Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System
Abstract
1. Introduction
2. Methodology
3. Therapeutic Benefits of Essential Oils
3.1. Role in Pain Management
- Clove Oil
- Lavender Oil
3.2. Role in Anxiety Relief and Stress Management
- Frankincense oil
- Lavender oil
- Lemongrass oil
3.3. Role in Depression Management
- Ylang ylang oil
- Cinnamon oil
3.4. Role in Memory Retention, Neuroprotection, and Alzheimer’s Disease Management
- Eucalyptus oil
- Peppermint oil
- Rosemary oil
- Sage oil
- Sandalwood oil
4. Essential-Oil-Based Nanomedicines/Pharmacotherapy
- Dendrimers
- Nanogels
- Carbon nanotubes (CNTs)
5. EO Therapy: Challenges and Research Gaps
- Lack of sufficient information pertaining to utility and dosage
- Patient Acceptability of EOs
- Sustainability
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R. Effects of essential oils on central nervous system: Focus on mental health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef]
- ChStratakos, A.; Koidis, A. Methods for Extracting Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 31–38. [Google Scholar]
- Asl, M.K.; Nazariborun, A.; Hosseini, M. Analgesic effect of the aqueous and ethanolic extracts of clove. Avicenna J. Phytomed. 2013, 3, 186–192. [Google Scholar]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Mertens, M.; Buettner, A.; Kirchhoff, E. The volatile constituents of frankincense—A review. Flavour Fragr. J. 2009, 24, 279–300. [Google Scholar] [CrossRef]
- Okano, S.; Honda, Y.; Kodama, T.; Kimura, M. The Effects of Frankincense Essential Oil on Stress in Rats. J. Oleo Sci. 2019, 68, 1003–1009. [Google Scholar] [CrossRef]
- Vuuren, S.F.V.; Kamatou, G.P.P.; Viljoen, A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. South Afr. J. Bot. 2010, 76, 686–691. [Google Scholar] [CrossRef]
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.; Donadio, M.V.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad Bras. Cienc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef]
- Scuteri, D.; Hamamura, K.; Sakurada, T.; Watanabe, C.; Sakurada, S.; Morrone, L.A.; Rombola, L.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Efficacy of Essential Oils in Pain: A Systematic Review and Meta-Analysis of Preclinical Evidence. Front. Pharmacol. 2021, 12, 640128. [Google Scholar] [CrossRef]
- Lehrner, J.; Marwinski, G.; Lehr, S.; Johren, P.; Deecke, L. Ambient odors of orange and lavender reduce anxiety and improve mood in a dental office. Physiol Behav. 2005, 86, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Conrad, P.; Adams, C. The effects of clinical aromatherapy for anxiety and depression in the high risk postpartum woman—A pilot study. Complementary Ther. Clin. Pract. 2012, 18, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Kaczmarzyk, J.R.; Dave, N.; Gabrieli, J.D.E.; Grossman, J.C. Sleep quality, duration, and consistency are associated with better academic performance in college students. NPJ Sci. Learn. 2019, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Lillehei, A.S.; Halcon, L.L.; Savik, K.; Reis, R. Effect of Inhaled Lavender and Sleep Hygiene on Self-Reported Sleep Issues: A Randomized Controlled Trial. J. Altern. Complementary Med. 2015, 21, 430–438. [Google Scholar] [CrossRef]
- Faydali, S.; Cetinkaya, F. The Effect of Aromatherapy on Sleep Quality of Elderly People Residing in a Nursing Home. Holist. Nurs. Pract. 2018, 32, 8–16. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Bialon, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical Composition of the Essential Oil of the New Cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef]
- Malcolm, B.J.; Tallian, K. Essential oil of lavender in anxiety disorders: Ready for prime time? Ment. Health Clin. 2017, 7, 147–155. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.E. Use of Cymbopogon citratus essential oil in food preservation: Recent advances and future perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 2541–2559. [Google Scholar] [CrossRef]
- Costa, C.A.; Cury, T.C.; Cassettari, B.O.; Takahira, R.K.; Florio, J.C.; Costa, M. Citrus aurantium L. essential oil exhibits anxiolytic-like activity mediated by 5-HT(1A)-receptors and reduces cholesterol after repeated oral treatment. BMC Complementary Altern. Med. 2013, 13, 42. [Google Scholar] [CrossRef]
- Costa, C.A.; Kohn, D.O.; de Lima, V.M.; Gargano, A.C.; Florio, J.C.; Costa, M. The GABAergic system contributes to the anxiolytic-like effect of essential oil from Cymbopogon citratus (lemongrass). J. Ethnopharmacol. 2011, 137, 828–836. [Google Scholar] [CrossRef]
- Goes, T.C.; Ursulino, F.R.; Almeida-Souza, T.H.; Alves, P.B.; Teixeira-Silva, F. Effect of Lemongrass Aroma on Experimental Anxiety in Humans. J. Altern. Complementary Med. 2015, 21, 766–773. [Google Scholar] [CrossRef]
- Tan, L.T.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evid. Based Complementary Alternat. Med. 2015, 2015, 896314. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. The anxiolytic effect of essential oil of Cananga odorata exposure on mice and determination of its major active constituents. Phytomedicine 2016, 23, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.J.; Cha, J.Y.; Kim, S.E.; Ko, I.G.; Jee, Y.S. Effects of Ylang-Ylang aroma on blood pressure and heart rate in healthy men. J. Exerc. Rehabil. 2013, 9, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. Cananga odorata essential oil reverses the anxiety induced by 1-(3-chlorophenyl) piperazine through regulating the MAPK pathway and serotonin system in mice. J. Ethnopharmacol. 2018, 219, 23–30. [Google Scholar] [CrossRef]
- Giang, P.M.; Son, P.T. GC and GC-MS analysis of the fresh flower essential oil of Cananga odorata (Lam) Hook. f. et Th. var. fruticosa (Craib). J. Sincl. Am. J. Essent. Oils Nat. Prod. 2016, 4, 9–11. [Google Scholar]
- Hwang, E.S.; Kim, H.B.; Lee, S.; Kim, M.J.; Kim, K.J.; Han, G.; Han, S.Y.; Lee, E.A.; Yoon, J.H.; Kim, D.O.; et al. Antidepressant-like effects of beta-caryophyllene on restraint plus stress-induced depression. Behav. Brain Res. 2020, 380, 112439. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, R.; Pazgoohan, N.; Seresht, H.R.; Amin, B. Repeated systemic administration of the cinnamon essential oil possesses anti-anxiety and anti-depressant activities in mice. Iran. J. Basic. Med. Sci. 2017, 20, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Wang, Y.W.; Huang, W.S.; Lee, M.M.; Wood, W.G.; Leung, Y.M.; Tsai, H.Y. Trans-Cinnamaldehyde, An Essential Oil in Cinnamon Powder, Ameliorates Cerebral Ischemia-Induced Brain Injury via Inhibition of Neuroinflammation Through Attenuation of iNOS, COX-2 Expression and NFkappa-B Signaling Pathway. Neuromolecular. Med. 2016, 18, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Tanabe, M.; Kobata, K.; Watanabe, T. TRPA1 agonists--allyl isothiocyanate and cinnamaldehyde--induce adrenaline secretion. Biosci. Biotechnol. Biochem. 2008, 72, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical Composition and Antioxidant, Antimicrobial, and Antiproliferative Activities of Cinnamomum zeylanicum Bark Essential Oil. Evid. Based. Complementary Altern. Med. 2020, 2020, 5190603. [Google Scholar] [CrossRef]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Brett, A.E.; A.Webster, A. Chapter 132—Acetylcholinesterase and its Inhibitors. In Primer on the Autonomic Nervous System (Third Edition), 3rd ed.; Robertson, D., Burnstock, G., Paton, J.F.R., Biaggioni, I., Low, P.A., Eds.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Jun, Y.S.; Kang, P.; Min, S.S.; Lee, J.M.; Kim, H.K.; Seol, G.H. Effect of eucalyptus oil inhalation on pain and inflammatory responses after total knee replacement: A randomized clinical trial. Evid. Based Complementary Altern. Med. 2013, 2013, 502727. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Touj, N.; Hammami, I.; Dridi, K.; Al-Ayed, A.S.; Hamdi, N. Chemical Composition and in vivo Efficacy of the Essential Oil of Mentha piperita L. in the Suppression of Crown Gall Disease on Tomato Plants. J. Oleo Sci. 2019, 68, 419–426. [Google Scholar] [CrossRef]
