Identification of Bacterial Wilt (Erwinia tracheiphila) Resistances in USDA Melon Collection
Abstract
1. Introduction
2. Results
2.1. Identification of Melon Lines for BW Resistance
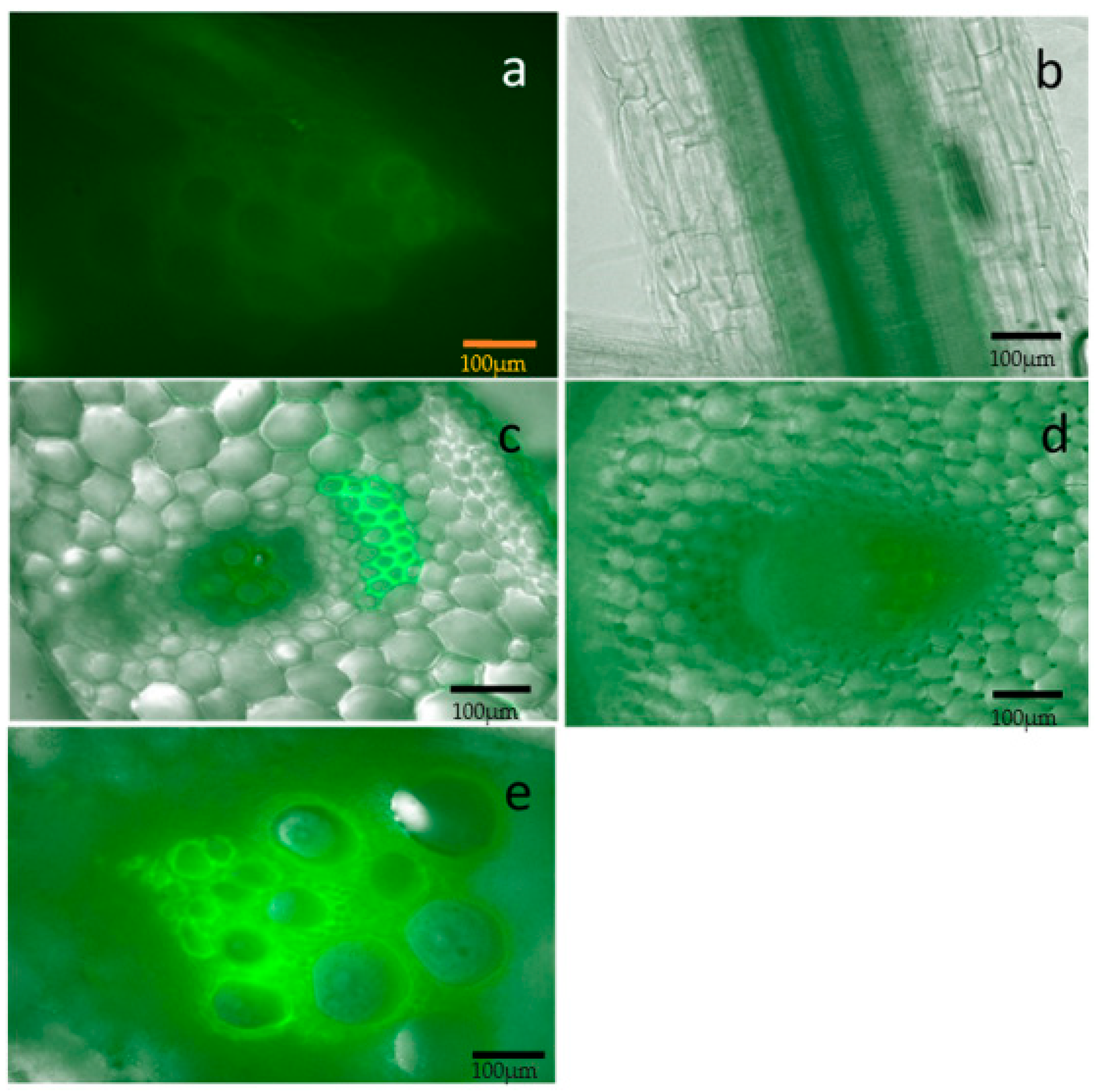
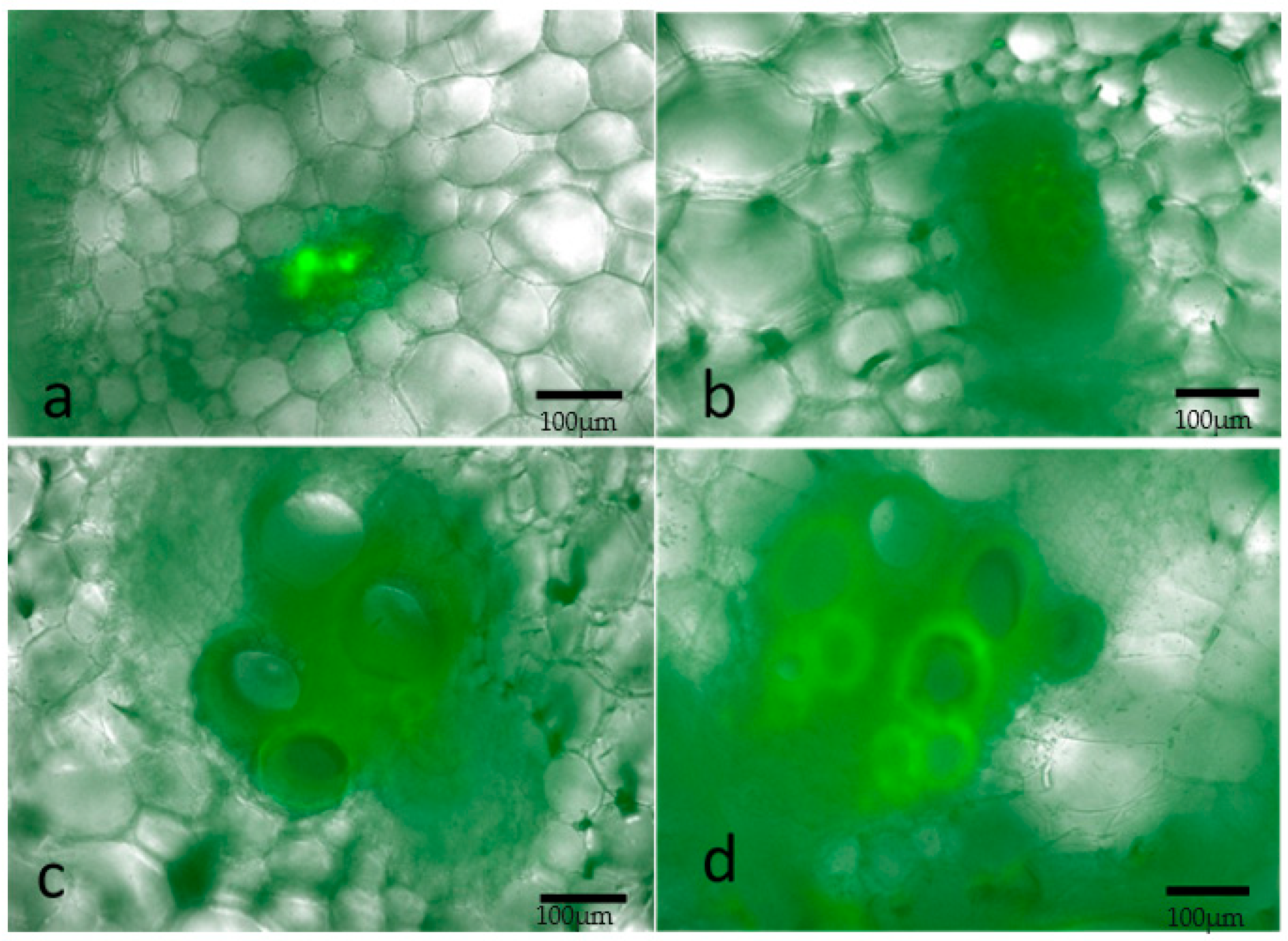
2.2. Localization of the Bacterium and Colonization in the Melon Host
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Bacterial Strain
4.2. Inoculation, Disease Screening and Statistical Analysis of Data
4.3. Fluorescence Microscopy
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deakin, J.R.; Bohn, G.W.; Whitaker, T.W. Interspecific hybridization in cucumis. Econ. Bot. 1971, 25, 195–211. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of cucurbits crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2019, 226, 1240–1255. [Google Scholar] [CrossRef]
- Dhaliwal, M.S. Cucurbits. In Handbook of Vegetable Crops; Kalyani Publishers: New Delhi, India, 2016; pp. 77–147. [Google Scholar]
- National Agricultural Statistics Service (N.A.S.S.). Vegetable 2019 Summary; USDA: Washington, DC, USA, 2020.
- Zitter, T.A.; Hopkins, D.L.; Thomas, C.E. Compendium of Cucurbit Diseases, 10th ed.; APS Press: St. Paul, MN, USA, 2010. [Google Scholar]
- Shapiro, L.R.; Batzer, J.C.; Beattie, G.A.; Fleischer, S.J.; Shapiro, L.R.; Williams, M.A.; Bessin, R.; Bruton, B.D.; Boucher, T.J.; Jesse, L.C.H.; et al. Draft genome sequence of Erwinia tracheiphila, an economically important bacterial pathogen of cucurbits. Genome Announc. 2015, 3, e00482-15. [Google Scholar] [CrossRef]
- Rojas, E.S.; Gleason, M.L.; Batzer, J.C.; Duffy, M. Feasibility of delaying removal of row covers to suppress bacterial wilt of muskmelon (Cucumis melo). Plant Dis. 2011, 95, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Brust, G.E. Differential susceptibility of pumpkins to bacterial wilt related to plant growth stage and cultivar. Crop Prot. 1997, 16, 411–414. [Google Scholar] [CrossRef]
- Shapiro, L.R.; Paulson, J.N.; Arnold, B.J.; Scully, E.D.; Zhaxybayeva, O.; Pierce, N.E.; Rocha, J.; Klepac-Ceraj, V.; Holton, K.; Kolter, R. An introduced crop plant is driving diversification of the virulent bacterial pathogen Erwinia tracheiphila. mBio 2018, 9, e01307-18. [Google Scholar] [CrossRef] [PubMed]
- Sasu, M.A.; Seidl-Adams, I.; Wall, K.; Winsor, J.A.; Stephenson, A.G. Floral transmission of Erwinia tracheiphila by cucumber beetles in a wild Cucurbita pepo. Environ. Entomol. 2010, 39, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.F.; Hanks, L.M. Insect frass as a pathway for transmission of bacterial wilt of cucurbits. Environ. Entomol. 2009, 38, 395–403. [Google Scholar] [CrossRef]
- Sanogo, S.; Etarock, B.F.; Clary, M. First report of bacterial wilt caused by Erwinia tracheiphila on pumpkin and watermelon in New Mexico. Plant Dis. 2011, 95, 1583. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, S.J.; de Mackiewicz, D.; Gildow, F.E.; Lukezic, F.L. Serological estimates of the seasonal dynamics of Erwinia tracheiphila in Acalymma vittata (Coleoptera: Chrysomelidae). Environ. Entomol. 1999, 28, 470–476. [Google Scholar] [CrossRef]
