 bcd | 1.43 b |
| S3 | 22.31 cd | 15.38 c | 6.02 cd | 5 e | 27.77 c | 0.22 bcd | 0.33 cd | 0.83 def |
| S4 | 29.13 a | 20.00 b | 7.63 bc | 6 de | 25.00 d | 0.21 bcd | 0.30 d | 0.79 ef |
| G1S1 | 16.89 ef | 5.13 g | 5.44 de | 7 cde | 1.16 e | 0.41 b | 1.37 a | 1.29 bc |
| G2S2 | 20.35 de | 10.77 de | 6.43 cd | 8 bcd | 0.33 e | 0.39 bc | 0.74 b | 1.25 bc |
| G3S3 | 25.05 bc | 13.33 cd | 9.05 ab | 8 bcd | 0.14 e | 0.32 bcd | 0.60 bcd | 0.88 cdef |
| G4S4 | 31.79 a | 22.82 a | 10.31 a | 10 ab | 0.10 e | 0.31 bcd | 0.44 cd | 0.97 cdef |
| C | Var. Pusa-578 | -- | -- | -- | ||||
| -- | -- | -- | -- | -- | ||||
| G1 | 12.28 e | 9.87 ef | 3.19 f | 1 c | 0.01 e | 0.08 cd | 0.10 fg | 0.31 de |
| G2 | 17.17 cde | 11.14 ef | 5.77 bc | 6 ab | 0.03 e | 0.35 ab | 0.54 a | 1.04 abc |
| G3 | 21.51 abc | 16.96 cd | 5.83 bc | 6 ab | 0.02 e | 0.28 ab | 0.35 bcd | 1.03 abc |
| G4 | 23.47 ab | 21.01 b | 8.48 a | 6 ab | 0.02 e | 0.26 ab | 0.29 bcdef | 0.71 cd |
| S1 | 11.99 e | 8.86 f | 1.05 g | 1 c | 16.66 d | 0.08 cd | 0.11 efg | 0.95 abc |
| S2 | 14.84 de | 13.42 de | 3.56 ef | 5 ab | 41.66 a | 0.34 bc | 0.37 abcd | 1.41 a |
| S3 | 19.18 bcd | 20.51 bc | 5.00 bcde | 4 b | 22.22 c | 0.21 bc | 0.20 def | 0.80 bc |
| S4 | 20.12 bcd | 23.29 ab | 6.62 b | 6 ab | 25.00 b | 0.30 ab | 0.26 cdef | 0.91 abc |
| G1S1 | 13.11 e | 10.89 ef | 4.05 def | 5 ab | 1.00 e | 0.38 a | 0.46 ab | 1.23 abc |
| G2S2 | 17.72 bcde | 13.67 de | 4.90 cde | 6 ab | 0.25 e | 0.34 ab | 0.44 abc | 1.22 abc |
| G3S3 | 21.12 bc | 21.77 b | 5.63 bcd | 7 ab | 0.13 e | 0.33 ab | 0.32 bcd | 1.24 abc |
| G4S4 | 26.88 a | 26.33 a | 8.43 a | 8 a | 0.06 e | 0.30 ab | 0.30 bcde | 0.95 abc |
| Doses | N | Var. Gomati VU-89 | % Mutated Plants | k | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plant Height Mutants | Growth Habit Mutants | Leaf Mutants | Flower Mutants | Pod Mutants | Seed Mutants | Total Mutated Plants | ||||
| C | 3050 | - | - | - | - | - | - | - | - | - |
| G1 | 3000 | 8 | - | 6 | 2 | 9 | 4 | 29 | 0.97 e | - |
| G2 | 2941 | 4 | 10 | 13 | 5 | - | 5 | 37 | 1.26 d | - |
| G3 | 2897 | - | 16 | 9 | 7 | - | 6 | 38 | 1.31 cd | - |
| G4 | 2845 | 8 | 8 | - | 2 | 6 | 3 | 27 | 0.95 e | - |
| Total | 11,683 | 20 | 34 | 28 | 16 | 15 | 18 | 131 | 4.49 | - |