- Masomeh, L.; Narges, M.; Hassan, R.A.H. Peppermint and Its Functionality: A Review. Arch. Clin. Microbiol. 2017, 7, 4. [Google Scholar] [CrossRef]
- Umezu, T. Evaluation of the effects of plant-derived essential oils on central nervous system function using discrete shuttle-type conditioned avoidance response in mice. Phytother. Res. 2012, 26, 884–891. [Google Scholar] [CrossRef]
- Kennedy, D.; Okello, E.; Chazot, P.; Howes, M.J.; Ohiomokhare, S.; Jackson, P.; Haskell-Ramsay, C.; Khan, J.; Forster, J.; Wightman, E. Volatile Terpenes and Brain Function: Investigation of the Cognitive and Mood Effects of Mentha × Piperita L. Essential Oil with In Vitro Properties Relevant to Central Nervous System Function. Nutrients 2018, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Bhadania, M.; Joshi, H.; Patel, P.; Kulkarni, V.H. Protective effect of menthol on beta-amyloid peptide induced cognitive deficits in mice. Eur. J. Pharmacol. 2012, 681, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Jirovetz, L. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha × piperita. Nat. Prod. Commun. 2009, 4, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Hewitt, S.; Moss, L.; Wesnes, K. Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. Int. J. Neurosci. 2008, 118, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of Essential Oils of Rosmarinus officinalis L. by Two Different Methods: Hydrodistillation and Microwave Assisted Hydrodistillation. Sci. World J. 2019, 2019, 3659432. [Google Scholar] [CrossRef]
- Villareal, M.O.; Ikeya, A.; Sasaki, K.; Arfa, A.B.; Neffati, M.; Isoda, H. Anti-stress and neuronal cell differentiation induction effects of Rosmarinus officinalis L. essential oil. BMC Complementary Altern. Med. 2017, 17, 549. [Google Scholar] [CrossRef]
- Habtemariam, S. The Therapeutic Potential of Rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer’s Disease. Evid. Based Complementary Altern. Med. 2016, 2016, 2680409. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Chalchat, J.C. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int. J. Food Sci. Nutr. 2008, 59, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Khedher, M.R.B.; Khedher, S.B.; Chaieb, I.; Tounsi, S.; Hammami, M. Chemical composition and biological activities of Salvia officinalis essential oil from Tunisia. EXCLI J. 2017, 16, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Rouse, M.; Moss, L. Aromas of Salvia Species Enhance Everyday Prospective Memory Performance in Healthy Young Adults. Adv. Chem. Eng. Sci. 2014, 4, 339–346. [Google Scholar] [CrossRef]
- Lopresti, A.L. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs R D 2017, 17, 53–64. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.A.; Sim, S.; Kohlenberg, B.; Lawrence, B.M. Hawaiian sandalwood: Oil composition of Santalum paniculatum and comparison with other sandal species. Nat. Prod. Commun. 2014, 9, 1365–1368. [Google Scholar] [CrossRef]
- Safwat, Y.N.; Elsayed, M.M. Sandalwood oil neuroprotective effects on middle cerebral artery occlusion model of ischemic brain stroke. Farmacogn. Mag. 2020, 16, 117–122. [Google Scholar]
- Misra, B.B.; Dey, S. Biological Activities of East Indian Sandalwood Tree, Santalum album. PeerJ 2013, 1, e96v1. [Google Scholar]
- . Misra, B.B.; Dey, S. Evaluation of in vivo anti-hyperglycemic and antioxidant potentials of alpha-santalol and sandalwood oil. Phytomedicine 2013, 20, 409–416. [Google Scholar] [CrossRef]
- Hoferl, M.; Hutter, C.; Buchbauer, G. A Pilot Study on the Physiological Effects of Three Essential Oils in Humans. Nat. Prod. Commun. 2016, 11, 1561–1564. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Mahfud, M. Chemical composition of essential oil of Indonesia sandalwood extracted by microwave-assisted hydrodistillation. AIP Conf. Proc. 2016, 1755, 050001. [Google Scholar]
- Moss, M.; Cook, J.; Wesnes, K.; Duckett, P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int. J. Neurosci. 2003, 113, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Hadi, N.; Hanid, A.A. Lavender Essence for Post-cesarean pain. Pak. J. Biol. Sci. 2011, 14, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Sayorwan, W.; Ruangrungsi, N.; Piriyapunyporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Siripornpanich, V. Effects of inhaled rosemary oil on subjective feelings and activities of the nervous system. Sci. Pharm. 2013, 81, 531–542. [Google Scholar] [CrossRef]
- Moss, L.; Rouse, M.; Wesnes, K.A.; Moss, M. Differential effects of the aromas of Salvia species on memory and mood. Hum. Psychopharmacol. 2010, 25, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Boehm, K.; Bussing, A.; Ostermann, T. Aromatherapy as an adjuvant treatment in cancer care--a descriptive systematic review. Afr. J. Tradit. Complementary Altern. Med. 2012, 9, 503–518. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Sheafer, H.; Tepper, D. The Effectiveness of Aromatherapy in Reducing Pain: A Systematic Review and Meta-Analysis. Pain Res. Treat. 2016, 2016, 8158693. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chang, M.C. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef]
- Wang, Z.J.; Heinbockel, T. Essential Oils and Their Constituents Targeting the GABAergic System and Sodium Channels as Treatment of Neurological Diseases. Molecules 2018, 23, 1061. [Google Scholar] [CrossRef]
- WHO. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 1 May 2021).
- Thibaut, F. Anxiety disorders: A review of current literature. Dialogues Clin. Neurosci. 2017, 19, 87–88. [Google Scholar]
- Ogata, K.; Ataka, K.; Suzuki, H.; Yagi, T.; Okawa, A.; Fukumoto, T.; Zhang, B.; Nakata, M.; Yada, T.; Asakawa, A. Lavender Oil Reduces Depressive Mood in Healthy Individuals and Enhances the Activity of Single Oxytocin Neurons of the Hypothalamus Isolated from Mice: A Preliminary Study. Evid. Based Complementary Altern. Med. 2020, 2020, 5418586. [Google Scholar] [CrossRef]
- WHO. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 1 May 2021).
- National Institute of Ageing. Treatment of Alzeimers Disease. Available online: https://www.nia.nih.gov/health/how-alzheimers-disease-treated (accessed on 1 May 2021).
