Antioxidant Activity and Discrimination of Organic Apples (Malus domestica Borkh.) Cultivated in the Western Region of Romania: A DPPH· Kinetics–PCA Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Radical Scavenging Activity of Apple Extracts
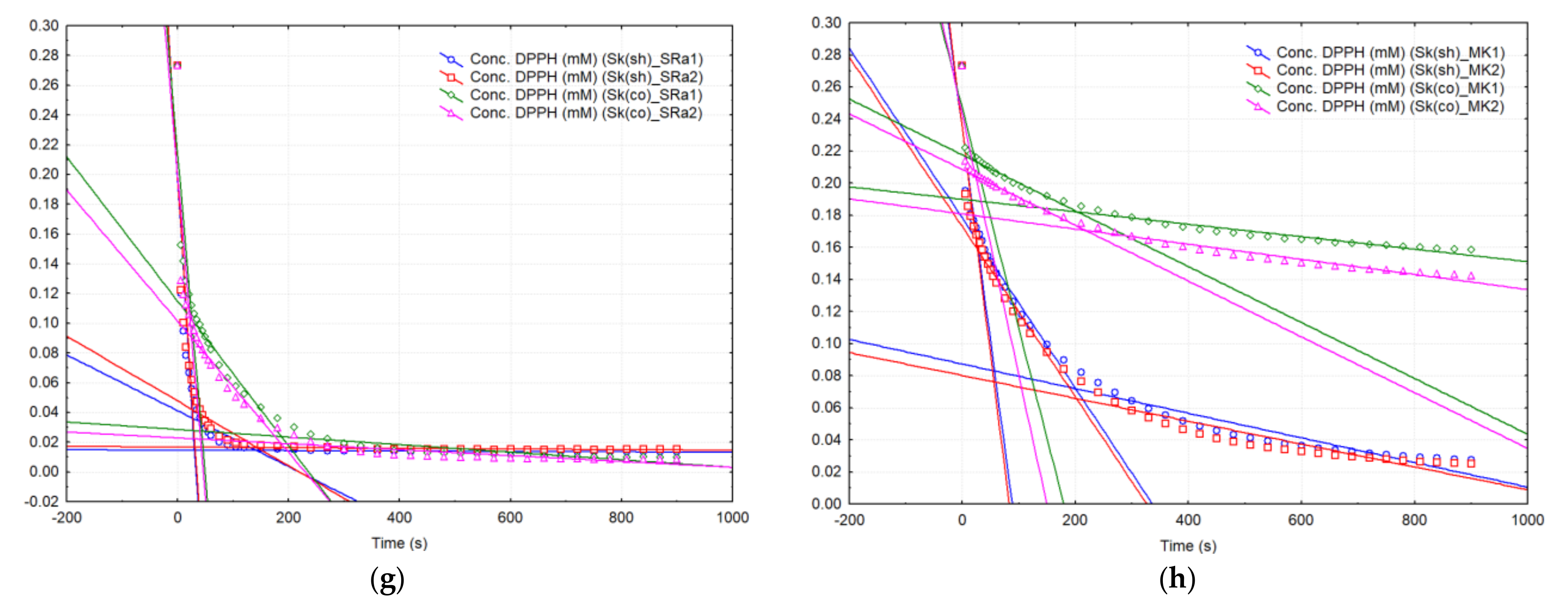
2.2. DPPH· Kinetics Approach for the Apple Extracts
2.3. Correlations and Principal Component Analysis (PCA) of the Antioxidant Activity and DPPH· Kinetics of Apple Extracts
3. Materials and Methods
3.1. Fruits and Chemicals
3.2. Obtaining of Apple Extracts
3.3. Evaluation of the Radical Scavenging Activity (RSA) by DPPH· Method
3.4. Evaluation of the DPPH· Kinetics
3.5. Statistical, Correlational and Principal Component Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmonds, M.S.J.; Howes, M.-J.R. Profile of compounds in different cultivars of apple (Malus x domestica). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Elsevier Inc.: London, UK, 2016; pp. 1–18. [Google Scholar]
- FAOSTAT. Crops and Livestock Products; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Bogdănescu, D.; Bordean, D.-M.; Poiană, M.A.; Hădărugă, N.; Tătaru, O.; Riviş, A. Characterization of the main Romanian apple varieties based on pH, refractometric index and moisture content. J. Hortic. For. Biotechnol. 2018, 22, 95–99. [Google Scholar]
- Soare, E.; Chiurciu, I.-A. Trends in the production and marketing of apples in Romania. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2018, 18, 465–472. [Google Scholar]
- DeEll, J.R. Pome fruits: Apple quality and storage. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Gil, M.I., Beaudry, R., Eds.; Elsevier Inc.: London, UK, 2020; pp. 293–298. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Quality evaluation of apples. In Computer Vision Technology for Food Quality Evaluation; Sun, D.-W., Ed.; Elsevier Inc.: London, UK, 2016; pp. 273–304. [Google Scholar]
- Massini, L.; Rico, D.; Martin-Diana, A.B. Quality attributes of apple juice: Role and effect of phenolic compounds. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Elsevier Inc.: London, UK, 2018; pp. 45–57. [Google Scholar] [CrossRef]
- Mühlbauer, W.; Müller, J. Apple (Malus domestica Borkh.). In Drying Atlas: Drying Kinetics and Quality of Agricultural Products; Elsevier Inc.: London, UK, 2020; pp. 259–268. [Google Scholar] [CrossRef]
- Peck, G.M.; Merwin, I.A. A Grower’s Guide to Organic Apples; NYS IPM Publication No. 223; New York State Department of Agriculture and Markets: New York, NY, USA, 2009; p. 72. [Google Scholar]
- Moruju, G.; Serboiu, L.; Stanciu, G.; Serboiu, A. Apple Tree (Malus pumila L. var domestica) Variety Named “Generos”. Patent RO87692 (A2), 31 October 1985. [Google Scholar]
- Drogoudi, P.D.; Pantelidis, G. Effects of position on canopy and harvest time on fruit physico-chemical and antioxidant properties in different apple cultivars. Sci. Hortic. 2011, 129, 752–760. [Google Scholar] [CrossRef]
- Mignard, P.; Beguería, S.; Reig, G.; Forcada, C.F.i.; Moreno, M.A. Genetic origin and climate determine fruit quality and antioxidant traits on apple (Malus x domestica Borkh). Sci. Hortic. 2021, 285, 110142. [Google Scholar] [CrossRef]
- Das, I.; Arora, A. Post-harvest processing technology for cashew apple—A review. J. Food Eng. 2017, 194, 87–98. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Opara, U.L. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Martín-Belloso, O. Fresh-cut fruits: Apples and pears. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Gil, M.I., Beaudry, R., Eds.; Elsevier Inc.: London, UK, 2020; pp. 487–494. [Google Scholar] [CrossRef]
