Variability of Phenological Behaviours of Wild Fruit Tree Species Based on Discriminant Analysis
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanalli, C.; Casagrandi, R.; Gatto, M.; Bevacqua, D. Shifts in the thermal niche of fruit trees under climate change: The case of peach cultivation in France. Agric. For. Meteorol. 2021, 300, 108327. [Google Scholar] [CrossRef]
- Dahal, N.; Lamichhaney, S.; Kumar, S. Climate change impacts on himalayan biodiversity: Evidence-based perception and current approaches to evaluate threats under climate change. J. Indian Inst. Sci. 2021, 101, 195–210. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.G.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Baciu, A.; Cichi, M.; Gruia, M. The effect of climate changes on phenological phases in plum tree Prunus domestica in south-western Romania. South-West J. Hortic. Biol. Environ. 2010, 1, 9–20. [Google Scholar]
- Cosmulescu, S.; Baciu, A.; Gruia, M. Influence of climatic factors on the phenology spring in Southern Oltenia Romania. J. Hortic. Sci. Biotechnol. 2015, 19, 147–158. [Google Scholar]
- Gordo, O.; Sanz, J.J. Phenology and climate change: A long-term study in a Mediterranean locality. Oecologia 2005, 146, 484–495. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Gruia, M. Climatic variability in Craiova Romania and its impacts on fruit orchards. South-West J. Hortic. Biol. Environ. 2016, 7, 15–26. [Google Scholar]
- Cosmulescu, S.; Bîrsanu Ionescu, M. Phenological calendar in some walnut genotypes grown in Romania and its correlations with air temperature. Int. J. Climatol. 2018, 62, 2007–2013. [Google Scholar] [CrossRef]
- Paltineanu, C.; Chitu, E. Climate change impact on phenological stages of sweet and sour cherry trees in a continental climate environment. Sci. Hortic. 2020, 261, 109011. [Google Scholar] [CrossRef]
- Lorite, I.J.; Cabezas-Luque, J.M.; Arquero, O.; Gabaldón-Leal, C.; Santos, C.; Rodríguez, A.; Lovera, M. The role of phenology in the climate change impacts and adaptation strategies for tree crops: A case study on almond orchards in Southern Europe. Agric. For. Meteorol. 2020, 294, 108142. [Google Scholar] [CrossRef]