- Santarsieri, D.; Schwartz, T.L. Antidepressant efficacy and side-effect burden: A quick guide for clinicians. Drugs Context 2015, 4, 212290. [Google Scholar] [CrossRef]
- Filiptsova, O.V.; Gazzavi-Rogozina, L.V.; Timoshyna, I.A.; Naboka, O.I.; Dyomina, Y.V.; Ochkur, A.V. The effect of the essential oils of lavender and rosemary on the human short-term memory. Alex. J. Med. 2018, 54, 41–44. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef]
- Sá, R.D.C.D.S.E.; Lima, T.C.; da Nóbrega, F.R.; Brito, A.E.M.D.; de Sousa, D.P. Analgesic-Like Activity of Essential Oil Constituents: An Update. Int. J. Mol. Sci. 2017, 12, 2932. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef]
- Chung, G.; Rhee, J.N.; Jung, S.J.; Kim, J.S.; Oh, S.B. Modulation of CaV2.3 calcium channel currents by eugenol. J. Dent. Res. 2008, 87, 137–141. [Google Scholar] [CrossRef]
- Cho, J.S.; Kim, T.H.; Lim, J.M.; Song, J.H. Effects of eugenol on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2008, 1243, 53–62. [Google Scholar] [CrossRef]
- Aoshima, H.; Hamamoto, K. Potentiation of GABAA Receptors Expressed in Xenopus Oocytes by Perfume and Phytoncid. Biosci. Biotechnol. Biochem. 1999, 63, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Dal Bo, W.; Luiz, A.P.; Martins, D.F.; Mazzardo-Martins, L.; Santos, A.R. Eugenol reduces acute pain in mice by modulating the glutamatergic and tumor necrosis factor alpha (TNF-alpha) pathways. Fundam. Clin. Pharmacol. 2013, 27, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Malik, N.; Khatkar, A. Lead optimization for promising monoamine oxidase inhibitor from eugenol for the treatment of neurological disorder: Synthesis and in silico based study. BMC Chem. 2019, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Holanda Pinto, S.A.; Pinto, L.M.; Guedes, M.A.; Cunha, G.M.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Antinoceptive effect of triterpenoid alpha,beta-amyrin in rats on orofacial pain induced by formalin and capsaicin. Phytomedicine 2008, 15, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Venâncio, A.M.; Marchioro, M.; Estavam, C.S.; Melo, M.S.; Santana, M.T.; Onofre, A.S.C.; Guimarães, A.G.; Oliveira, M.G.B.; Alves, P.B.; Pimentel, H.D.C.; et al. Ocimum basilicum leaf essential oil and (-)-linalool reduce orofacial nociception in rodents: A behavioral and electrophysiological approach. Braz. J. Farmacogn. 2011, 21, 1043–1051. [Google Scholar] [CrossRef]
- Andersen, P.; Bliss, T.V.; Skrede, K.K. Unit analysis of hippocampal polulation spikes. Exp. Brain Res. 1971, 13, 208–221. [Google Scholar] [CrossRef]
- Peana, A.T.; De Montis, M.G.; Nieddu, E.; Spano, M.T.; D’Aquila, P.S.; Pippia, P. Profile of spinal and supra-spinal antinociception of (-)-linalool. Eur. J. Pharmacol. 2004, 485, 165–174. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Chessa, M.L.; Moretti, M.D.; Serra, G.; Pippia, P. (-)-Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 2003, 460, 37–41. [Google Scholar] [CrossRef]
- Tashiro, S.; Yamaguchi, R.; Ishikawa, S.; Sakurai, T.; Kajiya, K.; Kanmura, Y.; Kuwaki, T.; Kashiwadani, H. Odour-induced analgesia mediated by hypothalamic orexin neurons in mice. Sci. Rep. 2016, 6, 37129. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Rodriguez, D.; Allred, K. A Systematic Review of Essential Oils and the Endocannabinoid System: A Connection Worthy of Further Exploration. Evid. Based Complementary Altern. Med. 2020, 2020, 8035301. [Google Scholar] [CrossRef]
- Anxiety and Depression Association of America. Anxiety, Facts and Statistics. Available online: https://adaa.org/understanding-anxiety/facts-statistics (accessed on 1 May 2021).
- MayoClinic. Anxiety Disorders, Diagnosis and Treatment. Available online: https://www.mayoclinic.org/diseases-conditions/anxiety/diagnosis-treatment/drc-20350967 (accessed on 1 May 2021).
- NIH. Depression. Available online: https://www.nimh.nih.gov/health/topics/depression/ (accessed on 1 May 2021).
- MayoClinic. Depression (Major Depressive Disorder). Available online: https://www.mayoclinic.org/diseases-conditions/depression/symptoms-causes/syc-20356007 (accessed on 1 May 2021).