- Rojas, E.S.; Dixon, P.M.; Batzer, J.C.; Gleason, M.L. Genetic and virulence variability among Erwinia tracheiphila strains recovered from different cucurbit hosts. Phytopathology 2013, 103, 900–905. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snyder, W.E. Managing Cucumber Beetles in Organic Farming Systems; Oregon State University: Corvallis, OR, USA, 2019. [Google Scholar]
- Diver, S.; Hinman, T. Cucumber beetles: Organic and biorational integrated pest management. Natl. Cent. Appropr. Technol. 2008, 20. [Google Scholar]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, V.W.; Jasmin, J.J. The inheritance of resistance to bacterial wilt (Erwinia tracheiphila (E. F. SM) Holland) in cucumber. Can. J. Plant Sci. 1958, 38, 401–405. [Google Scholar] [CrossRef]
- Wilson, J.D.; John, C.A.; Walker, H.E.; Hoover, M.M. Two foreign cucumber resistant to bacterial wilt and powdery mildew. Plant Dis. Report. 1956, 40, 437–438. [Google Scholar]
- Wang, K.; Kang, L.; Anand, A.; Lazarovits, G.; Mysore, K.S. Monitoring in planta bacterial infection at both cellular and whole-plant levels using the green fluorescent protein variant GFPuv. New Phytol. 2007, 174, 212–223. [Google Scholar] [CrossRef]
- Gotz, M.; Gomes, N.C.; Dratwinski, A.; Costa, R.; Berg, G.; Peixoto, R.; Mendonça-Hagler, L.; Smalla, K. Survival of the gfp-tagged anatgonistic bacteria in the rhizosphere of the tomato plants and their effects on the indigenous bacterial community. FEMS Microbiol. Ecol. 2006, 56, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Sherf, A.F.; MacNab, A.A. Vegetable Diseases and Their Control; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Tomason, Y.; Nimmakayala, P.; Levi, A.; Reddy, U.K. Map-based molecular diversity, linkage disequilibrium and association mapping of fruit traits in melon. Mol. Breed. 2013, 31, 829–841. [Google Scholar] [CrossRef]
- Bahar, O.; Kritzman, G.; Burdman, S. Bacterial fruit blotch of melon: Screens for disease tolerance and role of seed transmission in pathogenicity. Eur. J. Plant Pathol. 2008, 123, 71–83. [Google Scholar] [CrossRef]
- Assunção, E.F.; da Conceição, C.S.; Alexandre, E.; Gama, M.A.S.; Nunes, G.H.; Souza, E.B. New sources of melon accessions with resistance to bacterial fruit blotch at different phenological stages of melon growth and to multiple strains of Acidovorax citrulli. Euphytica 2021, 217, 1–15. [Google Scholar] [CrossRef]
- Wechter, W.P.; Levi, A.; Ling, K.; Kousik, C.; Block, C. Identification of Resistance to Acidovorax avenae subsp. citrulli among Melon (Cucumis spp.) Plant Introductions. Hortscience 2011, 46, 207–212. [Google Scholar] [CrossRef]
