Genome-Wide Variation Analysis of Four Vegetable Soybean Cultivars Based on Re-Sequencing
Abstract
:1. Introduction
2. Results
2.1. Character Comparison
2.2. SSR Analysis
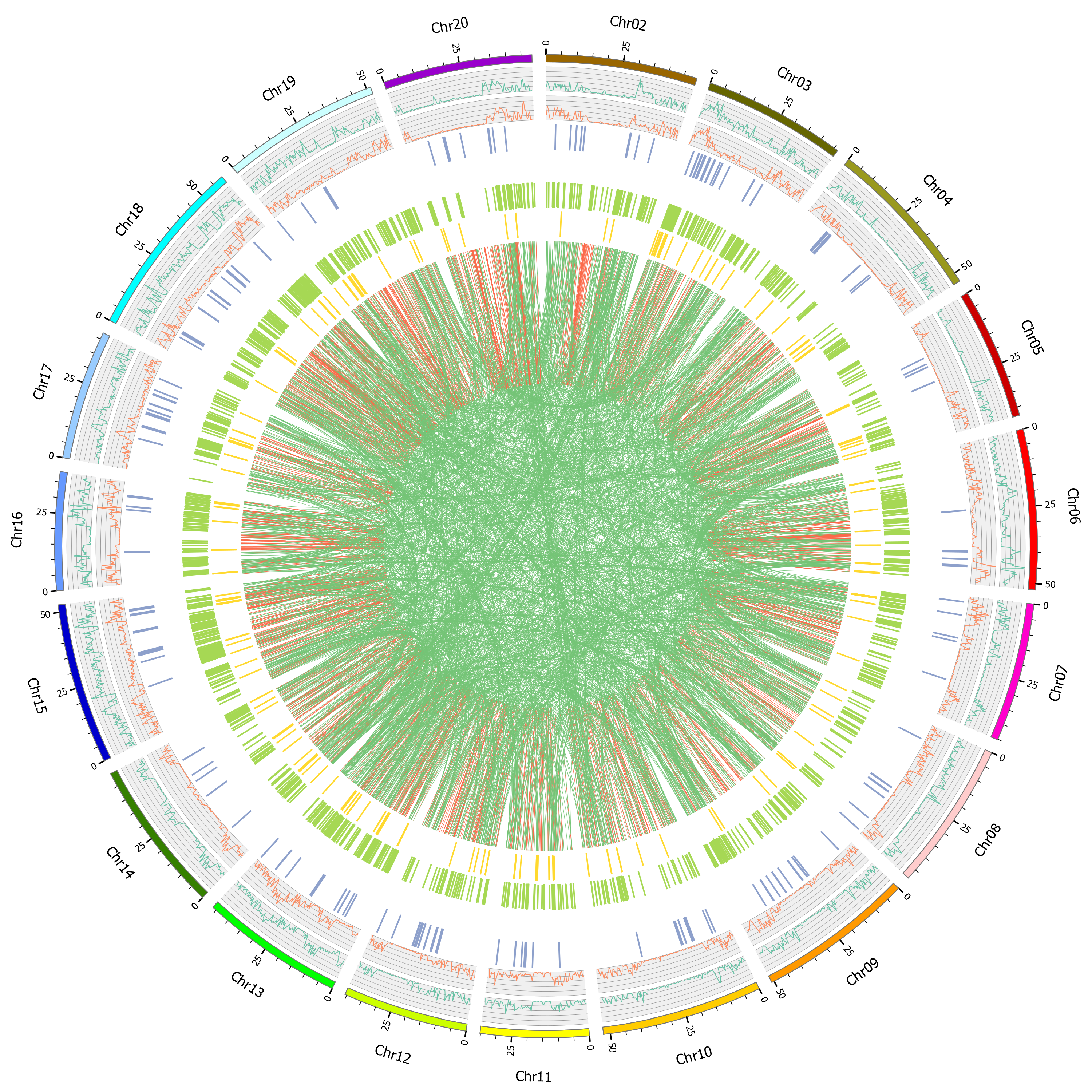
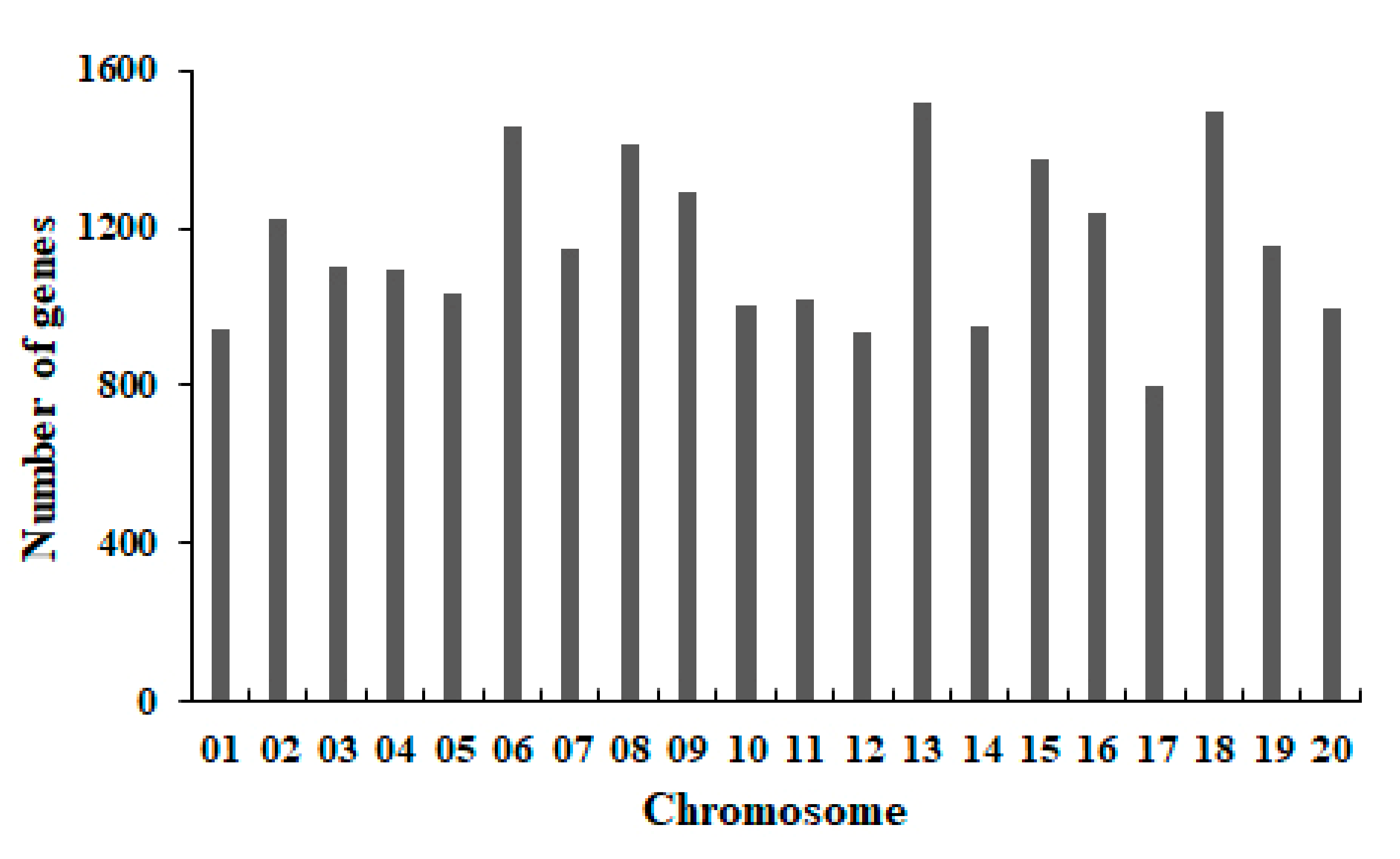
2.3. Variation Detection
2.4. Function Annotation
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Character Investigation
4.3. SSR Analysis
4.4. Sequencing and Variation Detection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Z. Soybean of ancient China. Agric. Hist. China 1987, 3, 50–57. [Google Scholar]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine Max (L.) merrill. Crop. Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Xu, Y.; Cartier, A.; Kibet, D.; Jordan, K.; Hakala, I.; Davis, S.; Sismour, E.; Kering, M.; Rutto, L. Physical and nutritional properties of edamame seeds as influenced by stage of development. J. Food Meas. Charact. 2016, 10, 193–200. [Google Scholar] [CrossRef]
- Jiang, G.; Rutto, L.K.; Ren, S.; Bowen, R.A.; Berry, H.; Epps, K. Genetic analysis of edamame seed composition and trait relationships in soybean lines. Euphytica 2018, 214, 158. [Google Scholar] [CrossRef]
- Young, G.; Mebrahtu, T.; Johnson, J. Acceptability of green soybeans as a vegetable entity. Plant. Food. Hum. Nutr. 2000, 55, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, Y. Vegetable soybean (Glycine max (L.) Merr.) leaf extracts: Functional components and antioxidant and anti-inflammatory activities. J. Food. Sci. 2021, 86, 2468–2480. [Google Scholar]
