Morphological, Physiological and Photophysiological Responses of Critically Endangered Acer catalpifolium to Acid Stress
Abstract
:1. Introduction
2. Results
2.1. Response of Morphological Characteristics of Acer catalpifolium to Different Forms of Acid Stress
2.1.1. Response of Plant Height to Different Forms of Acid Stress
2.1.2. Response of Diameter of Ground Stem to Different Forms of Acid Stress
2.1.3. Response of Crown to Different Forms of Acid Stress
2.1.4. Leaf Morphological Characteristics
2.2. Growth Response of Acer catalpifolium to Different Forms of Acid Stress
2.3. Effects of Different Forms of Acid Stress on Photosynthetic Characteristics of Acer catalpifolium
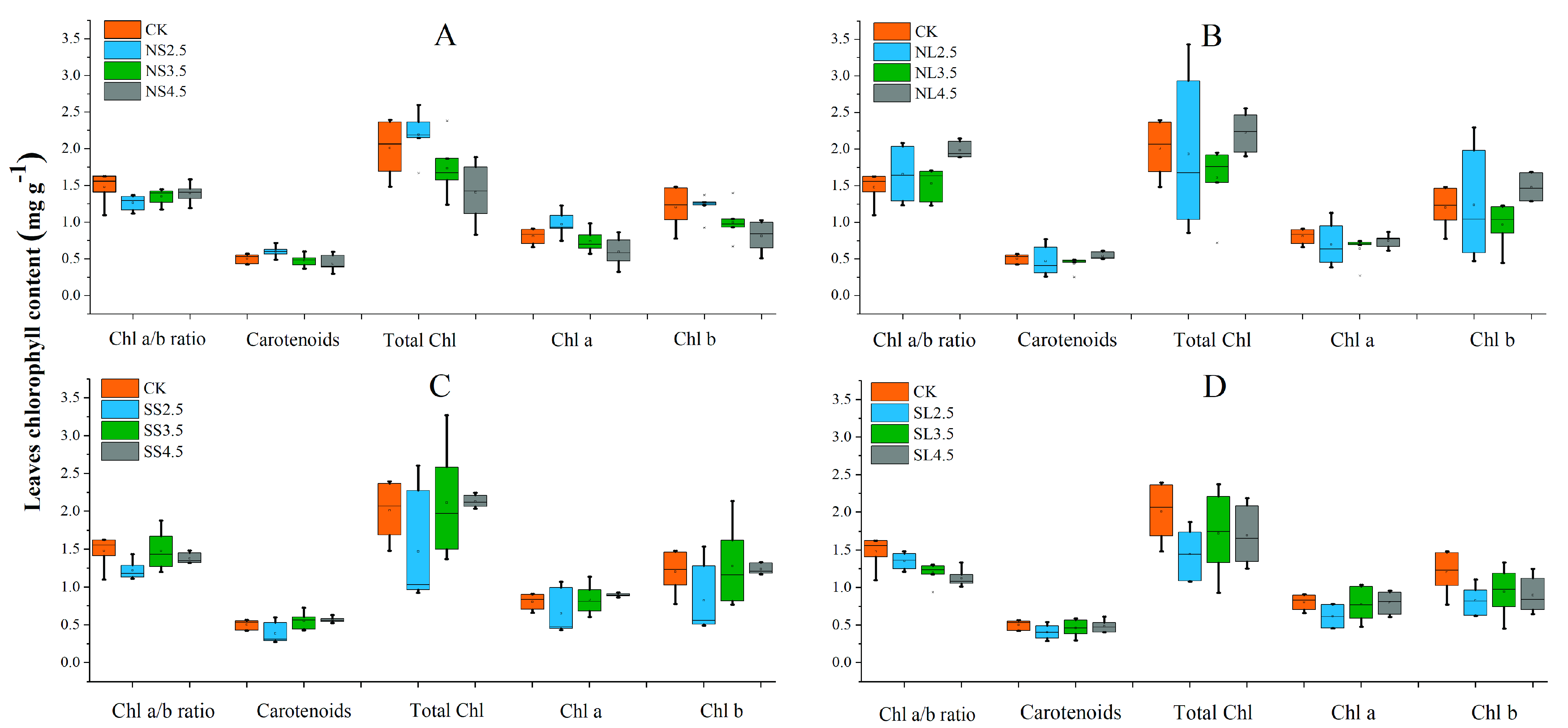
2.4. Effects of Different Forms of Acid Stress on Chlorophyll Content of Acer catalpifolium
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Growth Parameters
4.4. Photosynthetic Characteristics
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, X.Q.; Zhou, J.; Corsetti, G. How well have China’s recent five-year plans been implemented for energy conservation and air pollution control? Environ. Sci. Technol. 2014, 48, 10036–10044. [Google Scholar] [CrossRef]
- Du, E.Z.; Dong, D.; Zeng, X.T.; Sun, Z.Z.; Jiang, X.F.; Vries, W. Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China. Sci. Total Environ. 2017, 605–606, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.F.; Ding, H.M.; Feng, L.L.; Chen, J.J.; Yang, S.Y.; Wang, X.H. Photosynthetic and growth responses of Schima superba seedlings to sulfuric and nitric acid depositions. Environ. Sci. Pollut. R 2016, 23, 8644–8658. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, J.; Im, K.; Kim, K.; Lee, J.; Lee, K.; Park, J.; Lee, T.; Park, D.; Yang, J.; et al. Arabidopsis leaf necrosis caused by simulated acid rain is related to the salicylic acid signaling pathway. Plant Physiol. Biochem. 2006, 44, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.H.; Liu, T.W.; Wu, F.H.; Zheng, H.L. Photosynthetic and antioxidant responses of Liquidambar formosana and Schima superba seedlings to sulfuric-rich and nitric-rich simulated acid rain. Plant Physiol. Biochem. 2013, 64, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Neves, N.R.; Oliva, M.A.; Centeno, D.C.; Costa, A.; Ribas, R.F.; Pereira, E.G. Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: Potential use in environmental risk assessment. Sci. Total Environ. 2009, 407, 3740–3745. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Zhou, G.Y.; Yang, C.W.; Ou, Z.Y.; Peng, C.L. Responses of chlorophyll fluorescence and xanthophyll cycle in leaves of Schima superba Gardn. & Champ. and Pinus massoniana Lamb. to simulated acid rain at Dinghushan Biosphere Reserve, China. Acta Physiol. Plant. 2007, 29, 33–38. [Google Scholar]