- Islam, M.R.; Hossain, M.R.; Jesse, D.M.I.; Jung, H.-J.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Development of Molecular Marker Linked with Bacterial Fruit Blotch Resistance in Melon (Cucumis melo L.). Genes 2020, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Vrisman, C.M.; Deblais, L.; Rajashekara, G.; Miller, S.A. Differential colonization dynamics of cucurbit hosts by Erwinia tracheiphila. Phytopathology 2016, 106, 684–692. [Google Scholar] [CrossRef]
- Liu, Q.; Beattie, G.A.; Saalau Rojas, E.; Gleason, M.L. Bacterial wilt symptoms are impacted by host age and involve net downward movement of Erwinia tracheiphila in muskmelon. Eur. J. Plant Pathol. 2018, 151, 803–810. [Google Scholar] [CrossRef]
- Chatterjee, S.; Almeida, R.P.; Lindow, S. Living in two worlds: The plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 2008, 46, 243–271. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Purcell, A.H. Xylella fastidiosa: Cause of Pierce’s Disease of grapevine and other emergent diseases. Plant Dis. 2002, 86, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; González, V.M.; Hénaff, E.; Câmara, F.; Cozzuto, L.; Lowy, E.; et al. The genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA 2012, 109, 11872–11877. [Google Scholar] [CrossRef] [PubMed]
- Argyris, J.M.; Ruiz-Herrera, A.; Madriz-Masis, P.; Sanseverino, W.; Morata, J.; Pujol, M.; Ramos-Onsins, S.E.; Garcia-Mas, J. Use of targeted SNP selection for an improved anchoring of the melon (Cucumis melo L.) scaffold genome assembly. BMC Genom. 2015, 16, 4. [Google Scholar] [CrossRef]
- Ruggieri, V.; Alexiou, K.G.; Morata, J.; Argyris, J.; Pujol, M.; Yano, R.; Nonaka, S.; Ezura, H.; Latrasse, D.; Boualem, A.; et al. An improved assembly and annotation of the melon (Cucumis melo L.) reference genome. Sci. Rep. 2018, 8, 8088. [Google Scholar] [CrossRef]
- Nazareno, E.S.; Dumenyo, C.K. Modified inoculation and disease assessment methods reveal host specificity in Erwinia tracheiphila-Cucurbitaceae interactions. Microb. Pathog. 2015, 89, 184–187. [Google Scholar] [CrossRef] [PubMed]

| Summer 2019 (Experiment 1) | Autumn 2019 (Experiment 2) | ||||||
|---|---|---|---|---|---|---|---|
| Accessions | † DWILMean ± SD | † DWWP Mean ± SD | † DDP Mean ± SD | † DWIL Mean ± SD | † DWWP Mean ± SD | † DDP Mean ± SD | * Resistance Classification |
| Ames 13299 | 12.67 ± 2.31 a | 17.67 ± 6.66 a | 21 ± 9.64 abc | 6.67 ± 1.15 cdefghi | 16 ± 3 abcd | 19 ± 1.73 abcdef | H |
| PI 200814 | 4.0 ± 0 b | 8.67 ± 1.15 ghijkl | 11.33 ± 2.31 ijkl | 10.33 ± 0.58 abc | 14.33 ± 2.31 abcdefgh | 20.67 ± 3.21 abc | H |
| PI 230186 | 4.0 ± 0 b | 12 ± 0 cde | 18.0 ± 0 bcde | 12.67 ± 8.14 ab | 16 ± 6.93 abcd | 18.33 ± 7.57 abcdefg | H |
| PI 370441 | 4.67 ± 1.15 b | 9.67 ± 0.58 defghijkl | 11 ± 10.54 jkl | 13.33 ± 14.47 a | 18 ± 10.44 ab | 19.33 ± 9.24 abcde | H |
| PI 206043 ** | 4 ± 0 b | 12 ± 0 cde | 14.67 ± 0.58 efghij | 8 ± 0 cdefghi | 11.33 ± 1.53 efghijklmno | 17.33 ± 2.52 abcdefghij | L |
| Ames 2830 | 4.0 ± 0 b | 7.33 ± 0.58 lk | 10.0 ± 0 l | 7.33 ± 1.15 cdefghi | 14.67 ± 1.53 abcdefg | 15 ± 2 defghijklmnop | L |