- Vidović, S.; Tepić, A.; Horecki, J.V.; Šumić, Z.; Gavarić, A.; Vakula, A. Apple. In Valorization of Fruit Processing By-Products; Galanakis, C., Ed.; Elsevier Inc.: London, UK, 2020; pp. 17–42. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. A critical review on the development stage of biorefinery systems towards the management of apple processing-derived waste. Renew. Sustain. Energy Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Szczepańska, J.; Pinto, C.A.; Skąpska, S.; Saraiva, J.A.; Marszałek, K. Effect of static and multi-pulsed high pressure processing on the rheological properties, microbial and physicochemical quality, and antioxidant potential of apple juice during refrigerated storage. LWT—Food Sci. Technol. 2021, 150, 112038. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Ito, V.C.; Alberti, A.; Avila, S.; Spoto, M.; Nogueira, A.; Wosiacki, G. Effects of gamma radiation on the phenolic compounds and In Vitro antioxidant activity of apple pomace flour during storage using multivariate statistical techniques. Innov. Food Sci. Emerg. Technol. 2016, 33, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; del-Barrio, G.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, C.; Liang, D.; Zou, Y.; Li, P.; Ma, F. Phenolic compounds and antioxidant activity in red-fleshed apples. J. Funct. Foods 2015, 18, 1086–1094. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Sánchez-Moreno, C.; De-Ancos, B. Effect of high-pressure processing on flavonoids, hydroxycinnamic acids, dihydrochalcones and antioxidant activity of apple ‘Golden Delicious’ from different geographical origin. Innov. Food Sci. Emerg. Technol. 2019, 51, 20–31. [Google Scholar] [CrossRef]
- Khanizadeh, S.; Tsao, R.; Rekika, D.; Yang, R.; Charles, M.T.; Rupasinghe, H.P.V. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J. Food Compos. Anal. 2008, 21, 396–401. [Google Scholar] [CrossRef]
- Kidoń, M.; Grabowska, J. Bioactive compounds, antioxidant activity, and sensory qualities of red-fleshed apples dried by different methods. LWT—Food Sci. Technol. 2021, 136, 110302. [Google Scholar] [CrossRef]
- Kim, A.-N.; Lee, K.-Y.; Rahman, M.S.; Kim, H.-J.; Kerr, W.L.; Choi, S.-G. Thermal treatment of apple puree under oxygen-free condition: Effect on phenolic compounds, ascorbic acid, antioxidant activities, color, and enzyme activities. Food Biosci. 2021, 39, 100802. [Google Scholar] [CrossRef]
- Łata, B. Apple peel antioxidant status in relation to genotype, storage type and time. Sci. Hortic. 2008, 117, 45–52. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Michalska-Ciechanowska, A.; Wojdyło, A. Postharvest changes in phenolic compounds and antioxidant capacity of apples cv. Jonagold growing in different locations in Europe. Food Chem. 2020, 310, 125912. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv. Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Santarelli, V.; Neri, L.; Sacchetti, G.; Di-Mattia, C.D.; Mastrocola, D.; Pittia, P. Response of organic and conventional apples to freezing and freezing pretreatments: Focus on polyphenols content and antioxidant activity. Food Chem. 2020, 308, 125570. [Google Scholar] [CrossRef]