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Galaj, E.; Xi, Z.X. Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders. CNS Drugs 2019, 33, 1001–1030. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.G. Late-onset neurodegenerative diseases--the role of protein insolubility. J. Anat. 2000, 196 Pt 4, 609–616. [Google Scholar] [CrossRef]
- Gertsch, J. Anti-inflammatory cannabinoids in diet: Towards a better understanding of CB(2) receptor action? Commun. Integr. Biol 2008, 1, 26–28. [Google Scholar] [CrossRef]
- Paula-Freire, L.I.; Andersen, M.L.; Gama, V.S.; Molska, G.R.; Carlini, E.L. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine 2014, 21, 356–362. [Google Scholar] [CrossRef]
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of peripheral cannabinoid and opioid receptors in beta-caryophyllene-induced antinociception. Eur. J. Pain 2013, 17, 664–675. [Google Scholar] [CrossRef]
- Association, A.S. What Is Alzheimer’s Disease? Available online: https://www.alz.org/alzheimers-dementia/what-is-alzheimers (accessed on 1 May 2021).
- National Institute on Ageing. Alzheimer’s Disease Fact Sheet. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 1 May 2021).
- Reiman, E.M.; Langbaum, J.B.; Fleisher, A.S.; Caselli, R.J.; Chen, K.; Ayutyanont, N.; Quiroz, Y.T.; Kosik, K.S.; Lopera, F.; Tariot, P.N. Alzheimer’s Prevention Initiative: A plan to accelerate the evaluation of presymptomatic treatments. J. Alzheimers Dis. 2011, 26 (Suppl. 3), 321–329. [Google Scholar] [CrossRef]
- Lawther, B.K.; Kumar, S.; Krovvidi, H. Blood–brain barrier. In Continuing Education in Anaesthesia, Critical Care and Pain; Oxford Academic: Oxford, UK, 2011; Volume 11. [Google Scholar]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K. Designing Dendrimers; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem. Neurosci. 2014, 5, 2–13. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Q.; Qu, Y.H.; Ke, W.L.; Zhu, J.H.; Pei, Y.Y.; Jiang, C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 2007, 21, 1117–1125. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Han, L.; Li, J.; Liu, S.; Jiang, C. Targeted delivery of chlorotoxin-modified DNA-loaded nanoparticles to glioma via intravenous administration. Biomaterials 2011, 32, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; Jia, X.R.; Du, J.; Ying, X.; Lu, W.L.; Lou, J.N.; Wei, Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, R.; Han, L.; Ke, W.; Shao, K.; Ye, L.; Lou, J.; Jiang, C. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials 2009, 30, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, M.H.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; et al. Natural bioactive molecules: An alternative approach to the treatment and control of glioblastoma multiforme. Biomed. Pharmacother. 2021, 141, 111928. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Zhang, Z.; Liaw, K.; Kambhampati, S.P.; Porterfield, J.E.; Lin, K.C.; DeRidder, L.B.; Kannan, S.; Kannan, R.M. Dense hydroxyl polyethylene glycol dendrimer targets activated glia in multiple CNS disorders. Sci. Adv. 2020, 6, eaay8514. [Google Scholar] [CrossRef] [PubMed]
- Sultana, F.; Manirujjaman; Imran-Ul-Haque, M.; Arafat, M.; Sharmin, S. An Overview of Nanogel Drug Delivery System. J. Appl. Pharm. Sci. 2013, 3, 95–105. [Google Scholar]
- Kabanov, A.V.; Gendelman, H.E. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog. Polym. Sci. 2007, 32, 1054–1082. [Google Scholar] [CrossRef]
- Vinogradov, S. The second annual symposium on nanomedicine and drug delivery: Exploring recent developments and assessing major advances. 19-20 August 2004, Polytechnic University, Brooklyn, NY, USA. Expert. Opin. Drug Deliv. 2004, 1, 181–184. [Google Scholar] [CrossRef][Green Version]
- Vinogradov, S.V.; Batrakova, E.V.; Kabanov, A.V. Nanogels for oligonucleotide delivery to the brain. Bioconjug. Chem. 2004, 15, 50–60. [Google Scholar] [CrossRef]
- Azadi, A.; Rouini, M.R.; Hamidi, M. Neuropharmacokinetic evaluation of methotrexate-loaded chitosan nanogels. Int. J. Biol. Macromol. 2015, 79, 326–335. [Google Scholar] [CrossRef]