| S1 | 2980 | 9 | - | 11 | 12 | - | 3 | 35 | 1.17 de | - |
| S2 | 2897 | 5 | - | 6 | 5 | 11 | 6 | 33 | 1.14 de | - |
| S3 | 2847 | - | 12 | 7 | 10 | - | 7 | 36 | 1.26 d | - |
| S4 | 2720 | 10 | 7 | 13 | 8 | 7 | 4 | 49 | 1.80 b | - |
| Total | 11,444 | 24 | 19 | 37 | 35 | 18 | 20 | 153 | 5.38 | - |
| G1S1 | 2820 | 6 | - | - | 15 | - | 9 | 30 | 1.06 de | 0.49 c |
| G2S2 | 2751 | 6 | - | 19 | 13 | 12 | 10 | 60 | 2.18 a | 0.90 a |
| G3S3 | 2649 | - | 16 | 9 | - | - | 4 | 29 | 1.09 de | 0.42 d |
| G4S4 | 2500 | - | 17 | 7 | - | 14 | 0 | 38 | 1.52 c | 0.55 b |
| Total | 10,720 | 12 | 33 | 35 | 28 | 26 | 23 | 157 | 5.86 | 2.36 |
| C | 2960 | Var. Pusa-578 | - | - | ||||||
| - | - | - | - | - | - | - | ||||
| G1 | 2910 | 10 | - | 8 | 2 | - | 9 | 29 | 1.00 f | - |
| G2 | 2851 | 2 | 9 | 14 | 4 | 11 | 10 | 50 | 1.75 bc | - |
| G3 | 2807 | - | 14 | 8 | 5 | - | 5 | 32 | 1.14 ef | - |
| G4 | 2755 | 7 | 10 | - | 4 | 8 | 3 | 32 | 1.16 ef | - |
| Total | 11,323 | 19 | 33 | 30 | 15 | 19 | 27 | 143 | 5.05 | 0.00 |
| S1 | 2890 | 10 | - | 11 | 12 | - | 4 | 37 | 1.28 ef | - |
| S2 | 2807 | 7 | 13 | 6 | 5 | 9 | 8 | 48 | 1.71 bcd | - |
| S3 | 2757 | - | - | 8 | 10 | 9 | 9 | 36 | 1.31 def | - |
| S4 | 2630 | 11 | 8 | 10 | 10 | - | 7 | 46 | 1.75 bc | - |
| Total | 11,084 | 28 | 21 | 35 | 37 | 18 | 28 | 167 | 6.05 | 0.00 |
| G1S1 | 2730 | 8 | - | - | 14 | - | 10 | 32 | 1.17 ef | 0.51 b |
| G2S2 | 2661 | 8 | - | 17 | 15 | 13 | 11 | 64 | 2.41 a | 0.70 a |
| G3S3 | 2559 | 3 | 15 | 11 | - | 2 | 5 | 36 | 1.41 cdef | 0.57 b |
| G4S4 | 2410 | - | 14 | 9 | - | 15 | 0 | 38 | 1.58 bcde | 0.58 b |
| Total | 10,360 | 19 | 29 | 37 | 29 | 30 | 26 | 170 | 6.56 | 2.42 |
| Characters | Morphological Mutants | Var. Gomati VU-89 | Grand Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| γ Rays | SA | γ Rays + SA | Total | ||||||||
| N | F% | N | F% | N | F% | N | F% | N | F% | ||
| Plant height | Tall | 13 | 0.11 | 15 | 0.13 | 9 | 0.08 | 37 | 0.11 | 56 | 0.17 ab |
| Dwarf | 5 | 0.04 | 8 | 0.07 | 2 | 0.02 | 15 | 0.04 | |||
| Semi-Dwarf | 2 | 0.02 | 1 | 0.01 | 1 | 0.01 | 4 | 0.01 | |||
| Growth habit | Bushy | 8 | 0.07 | 7 | 0.06 | 12 | 0.11 | 27 | 0.08 | 63 | 0.19 a |
| Prostrate | 1 | 0.01 | 2 | 0.02 | 5 | 0.05 | 8 | 0.02 | |||