- Stanger, M.C.; Steffens, C.A.; Soethe, C.; Moreira, M.A.; do-Amarante, C.V.T.; Both, V.; Brackmann, A. Phenolic compounds content and antioxidant activity of ‘Galaxy’ apples stored in dynamic controlled atmosphere and ultralow oxygen conditions. Postharvest Biol. Technol. 2018, 144, 70–76. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, L.; Wang, Z.; Nisar, T.; Gong, T.; Li, D.; Niu, P.; Guo, Y. The antioxidant property and α-amylase inhibition activity of young apple polyphenols are related with apple varieties. LWT—Food Sci. Technol. 2019, 111, 252–259. [Google Scholar] [CrossRef]
- Zheng, H.-Z.; Kim, Y.-I.; Chung, S.-K. A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chem. 2012, 131, 106–110. [Google Scholar] [CrossRef]
- Alvarez, L.V.H.; Zielinski, A.A.F.; Alberti, A.; Nogueira, A. Monitoring of the phenolic compounds and in vitro antioxidant activity of apple beverages according to geographical origin and their type: A chemometric study. LWT—Food Sci. Technol. 2017, 84, 385–393. [Google Scholar] [CrossRef]
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Janulis, V.; Viskelis, P. Phenolic antioxidant profiles in the whole fruit, flesh and peel of apple cultivars grown in Lithuania. Sci. Hortic. 2017, 216, 186–192. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH. assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Pękal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- López-Alarcóna, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Amboni, R.D.D.M.C.; Denardi, F.; Fett, R. Physico-chemical and antioxidant properties of six apple cultivars (Malus domestica Borkh) grown in southern Brazil. Sci. Hortic. 2009, 122, 421–425. [Google Scholar] [CrossRef]
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Di-Pietro, P.F.; da-Costa-Nunes, E.; Fett, R. Phenolic compounds and antioxidant activity of the apple flesh and peel of eleven cultivars grown in Brazil. Sci. Hortic. 2011, 128, 261–266. [Google Scholar] [CrossRef]
- Fernández-González, A.; Montejo-Bernardo, J.M.; Rodríguez-Prieto, H.; Castaño-Monllor, C.; Badía-Laíño, R.; Díaz-García, M.E. Easy-to-use analytical approach based on ATR–FTIR and chemometrics to identify apple varieties under Protected Designation of Origin (PDO). Comput. Electron. Agric. 2014, 108, 166–172. [Google Scholar] [CrossRef]
- Francini, A.; Romeo, S.; Cifelli, M.; Gori, D.; Domenici, V.; Sebastiani, L. 1H NMR and PCA-based analysis revealed variety dependent changes in phenolic contents of apple fruit after drying. Food Chem. 2017, 221, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M.; Woodcock, T.; O’Donnell, C.P.; Kelly, J.D.; Downey, G. Differentiation of apple juice samples on the basis of heat treatment and variety using chemometric analysis of MIR and NIR data. Food Res. Int. 2005, 38, 1109–1115. [Google Scholar] [CrossRef]
- Włodarska, K.; Piasecki, P.; Lobo-Prieto, A.; Pawlak-Lemańska, K.; Górecki, T.; Sikorska, E. Rapid screening of apple juice quality using ultraviolet, visible, and near infrared spectroscopy and chemometrics: A comparative study. Microchem. J. 2021, 164, 106051. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Alberti, A.; Braga, C.M.; da-Silva, K.M.; Canteri, M.H.G.; Mafra, L.I.; Granato, D.; Nogueira, A.; Wosiacki, G. Effect of mash maceration and ripening stage of apples on phenolic compounds and antioxidant power of cloudy juices: A study using chemometrics. LWT—Food Sci. Technol. 2014, 57, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Denver, S.; Jensen, J.D. Consumer preferences for organically and locally produced apples. Food Qual. Prefer. 2014, 31, 129–134. [Google Scholar] [CrossRef]