- Gulyaev, A.E.; Gelperina, S.E.; Skidan, I.N.; Antropov, A.S.; Kivman, G.Y.; Kreuter, J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm. Res. 1999, 16, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaudhary, R.K.; Singh, R.; Singh, S.P.; Wang, S.Y.; Hoe, Z.Y.; Pan, C.T.; Shiue, Y.L.; Wei, D.Q.; Kaushik, A.C.; et al. Nanotheranostic Applications for Detection and Targeting Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Koul, V.; Singh, Y.; Anand, A. Targeted drug delivery to central nervous system (CNS) for the treatment of neurodegenerative disorders: Trends and advances. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Kafa, H.; Wang, J.T.; Rubio, N.; Venner, K.; Anderson, G.; Pach, E.; Ballesteros, B.; Preston, J.E.; Abbott, N.J.; Al-Jamal, K.T. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials 2015, 53, 437–452. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Nasir, H.M.; Ahmad, A.; Setapar, S.H.M.; Ahmad, H.; Noor, M.H.M.; Rafatullah, M.; Khatoon, A.; Kausar, M.A.; Ahmad, I.; et al. Enrichment of Eucalyptus oil nanoemulsion by micellar nanotechnology: Transdermal analgesic activity using hot plate test in rats’ assay. Sci. Rep. 2019, 9, 13678. [Google Scholar] [CrossRef]
- Scuteri, D.; Cassano, R.; Trombino, S.; Russo, R.; Mizoguchi, H.; Watanabe, C.; Hamamura, K.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; et al. Development and Translation of NanoBEO, a Nanotechnology-Based Delivery System of Bergamot Essential Oil Deprived of Furocumarins, in the Control of Agitation in Severe Dementia. Pharmaceutics 2021, 13, 379. [Google Scholar] [CrossRef]
- Gaude, T.T.; Soares, G.A.B.E.; Priolkar, R.N.S.; Biradar, B.; Mamledesai1, S. Synthesis of 4-hydroxy-1-(phenyl/methyl)-3-[3-(substituted amino)-2-nitropropanoyl] quinolin-2(1H)-ones as an antimicrobial andantitubercular agents. Indian J. Heterocycl. Chem. 2017, 27, 223–228. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef]
- Conlon, P.M.; Haack, K.M.; Rodgers, N.J.; Dion, L.J.; Cambern, K.L.; Rohlik, G.M.; Nelson, D.E.; Barry, T.A.; Ayres, S.J.; Cutshall, S.M. Introducing Essential Oils into Pediatric and Other Practices at an Academic Medical Center. J. Holist. Nurs. 2017, 35, 389–396. [Google Scholar] [CrossRef] [PubMed]
- de Matos, S.P.; Teixeira, H.F.; de Lima, A.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- Sapra, B.; Jain, S.; Tiwary, A.K. Percutaneous permeation enhancement by terpenes: Mechanistic view. AAPS J. 2008, 10, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Lalko, J.; Api, A.M. Investigation of the dermal sensitization potential of various essential oils in the local lymph node assay. Food Chem. Toxicol. 2006, 44, 739–746. [Google Scholar] [CrossRef]
- Opdyke, D.L.J. Inhibition of sensitization reactions induced by certain aldehydes. Food Cosmet. Toxicol. 1976, 14, 197–198. [Google Scholar] [CrossRef]
- Nilsson, A.M.; Gafvert, E.; Salvador, L.; Luthman, K.; Bruze, M.; Gruvberger, B.; Nilsson, J.L.; Karlberg, A.T. Mechanism of the antigen formation of carvone and related alpha, beta-unsaturated ketones. Contact Dermat. 2001, 44, 347–356. [Google Scholar] [CrossRef]
- Nilsson, A.M.; Jonsson, C.; Luthman, K.; Nilsson, J.L.; Karlberg, A.T. Inhibition of the sensitizing effect of carvone by the addition of non-allergenic compounds. Acta Derm. Venereol. 2004, 84, 99–105. [Google Scholar] [CrossRef]
- Karlberg, A.T.; Nilsson, A.M.; Luthman, K.; Nilsson, J.L. Structural analogues inhibit the sensitizing capacity of carvone. Acta Derm. Venereol. 2001, 81, 398–402. [Google Scholar] [CrossRef]
- Basketter, D. Quenching: Fact or fiction? Contact Dermat. 2000, 43, 253–258. [Google Scholar] [CrossRef]
- Marturano, V.; Bizzarro, V.; De Luise, A.; Calarco, A.; Ambrogi, V.; Giamberini, M.; Tylkowski, B.; Cerruti, P. Essential oils as solvents and core materials for the preparation of photo-responsive polymer nanocapsules. Nano Res. 2018, 11, 2783–2795. [Google Scholar] [CrossRef]
- Mashwani, Z.U.; Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar] [CrossRef]
- Perino-Issartier, S.; Ginies, C.; Cravotto, G.; Chemat, F. A comparison of essential oils obtained from lavandin via different extraction processes: Ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J. Chromatogr. A 2013, 1305, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Tucker, J.L. Green Chemistry, a Pharmaceutical Perspective. Org. Process. Res. Dev. 2006, 10, 315–319. [Google Scholar] [CrossRef]