- Thelin, G.; Rosengren-Brinck, U.; Bengt, N.; Barkman, A. Trends in needle and soil chemistry of Norway spruce and Scots pine stands in South Sweden 1985–1994. Environ. Pollut. 1998, 99, 149–158. [Google Scholar] [CrossRef]
- Huhn, G.; Schulz, H. Contents of free amino acids in Scots pine needles from field sites with different levels of nitrogen deposition. New Phytol. 1996, 134, 95–101. [Google Scholar] [CrossRef]
- Ma, L.Y.; Wang, B.; Yang, J.G. Spatial-temporal distribution of acid rain in Sichuan province. Environ. Sci. Manage. 2008, 33, 26–29. (in Chinese). [Google Scholar]
- Du, E.Z.; Vries, W.; Liu, X.J.; Fang, J.Y.; Galloway, J. Spatial boundary of urban ‘acid islands’ in China. Sci. Rep. 2015, 5, 12625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Wang, Y.; Li, H.Y.; Yang, X.Q.; Sun, L.; Wang, X.F.; Tao, W.; Wang, W.X. Organic acids in cloud water and rainwater at a mountain site in acid rain areas of South China. Environ. Sci. Pollut. R 2016, 23, 9529–9539. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, W.; Meng, M.J.; Fu, Z.Y.; Xu, L.H.; Zha, Y.; Yue, J.M.; Zhang, J.C. Comparative effects of simulated acid rain of different ratios of SO42- to NO3− on fine root in subtropical plantation of China. Sci. Total Environ. 2018, 618, 336–346. [Google Scholar] [CrossRef]
- Duan, L.; Chen, X.; Ma, X.X.; Zhao, B.; Larssen, T.; Wang, S.X.; Ye, Z.X. Atmospheric S and N deposition relates to increasing riverine transport of S and N in southwest China: Implications for soil acidification. Environ. Pollut. 2016, 218, 1191–1199. [Google Scholar] [CrossRef]
- Zhu, Q.; Vries, W.; Liu, X.J.; Zeng, M.F.; Hao, T.X.; Du, E.Z.; Zhang, F.S.; Shen, J.B. The contribution of atmospheric deposition and forest harvesting to forest soil acidification in China since 1980. Atmos. Environ. 2016, 146, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Duan, L.; Mo, J.M.; Du, E.Z.; Shen, J.L.; Lu, X.K.; Zhang, Y.; He, C.E.; Zhang, F.S. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef]
- Larssen, T.; Lydersen, E.; Tang, D.G.; He, Y.; Gao, J.X.; Liu, H.Y.; Duan, L.; Seip, H.M.; Vogt, R.D.; Mulder, J.; et al. Acid rain in China. Environ. Sci. Technol. 2006, 40, 418–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, K.; Peng, Y. Biodioersity ecologyical risk assessment in Wuling Mnontainous area. J. Minzu Univ. China 2013, 22, 89–93. (in Chinese). [Google Scholar]