| PI 197891 | 6.0 ± 2.31 b | 10.33 ± 0.58 defghij | 14.33 ± 0.58 efghijk | 9.67 ± 4.62 abcd | 14 ± 3.46 bcdefghi | 18 ± 3.46 abcdefgh | L |
| PI 199097 | 5.33 ± 2.31 b | 8.67 ± 1.15 ghijkl | 12.67 ± 2.31 ghijkl | 8.33 ± 1.15 cdefgh | 13 ± 2 cdefghijkl | 16.67 ± 1.53 abcdefghijkl | L |
| PI 200816 | 4.0 ± 0 b | 9.33 ± 1.15 efghijkl | 12.0 ± 2 hijkl | 8 ± 1 cdefghi | 13 ± 0 cdefghijkl | 18 ± 1.73 abcdefgh | L |
| PI 207660 | 6.0 ± 2.31 b | 9.33 ± 1.15 efghijkl | 12.67 ± 2.31 ghijkl | 8.33 ± 2.31 cdefgh | 13.67 ± 1.15 cdefghij | 19 ± 1.73 abcdef | L |
| PI 211936 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 6 ± 1.73 defghi | 12.33 ± 0.58 cdefghijklm | 16.33 ± 0.58 abcdefghijkl | L |
| PI 251778 | 4.0 ± 0 b | 8.67 ± 1.15 ghijkl | 10.67 ± 1.15 kl | 7.33 ± 0.58 cdefghi | 11.67 ± 3.51 efghijklmn | 17 ± 2.65 abcdefghijk | L |
| PI 255948 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 21 ± 0 abc | 7 ± 0 cdefghi | 8 ± 0 nop | 14.33 ± 1.15 efghijklmnop | L |
| PI 261644 | 5.33 ± 2.31 b | 14 ± 1.73 bc | 23 ± 0 a | 4.33 ± 0.58 i | 5.33 ± 0.58 p | 8 ± 0 s | L |
| PI 277281 | 4.0 ± 0 b | 8.67 ± 1.15 ghijkl | 10.67 ± 1.15 kl | 6 ± 0 defghi | 13.33 ± 0.58 cdefghijk | 18 ± 3.46 abcdefgh | L |
| PI 344068 | 4.0 ± 0 b | 10 ± 0 defghijk | 14.67 ± 2.52 efghij | 6.67 ± 1.15 cdefghi | 12.33 ± 4.04 cdefghijklm | 17 ± 5.20 abcdefghijk | L |
| PI 378558 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 21.67 ± 1.15 ab | 6 ± 2 defghi | 11.67 ± 2.31 edfghijklmn | 13.33 ± 2.08 ghijklmnopqr | L |
| PI 401603 | 4.0 ± 0 b | 10.67 ± 1.15 defghi | 19.67 ± 1.15 abcd | 6 ± 0 defghi | 9.67 ± 1.15 jklmno | 11.67 ± 1.15 lmnopqrs | L |
| Ames 512543 | 4.0 ± 0 b | 7.67 ± 0.58 lkj | 11.33 ± 2.31 ijkl | 5 ± 1.73 ghi | 11 ± 2 fghijklmno | 19.67 ± 4.93 abcd | L |
| PI 500365 ** | 5 ± 1.73 b | 16 ± 3.46 ab | 19.67 ± 2.31 abcd | 7.33 ± 1.15 cdefghi | 14 ± 3 bcdefghi | 19 ± 3.61 abcdef | M |
| PI 344436 | 6.0 ± 2.31 b | 9.67 ± 0.58 defghijkl | 12.67 ± 1.15 ghijkl | 6 ± 0 defghi | 18.33 ± 1.53 a | 20.67 ± 1.15 abc | M |
| PI 378060 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 8.33 ± 4.04 cdefgh | 16 ± 12.12 abcd | 17.33 ± 10.97 abcdefghij | M |
| PI 618838 | 5.33 ± 2.31 b | 7.67 ± 0.58 lkj | 10.0 ± 0 l | 6 ± 0 defghi | 16.33 ± 1.15 abc | 21.33 ± 3.79 a | M |
| Ames 13247 ** | 4 ± 0 b | 10.0 ± 2 defghijk | 12.33 ± 2.52 ghijkl | 7.33 ± 1.15 cdefghi | 10.67 ± 0.58 ghijklmno | 13.67 ± 1.15 ghijklmnopq | S |
| Ames 13251 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 12 ± 0 hijkl | 8 ± 0 cdefghi | 10.67 ± 0.58 ghijklmno | 16 ± 3.61 bcdefghijklm | S |
| Ames 13264 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 12.0 ± 0 hijkl | 6 ± 2 defghi | 7.33 ± 9.06 op | 11 ± 3 mnopqrs | S |
| Ames 13268 | 4.0 ± 0 b | 10.67 ± 1.15 defghi | 12.67 ± 1.15 ghijkl | 6.33 ± 2.89 defghi | 9.33 ± 2.89 klmnop | 12 ± 3.46 klmnopqrs | S |