- Keyes, S.; Tyedmers, P.; Beazley, K. Evaluating the environmental impacts of conventional and organic apple production in Nova Scotia, Canada, through life cycle assessment. J. Clean. Prod. 2015, 104, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Longo, S.; Mistretta, M.; Guarino, F.; Cellura, M. Life Cycle Assessment of organic and conventional apple supply chains in the North of Italy. J. Clean. Prod. 2017, 140, 654–663. [Google Scholar] [CrossRef]
- Meyerding, S.G.H.; Merz, N. Consumer preferences for organic labels in Germany using the example of apples—Combining choice-based conjoint analysis and eye-tracking measurements. J. Clean. Prod. 2018, 181, 772–783. [Google Scholar] [CrossRef]
- Moscetti, R.; Raponi, F.; Ferri, S.; Colantoni, A.; Monarca, D.; Massantini, R. Real-time monitoring of organic apple (var. Gala) during hot-air drying using near-infrared spectroscopy. J. Food Eng. 2018, 222, 139–150. [Google Scholar] [CrossRef]
- Raffo, A.; D’Aloise, A.; Lardschneider, E.; Paoletti, F.; Marini, F.; Bucci, R.; Kelderer, M. Effect of soil nutrition on aroma compound formation in organically grown apples (cv. Golden Delicious). In Flavour Science; Ferreira, V., Lopez, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 173–176. [Google Scholar]
- Raffo, A.; Baiamonte, I.; Bucci, R.; D’Aloise, A.; Kelderer, M.; Matteazzi, A.; Moneta, E.; Nardo, N.; Paoletti, F.; Peparaio, M. Effects of different organic and conventional fertilisers on flavour related quality attributes of cv. Golden Delicious apples. LWT—Food Sci. Technol. 2014, 59, 964–972. [Google Scholar] [CrossRef]
- Róth, E.; Berna, A.; Beullens, K.; Yarramraju, S.; Lammertyn, J.; Schenk, A.; Nicolaï, B. Postharvest quality of integrated and organically produced apple fruit. Postharvest Biol. Technol. 2007, 45, 11–19. [Google Scholar] [CrossRef]
- Song, W.; Jiang, N.; Wang, H.; Guo, G. Evaluation of machine learning methods for organic apple authentication based on diffraction grating and image processing. J. Food Compos. Anal. 2020, 88, 103437. [Google Scholar] [CrossRef]
- Roussos, P.A.; Gasparatos, D. Apple tree growth and overall fruit quality under organic and conventional orchard management. Sci. Hortic. 2009, 123, 247–252. [Google Scholar] [CrossRef]
- Heinmaa, L.; Moor, U.; Põldma, P.; Raudsepp, P.; Kidmose, U.; Lo-Scalzo, R. Content of health-beneficial compounds and sensory properties of organic apple juice as affected by processing technology. LWT—Food Sci. Technol. 2017, 85, 372–379. [Google Scholar] [CrossRef]
- Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S. Variation in polyphenolics and antioxidant activity of traditional apple cultivars from West Himalaya, Uttarakhand. Hortic. Plant J. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, D.; Xi, P.; Cai, T.; Cao, X.; Liu, H.; Li, J. Effects of temperature-controlled ultrasound treatment on sensory properties, physical characteristics and antioxidant activity of cloudy apple juice. LWT—Food Sci. Technol. 2021, 142, 111030. [Google Scholar] [CrossRef]
- Gligor-(Pane), D.; Hădărugă, D.I.; Hădărugă, N.G. Quality and authenticity of the forest fruits through antioxidant compounds—A review on chemometric tools. J. Agroaliment. Process. Technol. 2020, 26, 251–257. [Google Scholar]