- Sanders, D.; Thébault, E.; Kehoe, R.; Veen, F. Trophic redundancy reduces vulnerability to extinction cascades. Proc. Natl. Acad. Sci. USA 2018, 115, 201716825. [Google Scholar] [CrossRef] [Green Version]
- State Forestry Administration. China’s Key Protected Wild Plant Resources Investigation; China Forestry Publishing House: Beijing, China, 2009; p. 2. [Google Scholar]
- Zhang, Y.Y.; Ma, W.B.; Yu, T.; Ji, H.J.; Gao, J.; Li, J.Q.; Gao, S.; Ke, L. Population structure and community characteristics of Acer catalpifolium Rehd. Chin. J. Appl. Environ. Biol. 2018, 24, 697–703. (in Chinese). [Google Scholar]
- Ma, W.B.; Xu, G.; Ji, H.J.; Liu, X.L. Preliminary study on germination characteristics of the rare plant Acer catalpifolium. Seed 2014, 33, 87–90. (in Chinese). [Google Scholar]
- Liu, M.H.; Korpelainen, H.; Dong, L.C.; Yi, L.T. Physiological responses of Elaeocarpus glabripetalus seedlings exposed to simulated acid rain and cadmium. Ecotoxicol. Environ. Saf. 2019, 175, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Yi, L.T.; Yu, S.Q.; Yu, F.; Yin, X.M. Chlorophyll fluorescence characteristics and the growth response of Elaeocarpus glabripetalusto simulated acid rain. Photosynthetica 2015, 53, 23–28. [Google Scholar] [CrossRef]
- Ohno, T.; Amirbahman, A. Phosphorus availability in boreal forest soils: A geochemical and nutrient uptake modeling approach. Geoderma 2010, 155, 46–54. [Google Scholar] [CrossRef]
- Yang, J.E.; Lee, W.Y.; Yong, S.O.; Skousen, J. Soil nutrient bioavailability and nutrient content of pine trees (Pinus thunbergii) in areas impacted by acid deposition in Korea. Environ. Monit. Assess. 2009, 157, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, C. Enhancing tolerance of rice (Oryza sativa) to simulated acid rain by exogenous abscisic acid. Environ. Sci. Pollut. Res. 2017, 24, 4860–4870. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Z.Y.; Zhang, B.; Zhai, L.; Meng, M.J.; Lin, J.; Zhuang, J.Y.; Wang, G.G.; Zhang, J.C. Effects of sulfuric, nitric, and mixed acid rain on Chinese fir sapling growth in Southern China. Ecotoxicol. Environ. Saf. 2018, 160, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Irshad, M.; Mitra, S.; Li, M.; Rizwan, H.M.; Liu, S.; Pan, T.F.; Qiu, D.L. Acid rain deposition modulates photosynthesis, enzymatic and non-enzymatic antioxidant activities in tomato. Int. J. Environ. Res. 2018, 12, 203–214. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Sanavy, S.A.M.M.; Gholamhoseini, M.; Joghan, A.K.; Majdi, M.; Kashkooli, A.B. The role of calcium in improving photosynthesis and related physiological and biochemical attributes of spring wheat subjected to simulated acid rain. Physiol. Mol. Biol. Plants 2013, 19, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Wu, B.D.; Jiang, K.; Zhou, J.W. Differences in functional traits between invasive and native Amaranthus, species under simulated acid deposition with a gradient of pH levels. Acta Oecologica 2018, 89, 32–37. [Google Scholar] [CrossRef]
- Percy, K. The effects of simulated acid rain on germinative capacity, growth and morphology of forest tree seedlings. New Phytol. 1986, 104, 473–484. [Google Scholar] [CrossRef]
- Sant’Anna-Santos, B.F.; da Silva, L.C.; Azevedo, A.A.; de Araújo, J.M.; Alves, E.F.; da Silva, E.A.M.; Aguiara, S.R. Effects of simulated acid rain on the foliar micromorphology and the of tree tropical species. Environ. Exp. Bot. 2006, 58, 158–168. [Google Scholar] [CrossRef]
- Debnath, B.; Hussain, M.; Irshad, M.; Mitra, S.; Li, M.; Liu, S.; Qiu, D.L. Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 2018, 23, e388. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.Q.; Wang, L.H.; Liao, C.Y.; Fan, C.X.; Zhou, Q.; Huang, X.H. Combined effects of lead and acid rain on photosynthesis in soybean seedlings. Biol. Trace. Elem. Res. 2014, 161, 136–142. [Google Scholar] [CrossRef]
- Sun, J.W.; Hu, H.Q.; Li, Y.W.; Wang, L.H.; Zhou, Q.; Huang, X.H. Effects and mechanism of acid rain on plant chloroplast ATP synthase. Environ. Sci. Pollut. Res. 2016, 23, 18296–18306. [Google Scholar] [CrossRef]
- Velikova, V.; Tsonev, T.; Yordanov, I. Light and CO2 responses of photosynthesis and chlorophyll fluorescence characteristics in bean plants after simulated acid rain. Physiol. Plantarum. 1999, 107, 77–83. [Google Scholar] [CrossRef]
- Zeng, G.M.; Zhang, G.; Huang, G.H.; Jiang, Y.M.; Liu, H.L. Exchange of Ca2+, Mg2+ and K+ and uptake of H+, for the subtropical forest canopies influenced by acid rain in Shaoshan forest located in Central South China. Plant Sci. 2005, 168, 259–266. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.Y.; Zhong, Y.D.; Huang, J.H.; Fu, X.F.; Wang, L.H.; Teng, W.C. Growth and physiological response of an endangered tree, Horsfieldia hainanensis merr., to simulated sulfuric and nitric acid rain in southern China. Plant Physiol. Biochem. 2019, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.L.; Yao, F.F.; Wang, X.H.; Yang, Q.S.; Yang, H.B.; Ding, H.M. Effects of simulated acid rain with lower S/N ratio on gas exchange and membrane of three dominant species in subtropical forests. Acta Ecologica Sinica 2011, 31, 1911–1917. [Google Scholar]