| Ames 13270 | 4.0 ± 0 b | 10.67 ± 1.15 defghi | 12.67± 1.15 ghijkl | 6.67 ± 2.31 cdefghi | 10.67 ± 0.58 ghijklmno | 15 ± 4.58 defghijklmnop | S |
| Ames 13285 | 6.0 ± 2.31 b | 8.67 ± 1.15 ghijkl | 11.33 ± 2.31 ijkl | 5 ± 3.61 ghi | 9.67 ± 5.13 jklmno | 14.67 ± 7.64 defghijklmnop | S |
| Ames 13305 | 4.0 ± 0 b | 10.0 ± 3.46 defghijk | 12.67 ± 4.62 ghijkl | 5.33 ± 1.15 fghi | 12 ± 2.65 defghijklmn | 15 ± 1.73 defghijklmnop | S |
| Ames 13332 | 6.0 ± 2.31 b | 12 ± 2 cde | 17.33 ± 4.93 cdef | 8 ± 0 cdefghi | 12.33 ± 2.08 cdefghijklm | 14.67 ± 4.04 defghijklmnop | S |
| Ames 19036 | 4.0 ± 0 b | 9.67 ± 2.08 defghijkl | 14.33 ± 3.46 efghijk | 6.67 ± 1.15 cdefghi | 13.33 ± 0.58 cdefghijk | 15.67 ± 1.15 cdefghijklmn | S |
| Ames 20203 | 4.0 ± 0 b | 9.33 ± 1.15 efghijkl | 11.33 ± 1.15 ijkl | 6 ± 0 defghi | 9.67 ± 1.15 jklmno | 10.67 ± 2.08 nopqrs | S |
| Ames 20219 | 4.0 ± 0 b | 7.0 ± 0 l | 10.0 ± 0 l | 6.67 ± 1.15 cdefghi | 9 ± 0 lmnop | 13 ± 0 hijklmnopqrs | S |
| NSL 5648 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 8 ± 0 cdefghi | 9.33 ± 0.58 klmnop | 10.33 ± 0.58 opqrs | S |
| NSL 8521 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 5.33 ± 1.15 fghi | 9 ± 0 lmnop | 9 ± 0 qrs | S |
| PI 193495 | 5.0 ± 0.73 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 6 ± 0 defghi | 9 ± 0 lmnop | 9 ± 0 qrs | S |
| PI 197077 | 5.33 ± 2.31 b | 8.67 ± 1.15 ghijkl | 10.67 ± 1.15 kl | 5.33 ± 3.06 fghi | 10.33 ± 1.15 hijklmno | 13.67 ± 3.51 ghijklmnopq | S |
| PI 204691 | 4.0 ± 0 b | 8.67 ± 0.58 ghijkl | 11.33 ± 1.15 ijkl | 7.67 ± 1.15 cdefghi | 10.67 ± 1.15 ghijklmno | 13 ± 0 hijklmnopqrs | S |
| PI 210768 | 4.0 ± 0 b | 10 ± 0 defghijk | 18.0 ± 3 bcde | 7 ± 0 cdefghi | 12 ± 0 defghijklmn | 16 ± 0 bcdefghijklm | S |
| PI 211923 | 4.0 ± 0 b | 8.33 ± 0.58 hijkl | 11.33 ± 1.15 ijkl | 9 ± 1.73 bcdef | 11.33 ± 1.15 edfghijklmno | 15 ± 1.73 defghijklmnop | S |
| PI 211946 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 12.67 ± 1.15 ghijkl | 5 ± 1.73 ghi | 8.67 ± 0.58 mnop | 11.67 ± 2.31 lmnopqrs | S |
| PI 211957 | 4.0 ± 0 b | 12 ± 3 cde | 17.33 ± 4.73 cdef | 6 ± 0 defghi | 10 ± 0 ijklmno | 11 ± 0 mnopqrs | S |
| PI 213247 | 4.0 ± 0 b | 11.67 ± 2.89 cdef | 16 ± 4.58 defg | 4.67 ± 1.15 hi | 10.33 ± 2.31 hijklmno | 11 ± 2.65 mnopqrs | S |
| PI 218071 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 5.33 ± 1.15 fghi | 9 ± 0 lmnop | 9 ± 0 qrs | S |
| PI 222098 | 4.0 ± 0 b | 8.33 ± 0.58 hijkl | 10.67 ± 1.15 kl | 6.67 ± 1.15 cdefghi | 9.67 ± 1.15 jklmno | 13.33 ± 5.86 ghijklmnopqr | S |
| PI 223770 | 4.0 ± 0 b | 7.67 ± 0.58 lkj | 10.0 ± 0 l | 6 ± 0 defghi | 9.67 ± 0.58 jklmno | 15 ± 1.73 defghijklmno | S |
| PI 224770 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 5.33 ± 3.79 fghi | 9 ± 1.73 lmnop | 12 ± 0 klmnopqrs | S |
| PI 266932 | 4.0 ± 0 b | 12 ± 0 cde | 14.0 ± 0 fghijk | 7.33 ± 0.58 cdefghi | 10.33 ± 0.58 hijklmno | 14 ± 1.73 fghijklmnopoq | S |
| PI 266942 | 6.67 ± 2.31 b | 10.0 ± 2 defghijk | 15.33 ± 3.51 efgh | 6 ± 3 defghi | 7.33 ± 3.51 op | 8.33 ± 3.51 rs | S |