- Fraga, H.; Santos, J.A. Assessment of climate change impacts on chilling and forcing for the main fresh fruit regions in Portugal. Front. Plant Sci. 2021, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Schultz, H.R. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Zhou, X.; Geng, X.; Guo, Y.; Zhang, Y. Progress in plant phenology modeling under global climate change. Sci. China Earth Sci. 2020, 63, 1237–1247. [Google Scholar] [CrossRef]
- Markkula, I.; Turunen, M.; Rasmus, S. A review of climate change impacts on the ecosystem services in the Saami Homeland in Finland. Sci. Total Environ. 2019, 692, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gou, X.; Yin, D. Response of biodiversity, ecosystems, and ecosystem services to climate change in China: A Review. Ecologies 2021, 2, 313–331. [Google Scholar] [CrossRef]
- Badeck, F.W.; Bondeau, A.; Böttcher, K.; Doktor, D.; Lucht, W.; Schaber, J.; Sitch, S. Responses of spring phenology to climate change. New Phytol. 2004, 162, 295–309. [Google Scholar] [CrossRef]
- Caparros-Santiago, J.A.; Rodriguez-Galiano, V.; Dash, J. Land surface phenology as indicator of global terrestrial ecosystem dynamics: A systematic review. ISPRS J. Photogramm. Remote Sens. 2021, 171, 330–347. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species. (Agrostis spp.); Unwin Hyman: London, UK, 1988; pp. 58–65. [Google Scholar]
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Fichtner, A.; Wissemann, V. Biological flora of the British isles: Crataegus monogyna. J. Ecol. 2021, 109, 541–571. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Calusaru Gavrila, F. Influence of temperature on blackthorn (Prunus spinosa L.) phenophases in spring season. J. Agric. Meteorol. 2020, 76, 53–57. [Google Scholar] [CrossRef]
- Dönmez, A.A. The genus Crataegus, L. (Rosaceae) with special reference to hybridisation and biodiversity in Turkey. Turk. J. Bot. 2004, 28, 29–37. [Google Scholar]