- Hegheş, A.; Hădărugă, N.G.; Fuliaş, A.-V.; Bandur, G.N.; Hădărugă, D.I.; Dehelean, C.-A. Capsicum annuum extracts/β-cyclodextrin complexes. Thermal analyses—Karl Fischer water titration correlations and antioxidant activity. J. Therm. Anal. Calorim. 2015, 120, 603–615. [Google Scholar] [CrossRef]
- Ivanovici, M.; Sicoe, G.; Hădărugă, D.I. Kinetics and antiradical activity of natural and synthetic phenolic compounds by DPPH method: A comparative study. J. Agroaliment. Process. Technol. 2018, 24, 97–103. [Google Scholar]
- Oprinescu, C.; Hădărugă, D.I.; Hădărugă, N.G. A critical review on the antioxidant analysis and composition of Vitis species. J. Agroaliment. Process. Technol. 2020, 26, 429–440. [Google Scholar]
- Costescu, C.I.; Rus, D.; Hădărugă, N.G.; Pogor, V.; Badea, M.; Mateican, E.; Szakal, R.; Olaru, P.; Hădărugă, D.I. Solvent influence on the antioxidant activity of pomegranate extracts. J. Agroaliment. Process. Technol. 2015, 21, 21–27. [Google Scholar]
- Sicoe, G.; Oprinescu, C.I.; Golea, G.M.; Riviş, A.; Hădărugă, N.G. Kinetics on the DPPH· reaction with hydroalcoholic extracts from various pomegranate parts. J. Agroaliment. Process. Technol. 2017, 23, 271–280. [Google Scholar]
- Hădărugă, D.I.; Pantea, C.; Hădărugă, N.G. Antioxidant activity and kinetics on kiwi fruit (Actinidia deliciosa) ethanolic extracts by 2,2-diphenyl-1-picrylhydrazyl (DPPH·) method. J. Agroaliment. Process. Technol. 2016, 22, 207–211. [Google Scholar]
- Iordănescu, O.A.; Băla, M.; Gligor-(Pane), D.; Zippenfening, S.E.; Cugerean, M.I.; Petroman, M.I.; Hădărugă, D.I.; Hădărugă, N.G.; Riviş, M. A DPPH·kinetic approach on the antioxidant activity of various parts and ripening levels of papaya (Carica papaya L.) ethanolic extracts. Plants 2021, 10, 1679. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensmittel-Wissenschaft und -Technologie 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft und -Technologie 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH. method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Nićiforović, N.; Polak, T.; Makuc, D.; Ulrih, N.P.; Abramovič, H. A kinetic approach in the evaluation of radical-scavenging efficiency of sinapic acid and its derivatives. Molecules 2017, 22, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stoichiometric and kinetic studies of phenolic antioxidants from andean purple corn and red-fleshed sweetpotato. J. Agric. Food Chem. 2003, 51, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.; Serra, M.; Molinu, M.G.; Azara, E.; Barberis, A.; Sanna, D. Reaction time and DPPH concentration influence antioxidant activity and kinetic parameters of bioactive molecules and plant extracts in the reaction with the DPPH radical. J. Food Compos. Anal. 2014, 35, 112–119. [Google Scholar] [CrossRef]
- Savatović, S.M.; Ćetković, G.S.; Čanadanović-brunet, J.M.; Djilas, S.M. Kinetic behaviour of the DPPH radical-scavenging activity of tomato waste extracts. J. Serb. Chem. Soc. 2012, 77, 1381–1389. [Google Scholar] [CrossRef]
- López-Nicolá, J.M.; Núñez-Delicado, E.; Sánchez-Ferrer, Á.; García-Carmona, F. Kinetic model of apple juice enzymatic browning in the presence of cyclodextrins: The use of maltosyl-β-cyclodextrin as secondary antioxidant. Food Chem. 2007, 101, 1164–1171. [Google Scholar] [CrossRef]


| Code | Organic or Non-Organic Orchard 1 | RSA (1 min) (%) | RSA (3 min) (%) | RSA (5 min) (%) | RSA (15 min) (%) |
|---|---|---|---|---|---|
| Gd(sh)_SRa | O | 78.76 (±0.67) a | 92.13 (±0.29) a | 93.68 (±0.35) a | 95.25 (±0.27) a |
| Gd(co)_SRa | O | 63.95 (±2.21) a | 80.41 (±1.34) a | 87.56 (±1.29) a | 93.43 (±0.62) a |