| PI 277280 | 4.0 ± 0 b | 9.33 ± 2.31 efghijkl | 14.67 ± 1.15 efghij | 9 ± 1 bcdef | 10.67 ± 0.58 ghijklmno | 15 ± 1.73 defghijklmnop | S |
| PI 302446 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 6 ± 0 defghi | 12 ± 5.20 defghijklmn | 13.33 ± 5.77 ghijklmnopqr | S |
| PI 344345 | 4.0 ± 0 b | 9.33 ± 1.15 efghijkl | 15.33 ± 5.51 efgh | 5.33 ± 1.15 fghi | 9.33 ± 0.58 klmnop | 13.33 ± 0.58 ghijklmnopqr | S |
| PI 357756 | 6.33 ± 1.15 b | 10.0 ± 0 defghijk | 14.0 ± 1.73 fghijk | 5 ± 3.61 ghi | 9.33 ± 6.35 klmnop | 12.33 ± 3.79 jklmnopqrs | S |
| PI 357783 | 6.0 ± 0 b | 10.0 ± 0 defghijk | 12.0 ± 0 hijkl | 6 ± 0 defghi | 10.33 ± 1.15 hijklmno | 14.33 ± 0.58 efghijklmnop | S |
| PI 401600 | 4.0 ± 0 b | 9.33 ± 1.15 efghijkl | 15.33 ± 4.62 efgh | 6 ± 0 defghi | 11.67 ± 1.15 efghijklmn | 14 ± 1 fghijklmnop | S |
| PI 419220 | 4.0 ± 0 b | 11.32 ± 2.31 cdefg | 15 ± 1.73 efghi | 6 ± 0 defghi | 9 ± 0 lmnop | 13.33 ± 0.58 ghijklmnopqr | S |
| PI 482400 | 4.0 ± 0 b | 7.0 ± 0 l | 10.0 ± 0 l | 6 ± 0 defghi | 10.67 ± 2.89 ghijklmno | 15.33 ± 5.13 defghijklmno | S |
| PI 502329 | 5.67 ± 2.31 b | 12.33 ± 4.93 cd | 15 ± 5.20 efghi | 6 ± 3.46 defghi | 11 ± 0 fghijklmno | 14.33 ± 2.31 efghijklmnop | S |
| PI 505611 | 6.67 ± 2.31 b | 9.33 ± 2.31 efghijkl | 12.0 ± 2 hijkl | 6 ± 0 defghi | 9.67 ± 1.15 jklmno | 12 ± 2.65 klmnopqrs | S |
| PI 601164 | 4.67 ± 1.15 b | 8.67 ± 1.15 ghijkl | 11.33 ± 2.31 ijkl | 8 ± 0 cdefghi | 9 ± 0 lmnop | 13 ± 1.73 hijklmnopqrs | S |
| PI 614161 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 8 ± 0 cdefghi | 8.67 ± 0.58 mnop | 10 ± 1 pqrs | S |
| PI 618819 | 4.0 ± 0 b | 8.67 ± 1.15 ghijkl | 10.67 ± 1.15 kl | 8.67 ± 1.15 cdefg | 11.67 ± 1.15 efghijklm | 14.33 ± 2.31 efghijklmnop | S |
| PI 505598 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | 6.33 ± 2.89 defghi | 10.33 ± 2.31 hijklmno | 15.67 ± 4.62 cdefghijklmn | S |
| Accessions | † DWIL Mean ± SD | † DWWP Mean ± SD | † DDP Mean ± SD | Experiment | * Resistance Classification |
|---|---|---|---|---|---|
| PI 229309 ** | 8 ± 0 cdefghi | 12.33 ± 2.08 cdefghijklm | 14.33 ± 3.51 efghijklmnop | Autumn 2019 | S |
| PI 207659 | 4.0 ± 0 b | 14 ± 0 bc | 21 ± 0 abc | Summer 2019 | L |
| Ames 13248 | 5.33 ± 4.04 fghi | 9 ± 6.24 lmnop | 17.33 ± 3.51 abcdefghij | Autumn 2019 | L |
| Ames 13321 | 8 ± 0 cdefghi | 14 ± 5.20 bcdefghi | 19 ± 1.73 abcdef | Autumn 2019 | L |
| PI 207661 | 7.67 ± 0.58 cdefghi | 13 ± 0 cdefghijkl | 17 ± 0 abcdefghijk | Autumn 2019 | L |
| PI 211948 | 7 ± 0 cdefghi | 12 ± 0 defghijklmn | 18 ± 2 abcdefgh | Autumn 2019 | L |
| PI 236355 | 9.33 ± 2.08bcde | 14 ± 1.73 bcdefghi | 21 ± 4.36 ab | Autumn 2019 | L |
| PI 292312 | 6 ± 0 defghi | 13.33 ± 0.58 cdefghijk | 16.67 ± 2.89 abcdefghijkl | Autumn 2019 | L |
| PI 403994 | 7.33 ± 1.53 cdefghi | 11.33 ± 0.58 efghijklmno | 19.67 ± 4.51 abcd | Autumn 2019 | L |
| PI 211016 | 7 ± 0 cdefghi | 15.33 ± 2.52 abcde | 21 ± 3.46 ab | Autumn 2019 | M |