- Patel, K.D.; Davison, J.S.; Pittman, Q.J.; Sharkey, K.A. Cannabinoid CB(2) receptors in health and disease. Curr. Med. Chem. 2010, 17, 1393–1410. [Google Scholar] [CrossRef]
- Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef]
- Ramirez, B.G.; Blazquez, C.; Gomez del Pulgar, T.; Guzman, M.; de Ceballos, M.L. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005, 25, 1904–1913. [Google Scholar] [CrossRef]
- Farooqui, A.A. Potential Treatment Strategies for the Treatment of Dementia With Chinese Medicinal Plants. In Molecular Mechanisms of Dementia; Academic Press: Cambridge, MA, USA, 2019; pp. 251–286. [Google Scholar]
- Koppel, J.; Bradshaw, H.; Goldberg, T.E.; Khalili, H.; Marambaud, P.; Walker, M.J.; Pazos, M.; Gordon, M.L.; Christen, E.; Davies, P. Endocannabinoids in Alzheimer’s disease and their impact on normative cognitive performance: A case-control and cohort study. Lipids Health Dis. 2009, 8, 2. [Google Scholar] [CrossRef]
- Sharma, C.; Sadek, B.; Goyal, S.N.; Sinha, S.; Kamal, M.A.; Ojha, S. Small Molecules from Nature Targeting G-Protein Coupled Cannabinoid Receptors: Potential Leads for Drug Discovery and Development. Evid. Based Complementary Altern. Med. 2015, 2015, 238482. [Google Scholar] [CrossRef] [PubMed]
- Gulluni, N.; Re, T.; Loiacono, I.; Lanzo, G.; Gori, L.; Macchi, C.; Epifani, F.; Bragazzi, N.; Firenzuoli, F. Cannabis Essential Oil: A Preliminary Study for the Evaluation of the Brain Effects. Evid. Based Complementary Altern. Med. 2018, 2018, 1709182. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kunos, G. Modulating the endocannabinoid system in human health and disease--successes and failures. FEBS J. 2013, 280, 1918–1943. [Google Scholar] [CrossRef] [PubMed]
- Shepard, B.D.; Pluznick, J.L. How does your kidney smell? Emerging roles for olfactory receptors in renal function. Pediatric Nephrol. 2016, 31, 715–723. [Google Scholar] [CrossRef] [PubMed]
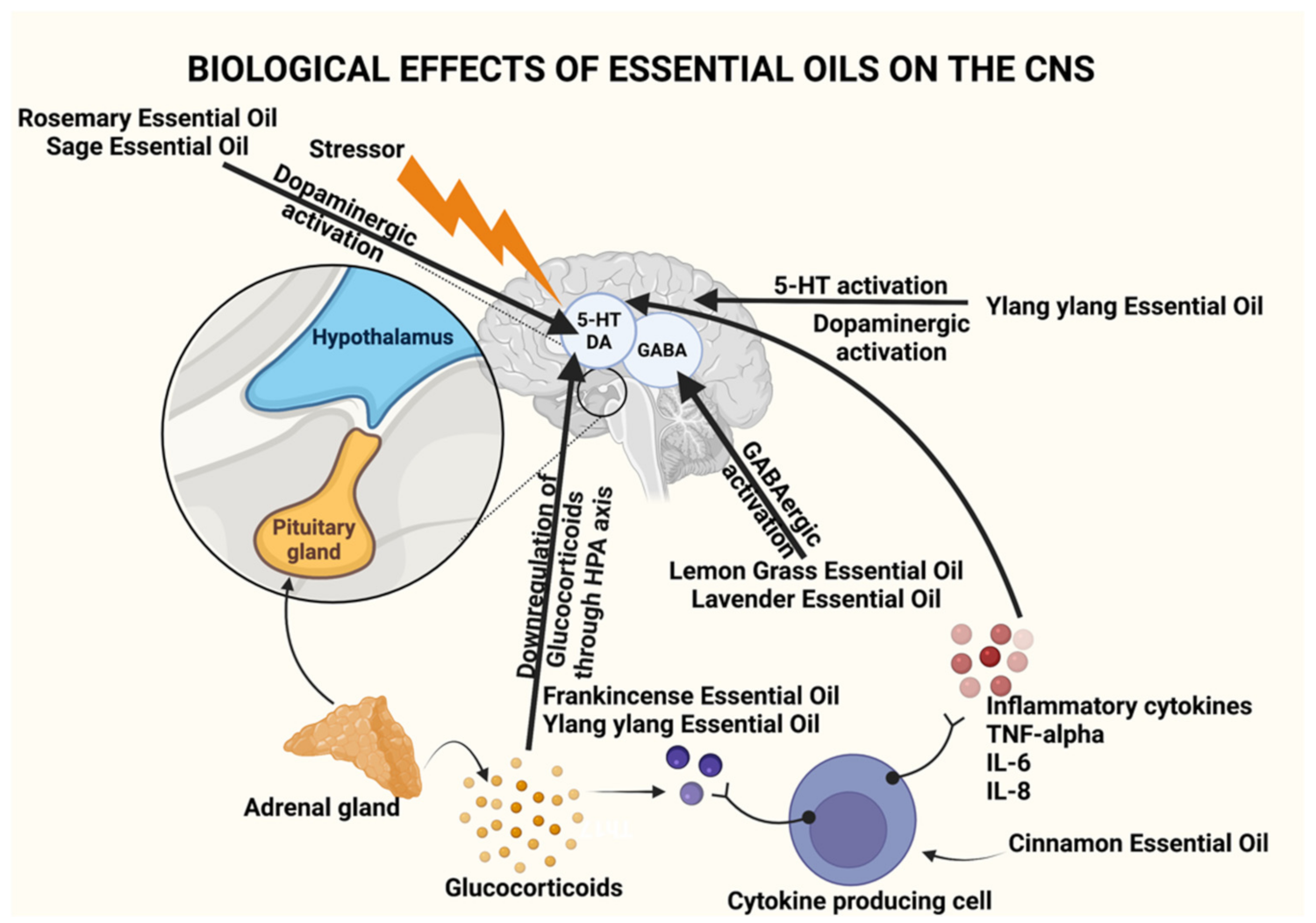

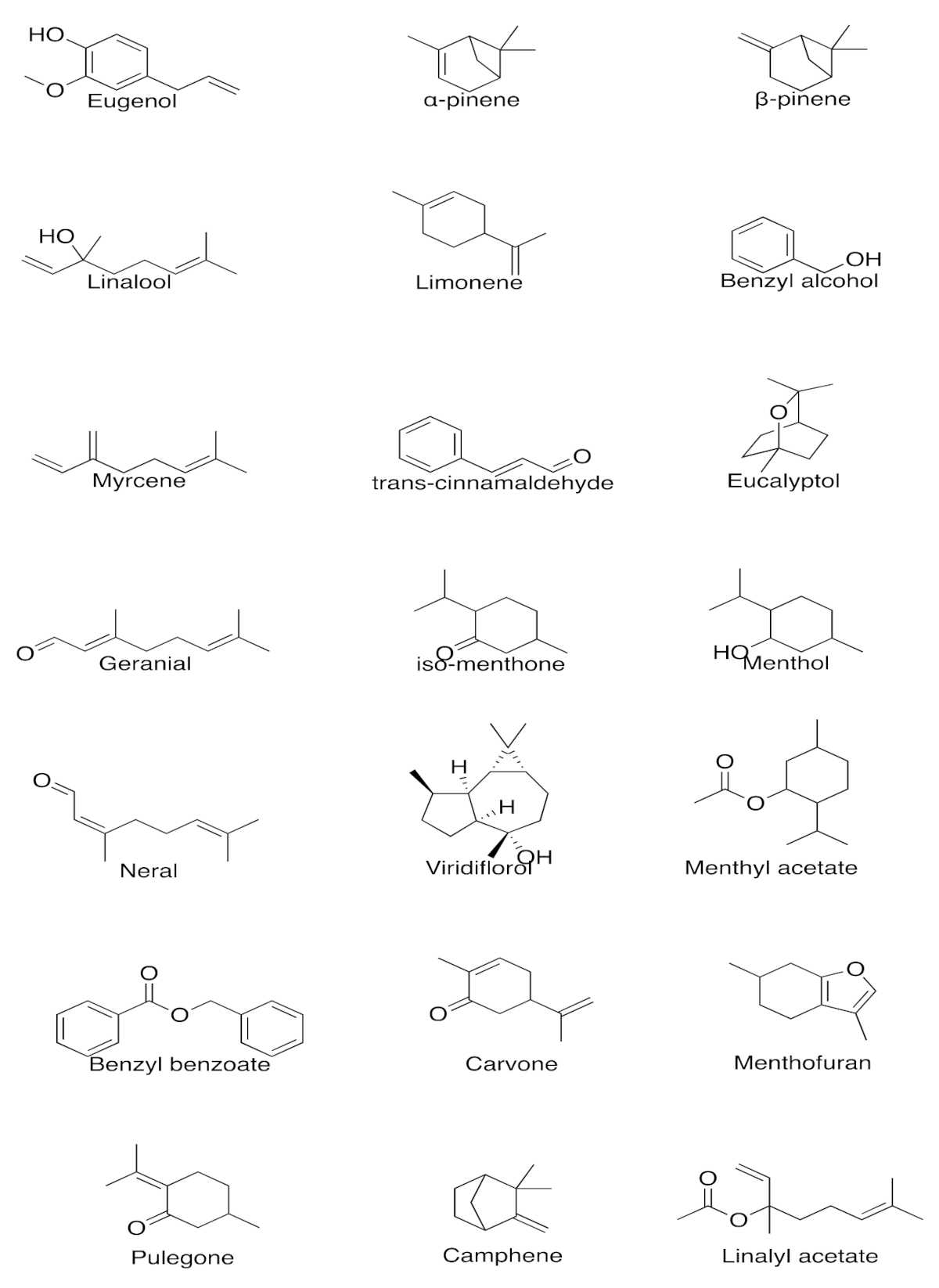
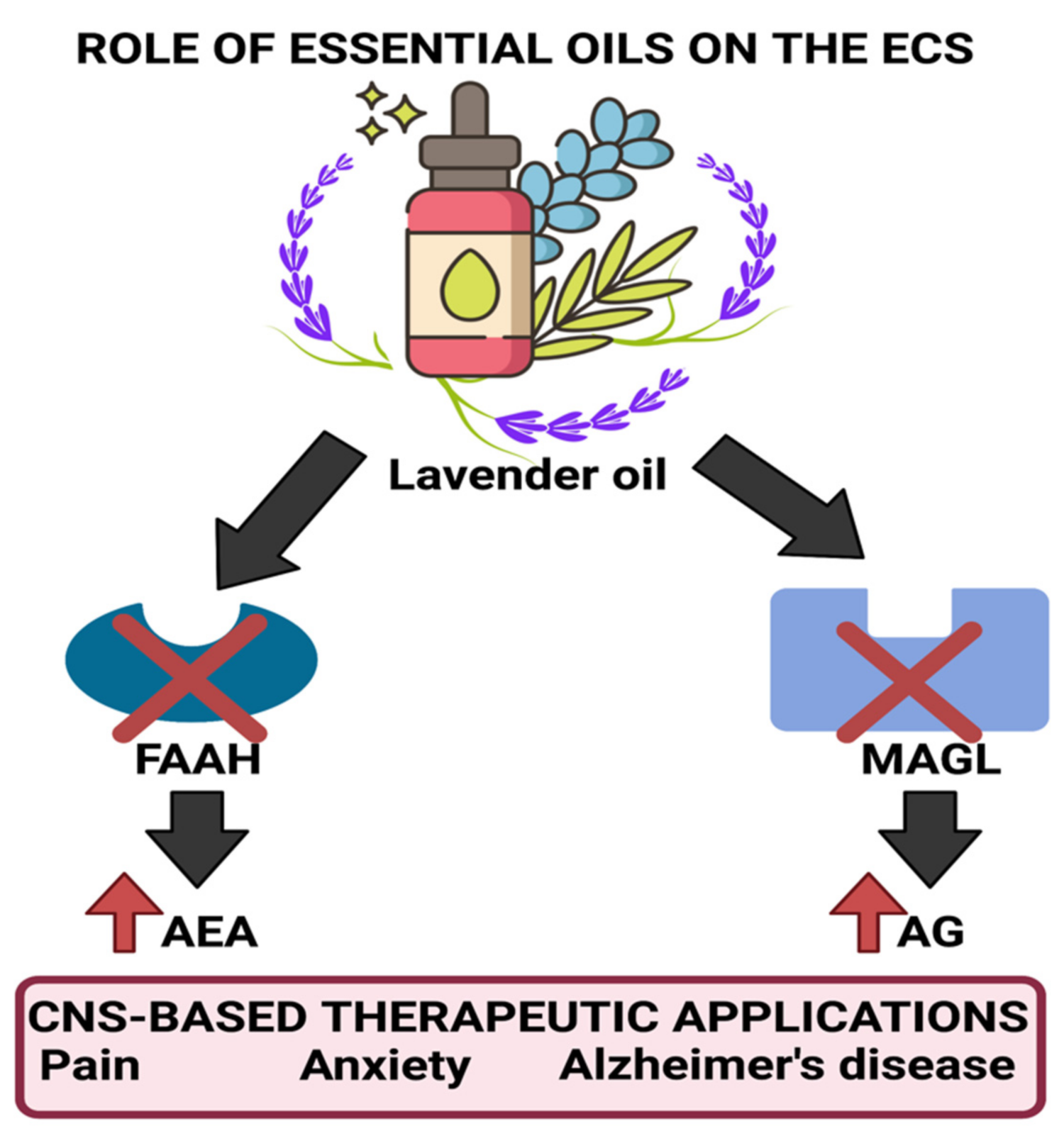
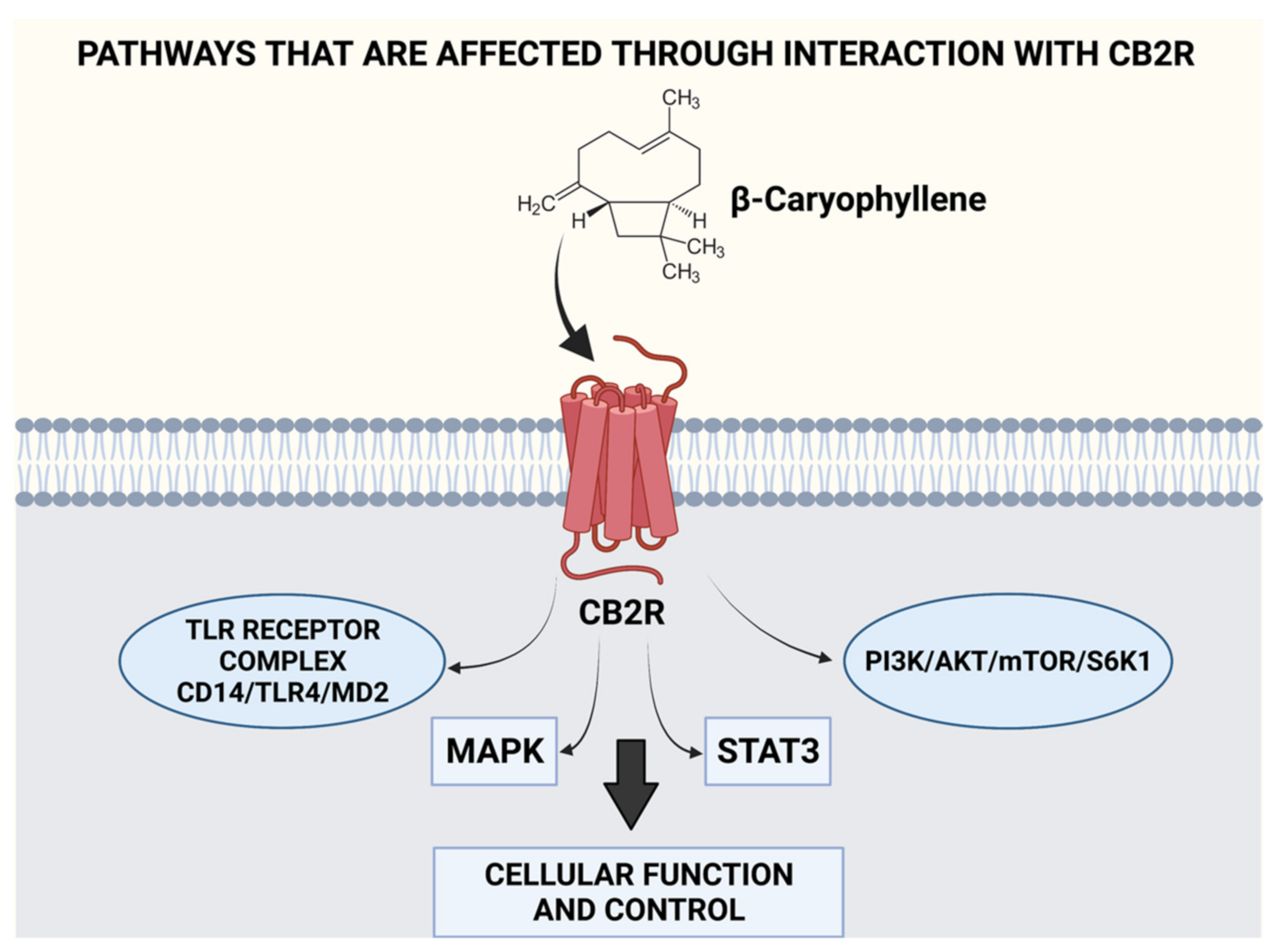
| Plant/Source | Active Constituents (>20%) | Extraction Method | Effective Dose/Preparation Technique | Biological Activity | Uses | References |
|---|---|---|---|---|---|---|
| Syzygium aromaticum | Eugenol (76.8%) | Water or steam distillation of the buds, stem, and leaves of clove tree | 50/100/200 mg/kg of aqueous extract/ethanolic extract of clove oil. Aqueous extract showed better results | GABAA receptor agonist | Analgesic | [4,5,6] |
|
Boswellia sacra, Boswellia frereana | α-Pinene (2–64.7%), α-thujene (0.3–52.4%), myrcene (1.1–22.4%), limonene (1.3–20.4%) | Hardened aromatic gum resins obtained from the tree | 50 μL in a 1:1000 dilution with jojoba oil on the nape of neck for 5 h with hourly intervals | Undetermined, believed to occur due to the synergistic effect of constituents | Anxiolytic and stress relief | [2,7,8,9] |
| Lavandula angustifolia | Linalyl acetate (7.4–44.2%), linalool 11.4–46.7%) | Steam distillation of flowers | 80 mg of standardized product (Silexan available in Germany) containing 36.8% of linalool and 34.2% linalyl acetate 160 mg/day for 8 weeks | GABAergic system interaction Antagonist of NK-1 receptor inhibiting release of substance P reduces peripheral and central nerve excitability Inhibition of voltage-gated calcium channels, reduction of 5-HT1A receptor activity, and increased parasympathetic tone | Anxiolytic, stress relief, mood enhancement, analgesic, and pain relief | [10,11,12,13,14,15,16,17,18] |
| Cymbopogon citratus | Citral (26.1%), neral (31.5%) | Steam distillation of fresh or partially dried grass | 1–10 mg/kg per day for 14 days | GABAergic system interaction | Anxiolytic, stress relief, and mood enhancement | [19,20,21,22] |
| Cananga odorata | β-Caryophyllene (26.8%) | Stem distillation of the flowers | 1% v/v of ylang ylang oil for 10 min. 25/50/100 mg/kg of β-Caryophyllene was injected intraperitoneally | Activation of ANS and has effects on the 5-HT and DAergic system Direct binding onto CB2R receptor | Mood adjustment, relaxation, and antidepressant activity | [23,24,25,26,27,28] |