- Worrell, R.; Ruhsam, M.; Renny, J.; Jessop, W.; Findlay, G. Scotland’s native wild apple–Malus sylvestris: Genetic issues and conservation. Scott. For. 2020, 72, 33–41. [Google Scholar]
- Tooke, F.; Battey, N.H. Temperate flowering phenology. J. Exp. Bot. 2010, 61, 2853–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šebek, G. The phenological and pomological traits of selected genotypes of wild pear [Pyrus pyraster (L.) Du Roi] important for the production of generative rootstocks. Acta Sci. Pol. Hortorum Cultus 2019, 18, 133–145. [Google Scholar]
- Cojoacă, F.D.; Niculescu, M. Diversity, distribution and ecology of the forest natural habitats in the Bratovoești Forest, Dolj County. Sci. Pap. Ser. A Agron. 2018, 61, 453–457. [Google Scholar]
- El Yamani, M.; Boussakouran, A.; Rharrabti, Y. Codification and description of almond (Prunus dulcis) vegetative and reproductive phenology according to the extended BBCH scale. Sci. Hortic. 2019, 247, 224–234. [Google Scholar]
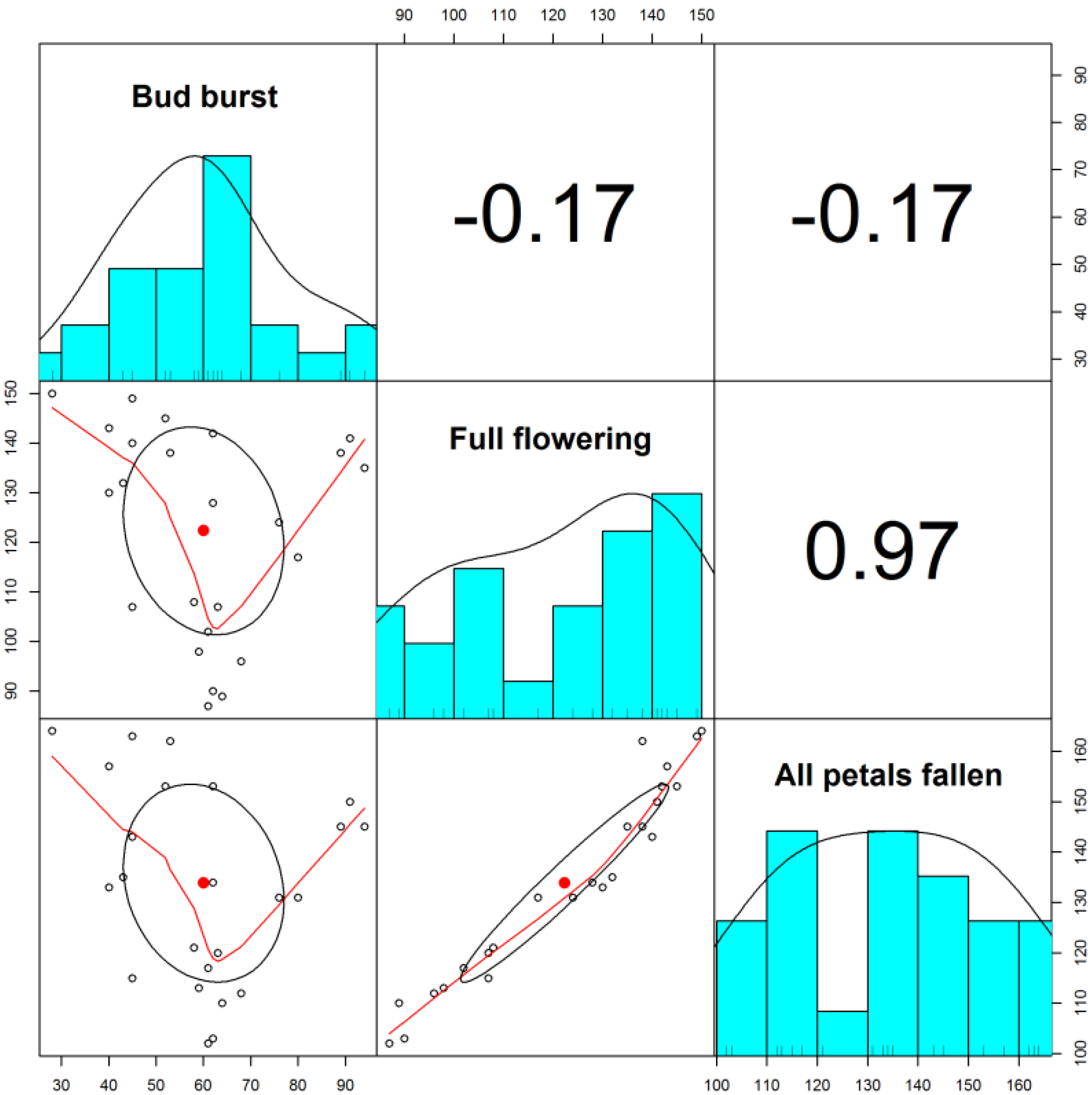

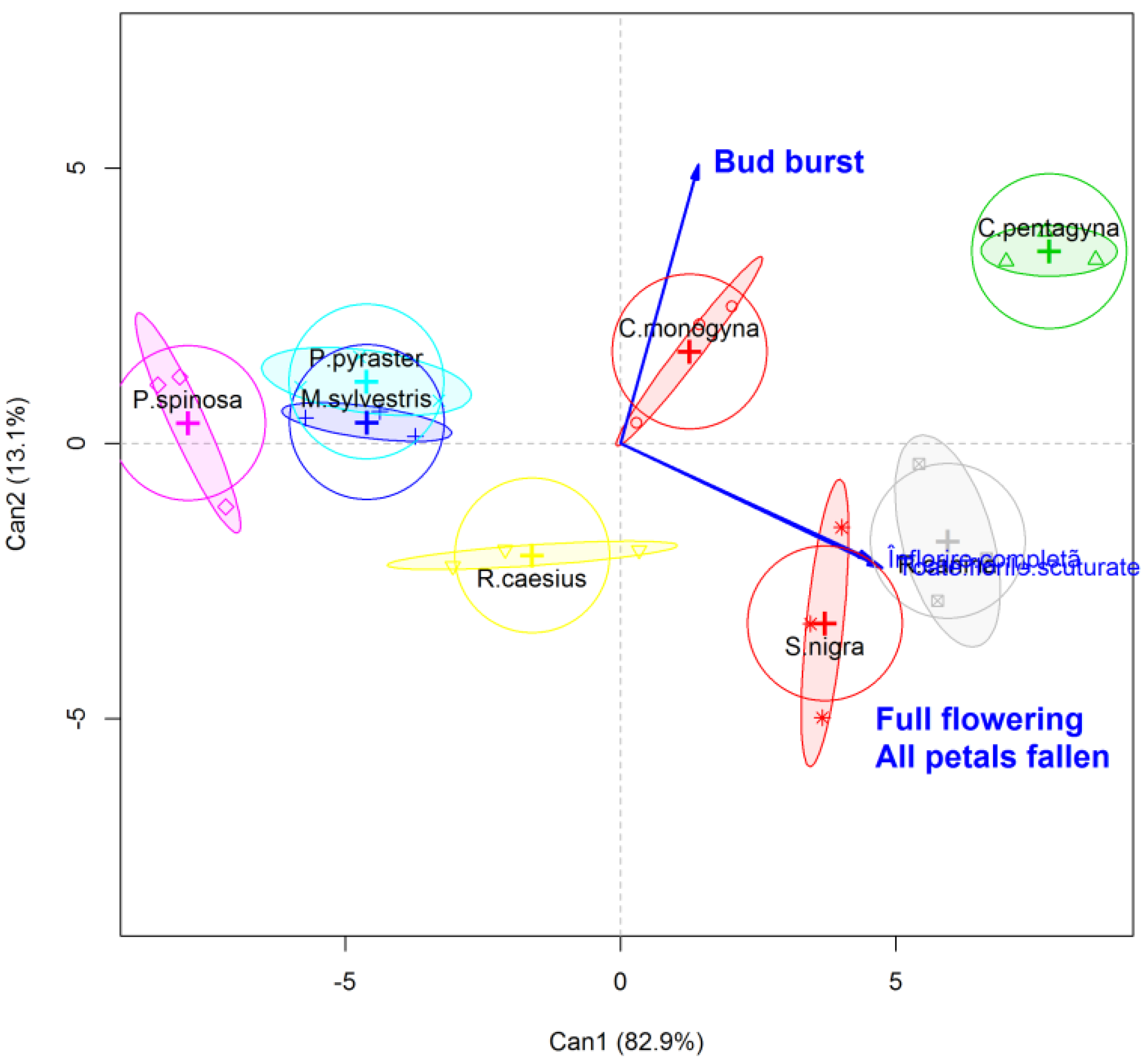

| Species | Descriptive Statistics | “Bud Burst” | “Full Flowering” | “All Petals Fallen” |
|---|---|---|---|---|
| Malus sylvestris | Mean ± SD | 59.33 ± 1.53 | 102.67 ± 5.03 | 117.00 ± 4.00 |
| Variation range | 58–61 | 98–108 | 113–121 | |
| CV% | 2.58 | 4.90 | 3.42 | |
| Pyrus pyraster | Mean ± SD | 65.00 ± 2.65 | 97.33 ± 9.07 | 114.00 ± 5.29 |
| Variation range | 63–68 | 89–107 | 110–120 | |
| CV% | 4.08 | 9.32 | 4.64 | |
| Crataegus monogyna | Mean ± SD | 72.67 ± 9.45 | 123.00 ± 5.57 | 132.00 ± 1.73 |
| Variation range | 62–80 | 117–128 | 131–134 | |
| CV | 13.00 | 4.53 | 1.31 | |
| Crataegus pentagyna | Mean ± SD | 91.33 ± 2.52 | 138.00 ± 3.00 | 146.67 ± 2.89 |
| Variation range | 89–94 | 135–141 | 145–150 | |
| CV% | 2.76 | 2.17 | 1.97 | |
| Rosa canina | Mean ± SD | 53.33 ± 8.50 | 143.00 ± 5.57 | 159.33 ± 5.51 |
| Variation range | 45–62 | 138–149 | 153–163 | |
| CV% | 15.94 | 3.90 | 3.46 | |
| Rubus caesius | Mean ± SD | 42.67 ± 2.52 | 179.67 ± 3.51 | 213.67 ± 4.51 |