| Gd(sh)_SRb | O | 88.65 (±1.45) a | 95.66 (±0.80) a | 96.24 (±0.76) a | 96.65 (±0.72) a |
| Gd(co)_SRb | O | 76.68 (±1.54) a | 91.36 (±0.64) a | 94.31 (±0.47) a | 95.20 (±0.28) a |
| Gd(sh)_AR | N | 69.26 (±2.62) a | 87.13 (±0.65) a | 89.02 (±0.17) a | 91.13 (±0.05) a |
| Gd(co)_AR | N | 58.36 (±13.23) a | 79.97 (±3.97) a | 84.17 (±0.18) a | 86.51 (±1.70) a |
| Gd(sh)_LG | N | 82.83 (±8.27) a | 89.35 (±2.14) a | 90.19 (±1.75) a | 90.78 (±1.01) a |
| Gd(co)_LG | N | 36.72 (±0.54) b | 67.55 (±0.23) b | 79.77 (±0.22) a | 86.90 (±0.84) a |
| Gd(sh)_MK | N | 31.64 (±0.43) b | 42.29 (±0.99) c | 48.80 (±1.27) b | 60.37 (±1.21) b |
| Gd(co)_MK | N | 34.21 (±13.6) b | 39.13 (±15.72) c | 41.59 (±17.03) b | 45.52 (±18.64) b |
| Code | Organic or Non-Organic Orchard 1 | RSA (1 min) (%) | RSA (3 min) (%) | RSA (5 min) (%) | RSA (15 min) (%) |
|---|---|---|---|---|---|
| Fl(sh)_SRa | O | 85.33 (±0.30) a | 92.47 (±0.02) a | 93.28 (±0.08) a | 93.45 (±0.06) a |
| Fl(co)_SRa | O | 64.43 (±2.52) b | 86.49 (±0.65) a | 91.28 (±0.14) a | 93.23 (±0.16) a |
| Fl(sh)_SRb | O | 61.25 (±0.49) b | 62.26 (±0.70) a,b | 62.29 (±0.67) b | 62.43 (±0.68) a |
| Fl(co)_SRb | O | 63.70 (±1.64) b | 68.90 (±1.70) a,b | 71.59 (±1.76) a,b | 74.98 (±0.72) a |
| Fl(sh)_LG | N | 89.09 (±1.95) a | 92.98 (±0.40) a | 93.33 (±0.48) a | 93.47 (±0.50) a |
| Fl(co)_LG | N | 45.86 (±2.17) b | 70.20 (±1.56) a,b | 81.89 (±1.15) a | 90.85 (±4.92) a |
| Fl(sh)_MK | N | 64.41 (±10.66) b | 71.16 (±13.00) a,b | 74.14 (±14.02) a | 79.30 (±15.62) a |
| Fl(co)_MK | N | 16.64 (±3.04) c | 20.21 (±3.49) c | 22.41 (±3.05) c | 25.64 (±3.05) b |
| Code | Organic or Non-Organic Orchard 1 | RSA (1 min) (%) | RSA (3 min) (%) | RSA (5 min) (%) | RSA (15 min) (%) |
|---|---|---|---|---|---|
| Gn(sh)_SR | O | 75.32 (±1.37) a | 78.45 (±0.04) a | 78.69 (±0.06) a | 79.12 (±0.14) a |
| Gn(co)_SR | O | 64.81 (±4.56) a | 78.13 (±3.08) a | 84.89 (±2.21) b | 94.69 (±0.27) b |
| Gn(sh)_LG | N | 75.80 (±2.09) a | 89.56 (±1.05) a | 93.00 (±0.74) c | 95.19 (±0.49) b |
| Gn(co)_LG | N | 41.28 (±2.03) b | 55.88 (±2.37) b | 64.33 (±1.89) d | 82.78 (±1.53) c |
| Code | Organic or Non-Organic Orchard 1 | RSA (1 min) (%) | RSA (3 min) (%) | RSA (5 min) (%) | RSA (15 min) (%) |
|---|---|---|---|---|---|
| Sk(sh)_SRa | O | 90.16 (±1.25) a | 94.04 (±0.57) a | 94.52 (±0.48) a | 94.71 (±0.39) a |
| Sk(co)_SRa | O | 71.77 (±2.75) b | 87.97 (±1.73) a | 93.67 (±1.03) a | 96.63 (±0.42) a |
| Sk(sh)_SRb | O | 91.78 (±0.10) a | 92.71 (±0.44) a | 92.89 (±0.95) a | 93.00 (±1.37) a |
| Sk(sh)_MK | N | 48.37 (±1.57) c | 68.23 (±1.47) b | 77.59 (±1.52) b | 90.39 (±0.65) a |
| Sk(co)_MK | N | 26.05 (±2.27) d | 32.71 (±2.56) c | 36.77 (±3.07) c | 44.89 (±4.16) b |
| Code | Organic or Non-Organic Orchard 1 | DPPH· Reacti on Rateon t1 Time Range, | DPPH· Reaction Rate on t2 Time Range, | DPPH· Reaction Rate on t3 Time Range, |
|---|---|---|---|---|
| Gd(sh)_SRa | O | 4.80 (±0.00) a | 0.50 (±0.00) a | 0.009 (±0.000) a |
| Gd(co)_SRa | O | 3.90 (±0.00) a | 0.50 (±0.00) a | 0.039 (±0.003) b |
| Gd(sh)_SRb | O | 5.65 (±0.07) a | 0.30 (±0.00) a,c | 0.003 (±0.000) a |
| Gd(co)_SRb | O | 4.75 (±0.07) a | 0.45 (±0.07) a | 0.019 (±0.010) a,b |
| Gd(sh)_AR | N | 4.25 (±0.21) a | 0.60 (±0.00) a | 0.013 (±0.000) a,b |
| Gd(co)_AR | N | 3.70 (±0.99) a,b | 0.70 (±0.14) a | 0.017 (±0.013) a,b |
| Gd(sh)_LG | N | 5.60 (±0.85) a | 0.30 (±0.28) a,c | 0.004 (±0.004) a |
| Gd(co)_LG | N | 2.10 (±0.00) b | 0.85 (±0.07) a,b | 0.052 (±0.003) b |