- Momen, B.; Anderson, P.D.; Helms, J.A. Temperature dependency of acid-rain effect on photosynthesis of Pinus ponderosa. For. Ecol. Manag. 1999, 113, 223–230. [Google Scholar] [CrossRef]
- Boyer, J.S. Soil Water Measurement, Plant Responses, and Breeding for Drought Resistance. In Water Deficits and Photosynthesis; Kozlowski, T.T., Ed.; Academic Press: London, UK, 1976; pp. 153–159. [Google Scholar]
- Liu, M.X.; Song, Y.; Xu, T.T.; Xu, Z.Y.; Wang, T.T.; Yin, L.F.; Jia, X.F.; Tang, J. Trends of precipitation acidification and determining factors in China during 2006–2015. J. Geophys. Res. Atmos. 2020, 125, e2019JD031301. [Google Scholar] [CrossRef]
- Zhao, X.L.; Yan, J.; Chen, Z.Y.; Huang, X.L.; Guo, X.; Sun, Y. Variation characteristics analysis of acid rain in Sichuan from 2006 to 2013. Meteorol. Environ. Sci. 2015, 38, 54–59. (in Chinese). [Google Scholar]
- Peng, L.; Chen, C.H.; Huang, C.; Cheng, Z.; Yang, L.; Jia, J.H. Effects of the “Eleventh five-year” desulphurization plan on sulfur deposition in Yangtze River Delta. Environ. Pollut. Prev. 2007, 29, 793–797. (in Chinese). [Google Scholar]
- Tu, L.H.; Hu, T.X.; Huang, L.H.; Li, R.H.; Dai, H.Z.; Luo, S.H.; Xiang, Y.B. Response of soil respiration to simulated nitrogen deposition in Pleioblastus amarus forest, rainy area of west China. Chin. J. Plant Ecol. 2009, 33, 728–738. (in Chinese). [Google Scholar]
- Eichelmann, H.; Oja, V.; Peterson, R.; Laisk, A. The rate of nitrite reduction in leaves as indicated by CO2 and O2 exchange during photosynthesis. J. Exp. Bot. 2011, 62, 2205–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnon, D.I. Copper enzyme in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Y. Study on the Effects of Different Environmental Factors on Morphology and Physiology of Acer catalpifolium, an Endangered Species in the Rainy Zone of West China. Dissertation of Ph.D., Beijing Forestry University, Beijing, China, 24 July 2020. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000; pp. 184–186. (in Chinese) [Google Scholar]




| Treatments | Leaf Weight (g) | Leaf Area (cm2) | SLA (cm2 g−1) | Leaf Length (cm) | Leaf Width (cm) | |
|---|---|---|---|---|---|---|
| CK | 1.25 ± 0.23 a | 85.72 ± 12.19 b | 69.19 ± 4.99 c | 14.50 ± 1.38 b | 13.88 ± 1.11 ab | |
| NS | 2.5 | 1.10 ± 0.36 ab | 87.66 ± 24.61 b | 81.80 ± 11.36 b | 16.28 ± 2.67 a | 14.58 ± 0.57 a |