- Dong, D.; Fu, X.; Yuan, F.; Chen, P.; Zhu, S.; Li, B.; Yang, Q.; Yu, X.; Zhu, D. Genetic diversity and population structure of vegetable soybean (Glycine max (L.) Merr.) in China as revealed by SSR markers. Genet. Resour. Crop. Evol. 2014, 61, 173–183. [Google Scholar] [CrossRef]
- Moseley, D.; Mozzoni, L.; Kaler, A.; Mason, R.E.; Shi, A.; Orazaly, M.; Lara, L.; da Silva, M.P.; Chen, P. Evaluation of genetic diversity and association mapping for seed weight and size in vegetable soybean germplasm. Crop. Sci. 2021, 61, 3516–3528. [Google Scholar] [CrossRef]
- Wang, Z.; Senga, E.F.B.; Wang, D. Vegetable soy bean (Glycine max (L.) Merrill) from production to processing. Outlook Agric. 2005, 34, 167–172. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Goyal, L.; Pratap, D.; Billore, S.D.; Chauhan, G.S. Evaluation of vegetable-type soybean for sucrose, taste-related amino acids, and isoflavones contents. Int. J. Food. Prop. 2011, 14, 1142–1151. [Google Scholar] [CrossRef]
- Yu, X.; Yuan, F.; Fu, X.; Zhu, D. Profiling and relationship of water soluble sugar and protein compositions in soybean seeds. Food. Chem. 2016, 196, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Saldivar, X.; Wang, Y.; Chen, P.; Mauromoustakos, A. Effects of blanching and storage conditions on soluble sugar contents in vegetable soybean. LWT-Food. Sci. Technol. 2010, 43, 1368–1372. [Google Scholar] [CrossRef]
- Keatinge, J.D.H.; Easdown, W.J.; Yang, R.; Chadha, M.L.; Shanmugasundaram, S. Overcoming chronic malnutrition in a future warming world: The key importance of mungbean and vegetable soybean. Euphytica 2011, 180, 129–141. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, S.; Mao, W.; Hu, Q.; Gong, Y. Determination of the genetic diversity of vegetable soybean [Glycine max (L.) Merr.] using EST-SSR markers. J. Zhejiang Univ. Sci. B 2013, 14, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Mimura, M.; Coyne, C.J.; Bambuck, M.W.; Lumpkin, T.A. SSR diversity of vegetable soybean [Glycine max (L.) Merr.]. Genet. Resour. Crop. Evol. 2007, 54, 497–508. [Google Scholar] [CrossRef]
- Grant, D.; Nelson, R.T.; Cannon, S.B.; Shoemaker, R.C. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res. 2010, 38, D843–D846. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Nayak, S.N.; May, G.D.; Jackson, S.A. Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol. 2009, 27, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.; Moharana, K.C.; Shankar, R.; Kumari, R.; Garg, R. Genomewide discovery of DNA polymorphisms in rice cultivars with contrasting drought and salinity stress response and their functional relevance. Plant Biotechnol. J. 2014, 12, 253–264. [Google Scholar] [CrossRef]
- Lam, H.M.; Xu, X.; Liu, X.; Chen, W.; Yang, G.; Wong, F.L.; Li, M.W.; He, W.; Qin, N.; Wang, B.; et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 2010, 42, 1053–1059. [Google Scholar] [CrossRef]
- Maldonado dos Santos, J.V.; Valliyodan, B.; Joshi, T.; Khan, S.M.; Liu, Y.; Wang, J.; Vuong, T.D.; de Oliveira, M.F.; Marcelino-Guimaraes, F.C.; Xu, D.; et al. Evaluation of genetic variation among Brazilian soybean cultivars through genome resequencing. BMC Genom. 2016, 17, 100. [Google Scholar] [CrossRef] [Green Version]
- Albert, G.I.; Naumann, R.; Herse, F.; Knobeloch, K.P.; Freund, C. Characterization of the GYF-domain protein CD2BP2. Transgenic Res. 2011, 20, 1161. [Google Scholar]