| Gd(sh)_MK | N | 1.75 (±0.07) b | 0.30 (±0.00) a,c | 0.099 (±0.002) c |
| Gd(co)_MK | N | 2.05 (±0.64) b | 0.13 (±0.11) a,c | 0.022 (±0.010) a,b |
| Code | Organic or Non-Organic Orchard 1 | DPPH· Reacti on Rateon t1 Time Range, | DPPH· Reaction Rate on t2 Time Range, | DPPH· Reaction Rate on t3 Time Range, |
|---|---|---|---|---|
| Fl(sh)_SRa | O | 5.60 (±0.00) a | 0.30 (±0.00) a | 0.002 (±0.000) a |
| Fl(co)_SRa | O | 3.85 (±0.07) b | 0.65 (±0.07) b | 0.016 (±0.003) a |
| Fl(sh)_SRb | O | 3.75 (±0.07) b | 0.03 (±0.01) a,c | 0.001 a |
| Fl(co)_SRb | O | 3.75 (±0.07) b | 0.10 (±0.00) a,c | 0.021 (±0.005) a |
| Fl(sh)_LG | N | 6.10 (±0.42) a | 0.20 (±0.14) a,c | 0.002 (±0.001) a |
| Fl(co)_LG | N | 2.75 (±0.07) b,c | 0.70 (±0.00) b | 0.136 (±0.090) a |
| Fl(sh)_MK | N | 3.85 (±0.64) b | 0.15 (±0.07) a,c | 0.028 (±0.009) a |
| Fl(co)_MK | N | 0.95 (±0.21) d | 0.09 (±0.01) a,c | 0.018 (±0.001) a |
| Code | Organic or Non-Organic Orchard 1 | DPPH· Reaction Rate on t1 Time Range, | DPPH· Reaction Rate on t2 Time Range, | DPPH· Reaction Rate on t3 Time Range, |
|---|---|---|---|---|
| Gn(sh)_SR | O | 4.95 (±0.21) a | 0.15 (±0.07) a | 0.003 (±0.002) a |
| Gn(co)_SR | O | 3.95 (±0.21) b | 0.40 (±0.00) b | 0.066 (±0.001) a |
| Gn(sh)_LG | N | 4.65 (±0.07) a | 0.40 (±0.00) b | 0.015 (±0.002) a |
| Gn(co)_LG | N | 2.60 (±0.14) c | 0.40 (±0.00) b | 0.053 (±0.062) a |
| Code | Organic or Non-Organic Orchard 1 | DPPH· Reaction Rate on t1 Time Range, | DPPH· Reaction Rate on t2 Time Range, | DPPH· Reaction Rate on t3 Time Range, |
|---|---|---|---|---|
| Sk(sh)_SRa | O | 5.90 (±0.14) a | 0.20 (±0.00) a | 0.002 (±0.001) a |
| Sk(co)_SRa | O | 4.30 (±0.00) b | 0.45 (±0.07) b | 0.023 (±0.004) b |
| Sk(sh)_SRb * | O | 5.90 (±0.42) a | 0.04 c | 0.001 a |
| Sk(sh)_MK | N | 2.70 (±0.14) c | 0.50 (±0.00) b | 0.074 (±0.004) c |
| Sk(co)_MK | N | 1.50 (±0.14) d | 0.20 (±0.00) a | 0.043 (±0.006) d |
| Code | Organic or Non-Organic Orchard 1 | Description |
|---|---|---|
| Gd(sh)_SRa | O | “Golden Delicious” variety, shell sample “a”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Gd(co)_SRa | O | “Golden Delicious” variety, core sample “a”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Gd(sh)_SRb | O | “Golden Delicious” variety, shell sample “b”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Gd(co)_SRb | O | “Golden Delicious” variety, core sample “b”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Gd(sh)_AR | N | “Golden Delicious” variety, shell sample, harvested directly from the conventional orchard (Arad, Arad county, Romania, 46°10′ N, 21°19′ E) |
| Gd(co)_AR | N | “Golden Delicious” variety, core sample, harvested directly from the conventional orchard (Arad, Arad county, Romania, 46°10′ N, 21°19′ E) |
| Gd(sh)_LG | N | “Golden Delicious” variety, shell sample, harvested from the conventional orchard (Lugoj, Timiş county, Romania, 45°41′10″ N, 21°52′2″ E) |
| Gd(co)_LG | N | “Golden Delicious” variety, core sample, harvested from the conventional orchard (Lugoj, Timiş county, Romania, 45°41′10″ N, 21°52′2″ E) |
| Gd(sh)_MK | N | “Golden Delicious” variety, shell sample, purchased from the supermarket (Timişoara, Timiş county, Romania) |
| Gd(co)_MK | N | “Golden Delicious” variety, core sample, purchased from the supermarket (Timişoara, Timiş county, Romania) |