| Cinnamomum verum | Trans-cinnamaldehyde (71.50%) | Brown bark | 0.5–2 mg/kg body weight three times a day or once daily for 14 days | Undetermined | Mood elevation and antidepressant action | [29,30,31,32] |
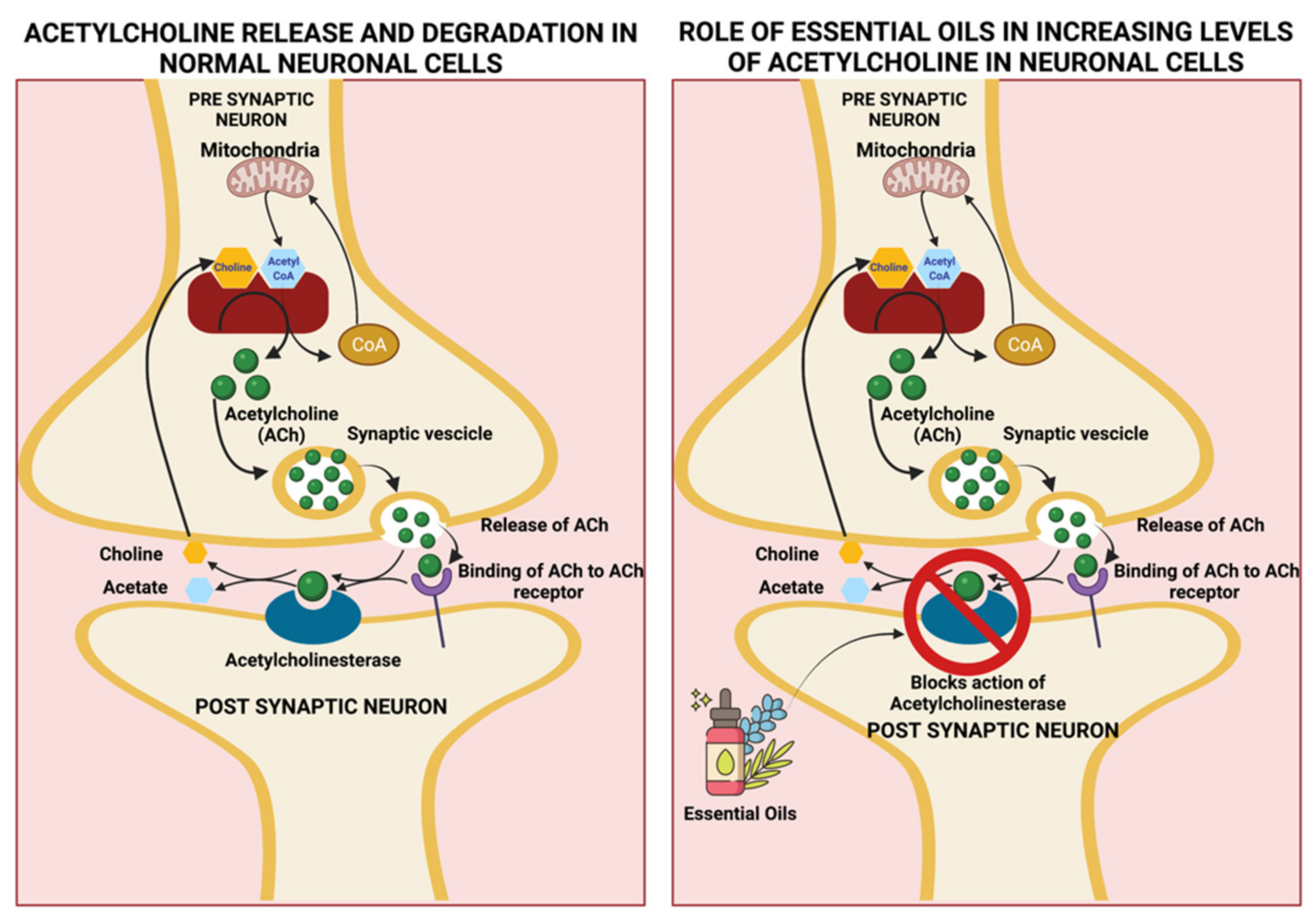
| Eucalyptus globulus | 1,8-cineole (49.07–83.59%), α-pinene (1.27–26.35%) | Steam distillation of the leaves | 3% v/v dissolved in almond oil, 30 min daily for 3 days | Acetylcholinesterase inhibition | Anti-inflammatory, improves memory, and improves symptoms of Alzheimer’s disease | [33,34,35,36,37,38] |
| Mentha piperita | Menthol (40.7%), iso-menthone (23.4%) | Stem distillation of the leaves | 4 drops of oil in a diffuser pad followed by 5 min of inhalation 2500 µL capsules containing 50–100 µL of peppermint oil in vegetable oil | Binds to the nicotinic/GABAA receptor and inhibits acetylcholinesterase | CNS stimulation, antioxidant, and memory retention | [39,40,41,42,43,44,45] |
| Rosmarinus officinalis | p-Cymene (44.02%), linalool (20.5%) 1,8-cineole (26.54%), α-pinene (20.14%), | Hydro distillation of the aerial parts | 4 drops of oil in a diffuser pad followed by 5 min of inhalation | Improves DA activation and secretion | Anxiolytic, improves mood and cognitive function | [46,47,48,49,50] |
| Salvia sclarea | Camphor (12.8–21.4%), α-thujone (17.2–27.4%), 1–8, cineole (11.9–26.9%), | Hydro distillation of the aerial parts | 5 drops of EO in 5 mL of water along with an aroma stone | Acetylcholinesterase inhibition | Improves memory, mood, attention and is beneficial for mild to moderate severity of Alzheimer’s disease | [51,52,53,54,55] |
| Santalum paniculatum | α-santalol (34.5–40.4%) and β-santalol (16–24.10%) | Steam distillation of the heartwood and roots | 1 g/kg body weight of sandalwood oil in 5% Tween 80 in saline for a week | Acetylcholinesterase inhibition | Improves memory, prevents dementia, beneficial in Alzheimer’s disease | [56,57,58,59,60,61,62] |
| Plant/Source | Essential Oil | Test Subjects/Animal Model | Route of Administration Tested | Effective Dose/Preparation Technique | Experimental Outcome | Purpose of Use | References |
|---|---|---|---|---|---|---|---|
| Syzygium aromaticum | Clove oil | 90 BALB/male mice (27–32 g) | Intraperitoneal injection | 50,100, and 200 mg/kg of aqueous/ethanolic extract of clove in a final volume of 10 mL/kg | Maximal percent effect (MPE) of animals that were tested on hot plate and treated with oil was higher than that of the control group | Analgesic | [4] |
| Boswellia sacra, Boswellia frereana | Frankincense oil | Adult male Sprague Dawley sleep-deprived rats | Topical application | 50 μL in a 1:1000 dilution with jojoba oil on the nape of neck for 5 h with hourly intervals | Corticosterone and glutathione levels declined, wakefulness time increased, and non-rapid eye movement time declined | Antidepressant, mood elevation, anxiolytic, and stress relief | [8] |
| Lavandula angustifolia | Lavender oil | 200 pregnant women undergoing cesarean section | Olfactory administration | 2 drops (1% cc) of 2% lavender essence applied with a cotton swab to oxygen face mask, which was used for 3 min, repeated thrice over different time periods | Mean Visual Analogue Scale (VAS) decreased significantly, indicating amelioration of pain | Analgesic | [63] |
| Cymbopogon citratus | Lemongrass oil | 30-day old adult swiss male mice | Oral administration | Doses of 1, 5, and 10 mg/kg provided as well as repeated dosing for 14 days | Anxiolytic effects observed through results obtained in light/dark box test | Anxiolytic | [20] |
| Cananga odorata | Ylang ylang oil | 29 male participants | Olfactory administration | Participants placed in a closed room for 60 min that was previously fragranced with ylang ylang oil for 20 min. | Decline in systolic and diastolic BP and reduction in heart rate | Sedative effect and mood adjustment | [25] |