| PI 502328 | 6 ± 0 defghi | 15 ± 2 abcdef | 17.67 ± 3.51 abcdefghi | Autumn 2019 | M |
| Ames 2822 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | Summer 2019 | S |
| Ames 2824 | 4.0 ±0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | Summer 2019 | S |
| Ames 2826 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 12.0 ± 0 hijkl | Summer 2019 | S |
| Ames 13261 | 4.0 ± 0 b | 9.0 ± 1.73 fghijkl | 12.67 ± 1.15 ghijkl | Summer 2019 | S |
| Ames 18738 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 15 ± 0 efghi | Summer 2019 | S |
| PI 183676 | 4.0 ± 0 b | 9.67 ± 1.53 defghijkl | 16 ± 6.56 defg | Summer 2019 | S |
| PI 211922 | 4.0 ± 0 b | 8.0± ijkl | 10.0 ± 0 l | Summer 2019 | S |
| PI 218070 | 4.0 ± 0 b | 11 ± 1 defgh | 15 ± 0 efghi | Summer 2019 | S |
| PI 223636 | 4.0 ± 0 b | 7.67 ± 0.58 lkj | 10.0 ± 0 l | Summer 2019 | S |
| PI 26443 | 4.67 ± 1.15 b | 9.33 ± 1.15 efghijkl | 12.0 ± 2 hijkl | Summer 2019 | S |
| PI 244713 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 14.0 ± 0 fghijk | Summer 2019 | S |
| PI 266946 | 4.0 ± 0 b | 10.0 ± 2 defghijk | 13.33 ± 1.15 ghijkl | Summer 2019 | S |
| PI 267083 | 4.0 ± 0 b | 11.33 ± 2.31 cdefg | 15 ± 5.20 efghi | Summer 2019 | S |
| PI 271329 | 4.0 ± 0 b | 7.0 ± 0 l | 10.0 ± 0 l | Summer 2019 | S |
| PI 344318 | 4.0 ± 0 b | 8.33 ± 0.58 hijkl | 10.67 ± 1.15 kl | Summer 2019 | S |
| PI 370021 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | Summer 2019 | S |
| PI 401655 | 4.0 ± 0 b | 10.0 ± 0 defghijk | 13.67 ± 1.53 fghijkl | Summer 2019 | S |
| PI 504527 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | Summer 2019 | S |
| PI 614159 | 4.0 ± 0 b | 10.67 ± 2.31 defghi | 12.67 ± 2.31 ghijkl | Summer 2019 | S |
| Ames 13302 | 4.0 ± 0 b | 8.67 ± 1.15 ghijkl | 11.33 ± 2.31 ijkl | Summer 2019 | S |
| Ames 13304 | 4.0 ± 0 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | Summer 2019 | S |
| Ames 13317 | 4.0 ± 0 b | 8.67 ± 1.15 ghijkl | 10.67 ± 1.15 kl | Summer 2019 | S |
| Ames 13318 | 5.33 ± 2.31 b | 8.0 ± 0 ijkl | 10.0 ± 0 l | Summer 2019 | S |
| Ames 13257 ** | 6 ± 0 defghi | 9.67 ± 1.15 jklmno | 14.67 ± 2.08 defghijklmnop | Autumn 2019 | S |
| Ames 13292 | 6.67 ± 1.15 cdefghi | 12 ± 1.73 defghijklmn | 13 ± 1.73 hijklmnopqrs | Autumn 2019 | S |
| Ames 13295 | 8 ± 0 cdefghi | 12 ± 1.73 defghijklmn | 15.67 ± 4.04 cdefghijklmn | Autumn 2019 | S |
| Ames 13303 | 6 ± 0 defghi | 10.33 ± 2.31 hijklmno | 14.33 ± 2.31 efghijklmnop | Autumn 2019 | S |
| Ames 13319 | 8 ± 0 cdefghi | 12 ± 1.73 defghijklmn | 16 ± 1.73 bcdefghijklm | Autumn 2019 | S |
| Ames 13325 | 5.67 ± 2.52 efghi | 12.67 ± 2.31 cdefghijklm | 15.33 ± 2.52 defghijklmno | Autumn 2019 | S |
| Ames 13337 | 6 ± 0 defghi | 9.67 ± 1.15 jklmno | 14 ± 0 fghijklmnopq | Autumn 2019 | S |
| PI 210541 | 8.33 ± 0.58 cdefgh | 12 ± 0 defghijklmn | 16 ± 0 bcdefghijklm | Autumn 2019 | S |
| PI 212639 | 7.33 ± 1.15 cdefghi | 10.33 ± 0.58 hijklmno | 13 ± 0 hijklmnopqrs | Autumn 2019 | S |