| Fl(sh)_SRa | O | “Florina” variety, shell sample “a”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Fl(co)_SRa | O | “Florina” variety, core sample “a”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Fl(sh)_SRb | O | “Florina” variety, shell sample “b”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Fl(co)_SRb | O | “Florina” variety, core sample “b”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Fl(sh)_LG | N | “Florina” variety, shell sample, harvested from the conventional orchard (Lugoj, Timiş county, Romania, 45°41′10″ N, 21°52′2″ E) |
| Fl(co)_LG | N | “Florina” variety, core sample, harvested from the conventional orchard (Lugoj, Timiş county, Romania, 45°41′10″ N, 21°52′2″ E) |
| Fl(sh)_MK | N | “Florina” variety, shell sample, purchased from the supermarket (Timişoara, Timiş county, Romania) |
| Fl(co)_MK | N | “Florina” variety, core sample, purchased from the supermarket (Timişoara, Timiş county, Romania) |
| Gn(sh)_SR | O | “Generos” variety, shell sample, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Gn(co)_SR | O | “Generos” variety, core sample, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Gn(sh)_LG | N | “Generos” variety, shell sample, harvested from the conventional orchard (Lugoj, Timiş county, Romania, 45°41′10″ N, 21°52′2″ E) |
| Gn(co)_LG | N | “Generos” variety, core sample, harvested from the conventional orchard (Lugoj, Timiş county, Romania, 45°41′10″ N, 21°52′2″ E) |
| Sk(sh)_SRa | O | “Starkrimson” variety, shell sample “a”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Sk(co)_SRa | O | “Starkrimson” variety, core sample “a”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Sk(sh)_SRb | O | “Starkrimson” variety, shell sample “b”, harvested from the organic orchard (Şiria, Arad county, Romania, 46°16′2″ N, 21°38′18″ E) |
| Sk(sh)_MK | N | “Starkrimson” variety, shell sample, purchased from the supermarket (Timişoara, Timiş county, Romania) |
| Sk(co)_MK | N | “Starkrimson” variety, core sample, purchased from the supermarket (Timişoara, Timiş county, Romania) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordănescu, O.A.; Băla, M.; Iuga, A.C.; Gligor, D.; Dascălu, I.; Bujancă, G.S.; David, I.; Hădărugă, N.G.; Hădărugă, D.I. Antioxidant Activity and Discrimination of Organic Apples (Malus domestica Borkh.) Cultivated in the Western Region of Romania: A DPPH· Kinetics–PCA Approach. Plants 2021, 10, 1957. https://doi.org/10.3390/plants10091957
Iordănescu OA, Băla M, Iuga AC, Gligor D, Dascălu I, Bujancă GS, David I, Hădărugă NG, Hădărugă DI. Antioxidant Activity and Discrimination of Organic Apples (Malus domestica Borkh.) Cultivated in the Western Region of Romania: A DPPH· Kinetics–PCA Approach. Plants. 2021; 10(9):1957. https://doi.org/10.3390/plants10091957
Chicago/Turabian StyleIordănescu, Olimpia Alina, Maria Băla, Alina Carmen Iuga, Dina Gligor (Pane), Ionuţ Dascălu, Gabriel Stelian Bujancă, Ioan David, Nicoleta Gabriela Hădărugă, and Daniel Ioan Hădărugă. 2021. "Antioxidant Activity and Discrimination of Organic Apples (Malus domestica Borkh.) Cultivated in the Western Region of Romania: A DPPH· Kinetics–PCA Approach" Plants 10, no. 9: 1957. https://doi.org/10.3390/plants10091957
APA StyleIordănescu, O. A., Băla, M., Iuga, A. C., Gligor, D., Dascălu, I., Bujancă, G. S., David, I., Hădărugă, N. G., & Hădărugă, D. I. (2021). Antioxidant Activity and Discrimination of Organic Apples (Malus domestica Borkh.) Cultivated in the Western Region of Romania: A DPPH· Kinetics–PCA Approach. Plants, 10(9), 1957. https://doi.org/10.3390/plants10091957