| Male and female mice weighing 25–30 g and 22–25 g, respectively | Olfactory administration | Stainless steel square inhalation apparatus (65 × 65 × 45 cm) with controllable heater to heat oil/water emulsion containing ylang ylang oil (1% v/v) and benzyl benzoate (2% v/v). Mice placed in chamber for 10 min | Male mice experienced more changes in concentration of neurotransmitters than female mice. Decline in DA in striatum and 5-HT concentration in hippocampus and decreased ratio of 5-HIAA/5-HT | Anxiolytic effect on male mice | [24] | ||
| Cinnamomum verum | Cinnamon oil | Male albino mice | Intraperitoneal injection | 0.5–2 mg/kg body weight three times a day or once daily for 14 days | Decreased immobility time in forced swim test (FST) and tail suspension test (TST) Mice treated with 2 mg/kg spent a longer time and showed more entries into the open arms of elevated plus-maze (EPM) | Antidepressant and anxiolytic | [29] |
| Eucalyptus globulus | Eucalyptus oil | 28 individuals with osteoarthritis that underwent total knee replacement surgery | Olfactory administration | 3% v/v was dissolved in almond oil, placed on 4 × 2 gauze pad, and inhaled for 30 min for 3 consecutive days | VAS scores after aromatherapy decreased. Heart rate increased to 0.3+/− 0.6 beats/min on day 1 and decreased to 1.7+/−1.7 beats/min and 0.6+/−1.0 beats/min on days 2 and 3, respectively | Analgesic, lowering BP, stress relief, and anxiolytic | [38] |
| Mentha piperita | Peppermint oil | 144 healthy individuals 24 participants (9 male/15 female) (mean age 25.2 years) | Olfactory administration Oral administration | 4 drops of oil in a diffuser pad followed by 5 min of inhalation 2500 µL capsules containing 50–100 µL of peppermint oil in vegetable oil | Enhanced alertness and memory 100 µL dose caused an improvement in rapid visual information processing task (RVIP) performance at 1 h and 3 h post-dose. Both doses decreased fatigue | Memory booster, modulated performance on cognitive tasks, and decreased mental fatigue | [42,45] |
| Rosemary oil | 20 healthy individuals | Olfactory administration | Inhalation of 10% v/v of the oil for 20 min using an oxygen pump attached to a respiratory mask whose airflow rate is 2 L/min | Decreased both powers of alpha1 and alpha2 waves | CNS stimulant | [64] | |
| Rosmarinus officinalis | 140 healthy individuals | Olfactory administration | 4 drops of oil in a diffuser pad followed by 5 min of inhalation | Mood elevation increased blood pressure, heart rate Improved mood and enhanced quality of memory | Memory enhancer | [62] | |
| Salvia sclarea | Sage oil | 45 healthy individuals 135 healthy individuals | Olfactory administration Olfactory administration | 5 drops of EO in 5 mL of water along with an aroma stone 5 drops of EO in 5 mL of water along with an aroma stone | Memory enhancement Improved and enhanced memory and secondary memory | Memory enhancement Memory enhancement | [52,65] |
| Santalum paniculatum | Sandalwood oil | D-galactose mediate oxidative stress-induced Swiss male albino mice (20–30 g) | Intraperitoneal administration | 1 g/kg body weight of sandalwood oil in 5% Tween 80 in saline for a week | Oxidative stress status ameliorated in group-administered sandalwood oil. Recovery of GSH, NO levels, catalase, and lipid peroxidation status in liver. Reduction in serum bilirubin, SGOT and SGPT. | Antioxidant | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, G.A.B.e.; Bhattacharya, T.; Chakrabarti, T.; Tagde, P.; Cavalu, S. Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants 2022, 11, 21. https://doi.org/10.3390/plants11010021
Soares GABe, Bhattacharya T, Chakrabarti T, Tagde P, Cavalu S. Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants. 2022; 11(1):21. https://doi.org/10.3390/plants11010021
Chicago/Turabian StyleSoares, Giselle A. Borges e, Tanima Bhattacharya, Tulika Chakrabarti, Priti Tagde, and Simona Cavalu. 2022. "Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System" Plants 11, no. 1: 21. https://doi.org/10.3390/plants11010021
APA StyleSoares, G. A. B. e., Bhattacharya, T., Chakrabarti, T., Tagde, P., & Cavalu, S. (2022). Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants, 11(1), 21. https://doi.org/10.3390/plants11010021