| PI 229750 | 7.67 ± 0.58 cdefghi | 9 ± 1 lmnop | 13.33 ± 2.31 ghijklmnopqr | Autumn 2019 | S |
| PI 266943 | 7.33 ± 0.58 cdefghi | 12 ± 0 defghijklmn | 15 ± 0 defghijklmnop | Autumn 2019 | S |
| PI 319217 | 6 ± 0 defghi | 10.33 ± 0.58 hijklmno | 13.33 ± 0.58 ghijklmnopqr | Autumn 2019 | S |
| PI 319218 | 6 ± 0 defghi | 10 ± 1.73 ijklmno | 12.33 ± 2.89 jklmnopqrs | Autumn 2019 | S |
| PI 344346 | 5.33 ± 2.31 fghi | 10.33 ± 1.15 hijklmno | 13.33 ± 0.58 ghijklmnopqr | Autumn 2019 | S |
| PI 355715 | 6 ± 0 defghi | 10 ± 1 ijklmno | 15.67 ± 1.15 cdefghijklmn | Autumn 2019 | S |
| PI 357758 | 6 ± 0 defghi | 12.33 ± 1.15 cdefghijklm | 15.67 ± 1.15 cdefghijklmn | Autumn 2019 | S |
| PI 378059 | 6 ± 2 defghi | 9.33 ± 6.35 klmnop | 10.33 ± 2.31 opqrs | Autumn 2019 | S |
| PI 391574 | 6.67 ± 2.31 cdefghi | 12 ± 1.73 defghijklmn | 13 ± 1.73 hijklmnopqrs | Autumn 2019 | S |
| PI 406737 | 7.67 ± 1.15 cdefghi | 10.33 ± 0.58 hijklmno | 12 ± 0 klmnopqrs | Autumn 2019 | S |
| PI 420146 | 6 ± 0 defghi | 9 ± 0 lmnop | 12.33 ± 2.89 jklmnopqrs | Autumn 2019 | S |
| PI 482396 | 6 ± 0 defghi | 12.67± 4.73 cdefghijklm | 13.33 ± 0.58 ghijklmnopqr | Autumn 2019 | S |
| PI 482397 | 6.67 ± 1.15 cdefghi | 9.33 ± 0.58 klmnop | 13.33 ± 0.58 ghijklmnopqr | Autumn 2019 | S |
| PI 482398 | 7.33 ± 4.04 cdefghi | 9.67 ± 2.89 jklmno | 14.33 ± 2.31 efghijlkmnop | Autumn 2019 | S |
| PI 505612 | 8 ± 0 cdefghi | 9 ± 0 lmnop | 12.67 ± 2.31 ijklmnopqrs | Autumn 2019 | S |
| PI 512442 | 6 ± 0 defghi | 9 ± 0 lmnop | 13.67 ± 3.06 ghijklmnopq | Autumn 2019 | S |
| Resistance Level | Number of Accessions Screened in Both Experiments | Number of Accessions Screened in One Experiment | Total |
|---|---|---|---|
| Susceptible (S) | 41 | 49 | 90 |
| Low (L) | 15 | 8 | 23 |
| Medium (M) | 4 | 2 | 6 |
| High (H) | 4 | 0 | 4 |
| Total | 64 | 59 | 123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, B.; Mackasmiel, L.; Taheri, A.; Ondzighi-Assoume, C.A.; Weng, Y.; Dumenyo, C.K. Identification of Bacterial Wilt (Erwinia tracheiphila) Resistances in USDA Melon Collection. Plants 2021, 10, 1972. https://doi.org/10.3390/plants10091972
Acharya B, Mackasmiel L, Taheri A, Ondzighi-Assoume CA, Weng Y, Dumenyo CK. Identification of Bacterial Wilt (Erwinia tracheiphila) Resistances in USDA Melon Collection. Plants. 2021; 10(9):1972. https://doi.org/10.3390/plants10091972
Chicago/Turabian StyleAcharya, Bimala, Lucas Mackasmiel, Ali Taheri, Christine A. Ondzighi-Assoume, Yiqun Weng, and C. Korsi Dumenyo. 2021. "Identification of Bacterial Wilt (Erwinia tracheiphila) Resistances in USDA Melon Collection" Plants 10, no. 9: 1972. https://doi.org/10.3390/plants10091972
APA StyleAcharya, B., Mackasmiel, L., Taheri, A., Ondzighi-Assoume, C. A., Weng, Y., & Dumenyo, C. K. (2021). Identification of Bacterial Wilt (Erwinia tracheiphila) Resistances in USDA Melon Collection. Plants, 10(9), 1972. https://doi.org/10.3390/plants10091972