- Guo, D.; Yuan, F.; Yu, X. Genome-wide variation analysis of grain and vegetable soybeans based on re-sequencing. Fenzi Zhiwu Yuzhong Mol. Plant Breed. 2019, 17, 7306–7312, (In Chinese with English abstract). [Google Scholar]
- Saleem, A.; Muylle, H.; Aper, J.; Ruttink, T.; Wang, J.; Yu, D.; Roldán-Ruiz, I. A genome-wide genetic diversity scan reveals multiple signatures of selection in a European soybean collection compared to Chinese collections of wild and cultivated soybean accessions. Front. Plant Sci. 2021, 12, 631767. [Google Scholar] [CrossRef]
- Yan, L.; Hofmann, N.; Li, S.; Ferreira, M.E.; Song, B.; Jiang, G.; Ren, S.; Quigley, C.; Fickus, E.; Cregan, P.; et al. Identification of QTL with large effect on seed weight in a selective population of soybean with genome-wide association and fixation index analyses. BMC Genom. 2017, 18, 529. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.F.; He, S.S.; Wang, C.S.; Lai, Z.Y.; Lin, D.G.; Chen, S. A modified Roger’s distance algorithm for mixed quantitative–qualitative phenotypes to establish a core collection for Taiwanese vegetable soybeans. Front. Plant Sci. 2021, 11, 612106. [Google Scholar] [CrossRef]
- Shilpashree, N.; Devi, S.N.; Manjunathagowda, D.C.; Muddappa, A.; Abdelmohsen, S.A.M.; Tamam, N.; Elansary, H.O.; El-Abedin, T.K.Z.; Abdelbacki, A.M.M.; Janhavi, V. Morphological characterization, variability and diversity among vegetable soybean (Glycine max L.) genotypes. Plants 2021, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Moloi, M.J.; van der Merwe, R. Drought tolerance responses in vegetable-type soybean involve a network of biochemical mechanisms at flowering and pod-filling stages. Plants 2021, 10, 1502. [Google Scholar] [CrossRef]