| 3.5 | 0.93 ± 0.22 b | 68.83 ± 3.01 d | 76.40 ± 13.92 bc | 15.20 ± 1.23 ab | 13.23 ± 2.00 ab | |
| 4.5 | 1.33 ± 0.27 a | 69.62 ± 15.55 bd | 52.18 ± 4.29 d | 13.50 ± 1.22 b | 12.00 ± 1.09 b | |
| SS | 2.5 | 1.03 ± 0.30 a | 53.32 ± 11.44 d | 52.85 ± 9.10 d | 16.85 ± 1.35 a | 14.28 ± 1.28 a |
| 3.5 | 1.12 ± 0.31 a | 72.67 ± 8.76 bc | 67.74 ± 11.75 c | 16.65 ± 1.17 a | 13.63 ± 0.86 a | |
| 4.5 | 1.22 ± 0.19 a | 73.15 ± 7.59 bc | 60.54 ± 4.02 c | 15.92 ± 2.59 ab | 11.43 ± 1.77 b | |
| NL | 2.5 | 1.20 ± 0.23 a | 62.51 ± 9.60 bcd | 52.38 ± 2.80 d | 12.00 ± 0.82 c | 11.71 ± 1.14 b |
| 3.5 | 0.85 ± 0.29 b | 62.21 ± 17.86 bcd | 75.78 ± 14.49 c | 12.30 ± 2.21 c | 12.02 ± 0.70 b | |
| 4.5 | 1.20 ± 0.33 a | 122.54 ± 27.07 a | 104.80 ± 16.62 a | 13.87 ± 2.09 bc | 13.53 ± 1.15 a | |
| SL | 2.5 | 1.30 ± 0.11 a | 77.14 ± 18.33 bc | 58.70 ± 10.20 d | 12.37 ± 0.72 c | 11.80 ± 1.88 b |
| 3.5 | 1.22 ± 0.23 a | 89.36 ± 15.80 b | 73.80 ± 4.69 c | 14.40 ± 1.38 b | 12.78 ± 1.57 b | |
| 4.5 | 1.03 ± 0.33 a | 73.44 ± 18.11 bc | 73.12 ± 8.52 c | 16.00 ± 2.28 a | 13.62 ± 1.65 ab | |
| Root Biomass (g) | Leaf Biomass (g) | Lateral Branch Biomass (g) | Stem Biomass (g) | Total Biomass (g) | ||
|---|---|---|---|---|---|---|
| CK | 92.50 ± 7.78 e | 23.05 ± 1.59 d | 13.55 ± 1.04 e | 85.55 ± 6.52 e | 214.65 ± 0.71 e | |
| NS | 2.5 | 180.20 ± 12.93 a | 43.90 ± 5.92 a | 27.80 ± 2.74 c | 187.45 ± 5.42 a | 439.35 ± 21.53 a |
| 3.5 | 110.70 ± 18.62 d | 30.50 ± 2.63 b | 15.70 ± 1.31 e | 139.45 ± 9.69 c | 296.35 ± 7.61 c | |
| 4.5 | 74.40 ± 15.23 f | 28.85 ± 2.03 b | 12.35 ± 2.46 f | 58.00 ± 18.07 hi | 173.60 ± 33.74 f | |
| SS | 2.5 | 139.80 ± 6.02 c | 31.30 ± 4.05 b | 19.15 ± 0.71 d | 151.30 ± 1.97 b | 341.55 ± 11.33 b |
| 3.5 | 120.00 ± 6.57 d | 23.65 ± 1.70 d | 14.20 ± 3.61 e | 100.35 ± 4.00 f | 258.20 ± 2.74 d | |
| 4.5 | 94.15 ± 3.12 e | 9.50 ± 2.74 e | 5.20 ± 0.66 g | 70.90 ± 2.30 g | 179.75 ± 2.03 f | |
| NL | 2.5 | 66.40 ± 8.76 f | 6.40 ± 2.30 f | 6.75 ± 0.71 g | 40.80 ± 6.90 i | 120.35 ± 12.65 g |
| 3.5 | 87.20 ± 7.12 e | 11.20 ± 0.77 e | 11.65 ± 1.26 f | 71.70 ± 6.24 g | 181.75 ± 1.37 f | |
| 4.5 | 151.85 ± 11.93 b | 35.80 ± 4.05 d | 30.75 ± 1.37 b | 90.70 ± 5.92 e | 309.10 ± 14.88 b | |
| SL | 2.5 | 58.15 ± 8.38 f | 26.15 ± 1.04 c | 13.45 ± 1.26 e | 66.00 ± 16.87 g | 163.75 ± 25.47 f |
| 3.5 | 88.70 ± 6.24 e | 38.70 ± 1.10 a | 33.50 ± 2.74 ab | 101.00 ± 16.76 de | 261.90 ± 6.68 d | |
| 4.5 | 116.80 ± 2.96 d | 40.65 ± 0.82 a | 39.75 ± 4.44 a | 119.10 ± 6.13 d | 316.30 ± 2.08 b | |
| Treatments | Pn (μmol m−2 s−1) | gs (mol CO2 m−2 s−1) | Ci (μmol−1 mol) | Tr (mmol H2O−2 s−1) | Ls | WUE | |