| Semi-Dwarf Spreading | 3 | 0.03 | 4 | 0.03 | 3 | 0.03 | 10 | 0.03 | |||
| One-Sided Branching | 7 | 0.06 | 1 | 0.01 | 1 | 0.01 | 9 | 0.03 | |||
| Axillary Branching | 3 | 0.03 | 2 | 0.02 | 4 | 0.04 | 9 | 0.03 | |||
| Leaf | Broad Leaf | 8 | 0.07 | 7 | 0.06 | 6 | 0.06 | 21 | 0.06 | 59 | 0.17 ab |
| Narrow Leaf | 5 | 0.04 | 5 | 0.04 | 7 | 0.07 | 17 | 0.05 | |||
| Altered Leaf Architecture | 2 | 0.02 | 2 | 0.02 | 5 | 0.05 | 9 | 0.03 | |||
| Elongated Rachis | 4 | 0.03 | 6 | 0.05 | 2 | 0.02 | 12 | 0.04 | |||
| Flower | Multi Flowering | 4 | 0.04 | 3 | 0.03 | 3 | 0.03 | 10 | 0.03 | 66 | 0.19 a |
| Flower Color | 2 | 0.02 | 5 | 0.04 | 4 | 0.04 | 11 | 0.03 | |||
| Open Flower | 7 | 0.06 | 7 | 0.06 | 6 | 0.06 | 20 | 0.06 | |||
| Non-Flowering | 1 | 0.01 | 1 | 0.01 | 2 | 0.02 | 4 | 0.01 | |||
| Late Flowering | 6 | 0.05 | 6 | 0.05 | 2 | 0.02 | 14 | 0.04 | |||
| Early Maturity | 4 | 0.03 | 2 | 0.02 | 1 | 0.01 | 7 | 0.02 | |||
| Pod | Narrow Pod | 7 | 0.06 | 8 | 0.07 | 10 | 0.09 | 25 | 0.07 | 45 | 0.13 b |
| Broad Pod | 5 | 0.04 | 6 | 0.05 | 9 | 0.08 | 20 | 0.06 | |||
| Seed | Coat Color | 7 | 0.06 | 8 | 0.07 | 7 | 0.07 | 22 | 0.06 | 75 | 0.22 a |
| Coat Pattern | 6 | 0.05 | 9 | 0.08 | 8 | 0.07 | 23 | 0.07 | |||
| Shape and Surface | 10 | 0.09 | 11 | 0.10 | 9 | 0.08 | 30 | 0.09 | |||
| Grand Total | 120 | 1.03 | 126 | 1.10 | 118 | 1.10 | 364 | 1.08 | |||
| Characters | Morphological Mutants | Var. Pusa-578 | Grand Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| γ Rays | SA | γ Rays + SA | Total | ||||||||
| N | F% | N | F% | N | F% | N | F% | N | F% | ||
| Plant height | Tall | 10 | 0.09 | 11 | 0.10 | 8 | 0.08 | 29 | 0.09 | 44 | 0.13 a |
| Dwarf | 4 | 0.04 | 7 | 0.06 | 1 | 0.01 | 12 | 0.04 | |||
| Semi-Dwarf | 1 | 0.01 | 1 | 0.01 | 1 | 0.01 | 3 | 0.01 | |||
| Growth habit | Bushy | 7 | 0.06 | 6 | 0.05 | 11 | 0.11 | 24 | 0.07 | 57 | 0.17 a |
| Prostrate | 4 | 0.04 | 1 | 0.01 | 6 | 0.06 | 11 | 0.03 | |||
| Semi-Dwarf Spreading | 2 | 0.02 | 3 | 0.03 | 2 | 0.02 | 7 | 0.02 | |||
| One-Sided Branching | 5 | 0.04 | 2 | 0.02 | 1 | 0.01 | 8 | 0.02 | |||
| Axillary Branching | 2 | 0.02 | 3 | 0.03 | 2 | 0.02 | 7 | 0.02 | |||
| Leaf | Broad Leaf | 7 | 0.06 | 6 | 0.05 | 5 | 0.05 | 18 | 0.05 | 50 | 0.15 a |
| Narrow Leaf | 4 | 0.04 | 4 | 0.04 | 5 | 0.05 | 13 | 0.04 | |||