- Yu, X.; Jin, H.; Yang, Q.; Fu, X.; Yuan, F. Genetic mapping of a soybean glabrous gene using specific-locus amplified fragment sequencing method. Legume Res. 2020, 43, 501–506. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Jia, G.; Zhu, Y.; Grant, D.; Nelson, R.T.; Hwang, E.Y.; Hyten, D.L.; Cregan, P.B. Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1.0) in soybean. Crop. Sci. 2010, 50, 1950–1960. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashburner, M.; Ball, C.A.; Judith, A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Wallis, J.W.; McLellan, M.D.; Larson, D.E.; Kalicki, J.M.; Pohl, C.S.; McGrath, S.D.; Wendi, M.C.; Zhang, Q.; Locke, D.P.; et al. BreakDancer: An algorithm for high-resolution mapping of genomic structural variation. Nat. Methods 2009, 6, 677–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence alignment/map (SAM) format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, 277–280. [Google Scholar] [CrossRef] [Green Version]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
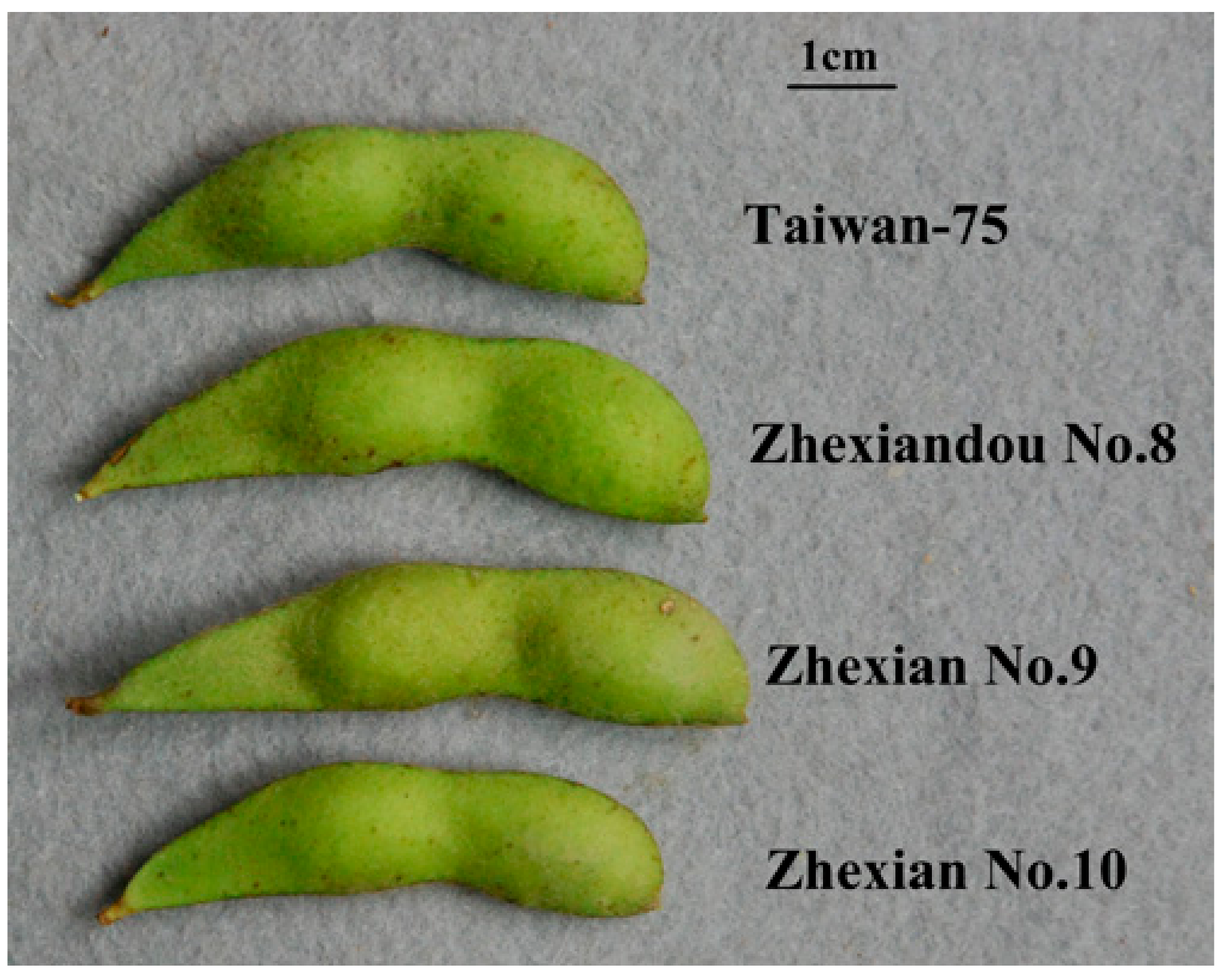

| Cultivar Name | Growth Duration (d) | Plant Height (cm) | Pod Length (cm) | Pod Width (cm) | Fresh 100-Seed Weight (g) | Soluble Sugar Content (g/100 g) | Starch Content (g/100 g) |
|---|---|---|---|---|---|---|---|
| Taiwan-75 | 84 a 1 | 40 b | 6.0 bc | 1.4 a | 83 bc | 3.1 a | 5.2 a |
| Zhexiandou No. 8 | 85 a | 36 c | 6.1 b | 1.4 a | 85 b | 2.8 bc | 5.1 ab |
| Zhexian No. 9 | 85 a | 35 c | 6.4 a | 1.4 a | 88 a | 2.9 ab | 4.8 c |
| Zhexian No. 10 | 85 a | 44 a | 5.9 c | 1.4 a | 80 c | 2.6 c | 4.9 bc |
| Cultivar Name | SNPs | InDels | ||||