|---|---|---|---|---|---|---|---|
| CK | 1.75 ± 0.019 f | 0.009 f | 170.80 ± 4.41 d | 0.144 ± 0.001 d | 0.571 ± 0.011 c | 12.18 ± 0.17 f | |
| NS | 2.5 | 2.87 ± 0.040 a | 0.014 a | 154.27 ± 4.17 e | 0.123 ± 0.001 g | 0.663 ± 0.011 b | 17.01 ± 0.246 b |
| 3.5 | 2.30 ± 0.085 c | 0.011 d | 162.21 ± 12.26 e | 0.143 ± 0.001 c | 0.592 ± 0.031 c | 16.12 ± 0.64 c | |
| 4.5 | 2.16 ± 0.047 d | 0.011 d | 156.13 ± 5.54 e | 0.162 ± 0.001 a | 0.557 ± 0.014 c | 13.39 ± 0.26 e | |
| SS | 2.5 | 2.43 ± 0.267 b | 0.008 g | 178.05 ± 13.53 d | 0.132 ± 0.002 e | 0.719 ± 0.029 a | 18.44 ± 1.12 a |
| 3.5 | 2.07 ± 0.057 e | 0.009 f | 124.40 ± 8.62 f | 0.126 ± 0.001 f | 0.686 ± 0.022 b | 16.39 ± 0.42 c | |
| 4.5 | 2.10 ± 0.041 e | 0.009 f | 128.03 ± 7.58 f | 0.144 ± 0.001 d | 0.677 ± 0.019 b | 14.64 ± 0.35 d | |
| NL | 2.5 | 1.44 ± 0.151 h | 0.012 c | 300.13 ± 4.15 a | 0.149 ± 0.005 c | 0.503 ± 0.017 d | 9.74 ± 1.04 g |
| 3.5 | 1.56 ± 0.032 g | 0.013 b | 271.02 ± 3.71 b | 0.157 ± 0.001 b | 0.570 ± 0.009 c | 16.28 ± 0.20 c | |
| 4.5 | 1.52 ± 0.035 g | 0.012 c | 260.76 ± 5.21 b | 0.145 ± 0.001 d | 0.595 ± 0.013 c | 17.41 ± 0.23 b | |
| SL | 2.5 | 1.241 ± 0.035 i | 0.009 f | 268.13 ± 5.38 b | 0.117 ± 0.001 h | 0.326 ± 0.013 f | 10.62 ± 0.26 g |
| 3.5 | 1.261 ± 0.034 i | 0.010 e | 282.72 ± 7.30 b | 0.134 ± 0.001 e | 0.290 ± 0.018 g | 9.43 ± 0.30 g | |
| 4.5 | 1.44 ± 0.036 h | 0.009 f | 239.70 ± 5.22 c | 0.135 ± 0.002 e | 0.398 ± 0.013 e | 10.69 ± 0.20 g | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, T.; Ma, W.; Dayananda, B.; Iwasaki, K.; Li, J. Morphological, Physiological and Photophysiological Responses of Critically Endangered Acer catalpifolium to Acid Stress. Plants 2021, 10, 1958. https://doi.org/10.3390/plants10091958
Zhang Y, Yu T, Ma W, Dayananda B, Iwasaki K, Li J. Morphological, Physiological and Photophysiological Responses of Critically Endangered Acer catalpifolium to Acid Stress. Plants. 2021; 10(9):1958. https://doi.org/10.3390/plants10091958
Chicago/Turabian StyleZhang, Yuyang, Tao Yu, Wenbao Ma, Buddhi Dayananda, Kenji Iwasaki, and Junqing Li. 2021. "Morphological, Physiological and Photophysiological Responses of Critically Endangered Acer catalpifolium to Acid Stress" Plants 10, no. 9: 1958. https://doi.org/10.3390/plants10091958
APA StyleZhang, Y., Yu, T., Ma, W., Dayananda, B., Iwasaki, K., & Li, J. (2021). Morphological, Physiological and Photophysiological Responses of Critically Endangered Acer catalpifolium to Acid Stress. Plants, 10(9), 1958. https://doi.org/10.3390/plants10091958