| Altered Leaf Architecture | 1 | 0.01 | 3 | 0.03 | 4 | 0.04 | 8 | 0.02 | |||
| Elongated Rachis | 5 | 0.04 | 5 | 0.05 | 1 | 0.01 | 11 | 0.03 | |||
| Flower | Multi Flowering | 3 | 0.03 | 2 | 0.02 | 4 | 0.04 | 9 | 0.03 | 64 | 0.20 a |
| Flower Color | 3 | 0.03 | 4 | 0.04 | 5 | 0.05 | 12 | 0.04 | |||
| Open Flower | 6 | 0.05 | 8 | 0.07 | 5 | 0.05 | 19 | 0.06 | |||
| Non-Flowering | 2 | 0.02 | 2 | 0.02 | 1 | 0.01 | 5 | 0.02 | |||
| Late Flowering | 5 | 0.04 | 5 | 0.05 | 1 | 0.01 | 11 | 0.03 | |||
| Early Maturity | 3 | 0.03 | 4 | 0.04 | 1 | 0.01 | 8 | 0.02 | |||
| Pod | Narrow Pod | 5 | 0.04 | 7 | 0.06 | 9 | 0.09 | 21 | 0.06 | 45 | 0.14 a |
| Broad Pod | 4 | 0.04 | 5 | 0.05 | 8 | 0.08 | 17 | 0.05 | |||
| Seed | Coat Color | 6 | 0.05 | 7 | 0.06 | 6 | 0.06 | 19 | 0.06 | 75 | 0.23 a |
| Coat Pattern | 5 | 0.04 | 8 | 0.07 | 7 | 0.07 | 20 | 0.06 | |||
| Shape and Surface | 9 | 0.08 | 10 | 0.09 | 8 | 0.08 | 27 | 0.08 | |||
| Grand Total | 103 | 0.91 | 114 | 1.03 | 102 | 0.98 | 319 | 0.97 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raina, A.; Laskar, R.A.; Wani, M.R.; Jan, B.L.; Ali, S.; Khan, S. Comparative Mutagenic Effectiveness and Efficiency of Gamma Rays and Sodium Azide in Inducing Chlorophyll and Morphological Mutants of Cowpea. Plants 2022, 11, 1322. https://doi.org/10.3390/plants11101322
Raina A, Laskar RA, Wani MR, Jan BL, Ali S, Khan S. Comparative Mutagenic Effectiveness and Efficiency of Gamma Rays and Sodium Azide in Inducing Chlorophyll and Morphological Mutants of Cowpea. Plants. 2022; 11(10):1322. https://doi.org/10.3390/plants11101322
Chicago/Turabian StyleRaina, Aamir, Rafiul Amin Laskar, Mohammad Rafiq Wani, Basit Latief Jan, Sajad Ali, and Samiullah Khan. 2022. "Comparative Mutagenic Effectiveness and Efficiency of Gamma Rays and Sodium Azide in Inducing Chlorophyll and Morphological Mutants of Cowpea" Plants 11, no. 10: 1322. https://doi.org/10.3390/plants11101322
APA StyleRaina, A., Laskar, R. A., Wani, M. R., Jan, B. L., Ali, S., & Khan, S. (2022). Comparative Mutagenic Effectiveness and Efficiency of Gamma Rays and Sodium Azide in Inducing Chlorophyll and Morphological Mutants of Cowpea. Plants, 11(10), 1322. https://doi.org/10.3390/plants11101322