| Variation range | 40–45 | 176–183 | 209–218 | |
| CV% | 5.91 | 1.95 | 2.11 | |
| Sambucus nigra | Mean ± SD | 40.00 ± 12.00 | 146.00 ± 3.61 | 158.00 ± 5.57 |
| Variation range | 28–52 | 143–150 | 153–164 | |
| CV% | 30.00 | 2.47 | 3.52 | |
| Prunus spinosa | Mean ± SD | 56.00 ± 9.54 | 94.67 ± 10.79 | 106.67 ± 7.23 |
| Variation range | 45–62 | 87–107 | 102–115 | |
| CV% | 17.04 | 11.39 | 6.78 |
| Phenology Stage | * LD1 (0.829) | LD2 (0.131) | LD3 (0.039) |
|---|---|---|---|
| “bud burst” | 0.138 | 0.122 | −0.003 |
| “full flowering” | −0.004 | 0.024 | 0.269 |
| “all petals fallen” | 0.273 | −0.056 | −0.276 |
| Climate Data | Year | January | February | March | April | May | June |
|---|---|---|---|---|---|---|---|
| Average temperature (°C) | 2019 | −3.39 | 5.36 | 11.72 | 13.67 | 17.05 | 24.76 |
| 2020 | 2.96 | 7.5 | 9.61 | 14.25 | 18.58 | 23.57 | |
| 2021 | 3.77 | 5.2 | 7.33 | 11.41 | 18.61 | 23.07 | |
| Total rainfall (mm) | 2019 | 64.5 | 26.7 | 2.5 | 23.1 | 22.2 | 85 |
| 2020 | 1 | 8.6 | 124.4 | 5.6 | 69.6 | 33.6 | |
| 2021 | 67.6 | 35.2 | 56 | 41.2 | 101.8 | 49.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosmulescu, S.; Ștefănescu, D.; Stoenescu, A.-M. Variability of Phenological Behaviours of Wild Fruit Tree Species Based on Discriminant Analysis. Plants 2022, 11, 45. https://doi.org/10.3390/plants11010045
Cosmulescu S, Ștefănescu D, Stoenescu A-M. Variability of Phenological Behaviours of Wild Fruit Tree Species Based on Discriminant Analysis. Plants. 2022; 11(1):45. https://doi.org/10.3390/plants11010045
Chicago/Turabian StyleCosmulescu, Sina, Dragoș Ștefănescu, and Ana-Maria Stoenescu. 2022. "Variability of Phenological Behaviours of Wild Fruit Tree Species Based on Discriminant Analysis" Plants 11, no. 1: 45. https://doi.org/10.3390/plants11010045
APA StyleCosmulescu, S., Ștefănescu, D., & Stoenescu, A.-M. (2022). Variability of Phenological Behaviours of Wild Fruit Tree Species Based on Discriminant Analysis. Plants, 11(1), 45. https://doi.org/10.3390/plants11010045