|---|---|---|---|---|---|---|
| Heterozygous | Homozygous | Total | Heterozygous | Homozygous | Total | |
| Taiwan-75 | 206,410 | 1,339,245 | 1,545,655 | 307,256 | 39,494 | 346,750 |
| Zhexiandou No. 8 | 132,889 | 1,143,801 | 1,276,690 | 252,400 | 24,069 | 276,469 |
| Zhexian No. 9 | 166,980 | 1,280,183 | 1,447,163 | 291,262 | 32,883 | 324,145 |
| Zhexian No. 10 | 175,910 | 1,209,567 | 1,385,477 | 287,939 | 35,274 | 323,213 |
| Cultivar Name | Genes with Non-Synonymous SNPs | Genes with InDels | Genes with SV | Total |
|---|---|---|---|---|
| Taiwan-75 | 15,338 | 4073 | 275 | 16,828 |
| Zhexiandou No. 8 | 13,939 | 3384 | 146 | 15,237 |
| Zhexian No. 9 | 14,709 | 3884 | 215 | 16,186 |
| Zhexian No. 10 | 14,794 | 3933 | 230 | 16,287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Fu, X.; Yang, Q.; Jin, H.; Zhu, L.; Yuan, F. Genome-Wide Variation Analysis of Four Vegetable Soybean Cultivars Based on Re-Sequencing. Plants 2022, 11, 28. https://doi.org/10.3390/plants11010028
Yu X, Fu X, Yang Q, Jin H, Zhu L, Yuan F. Genome-Wide Variation Analysis of Four Vegetable Soybean Cultivars Based on Re-Sequencing. Plants. 2022; 11(1):28. https://doi.org/10.3390/plants11010028
Chicago/Turabian StyleYu, Xiaomin, Xujun Fu, Qinghua Yang, Hangxia Jin, Longming Zhu, and Fengjie Yuan. 2022. "Genome-Wide Variation Analysis of Four Vegetable Soybean Cultivars Based on Re-Sequencing" Plants 11, no. 1: 28. https://doi.org/10.3390/plants11010028
APA StyleYu, X., Fu, X., Yang, Q., Jin, H., Zhu, L., & Yuan, F. (2022). Genome-Wide Variation Analysis of Four Vegetable Soybean Cultivars Based on Re-Sequencing. Plants, 11(1), 28. https://doi.org/10.3390/plants11010028






