Understanding Maize Response to Nitrogen Limitation in Different Light Conditions for the Improvement of Photosynthesis
Abstract
1. Introduction
2. Nitrogen, Light, and Photosynthesis in C3 and C4 Plants
3. Thylakoid Nitrogen Costs
4. Nitrogen and Distribution of Organic Acids between Mesophyll and BS Cells in Different Light Environments
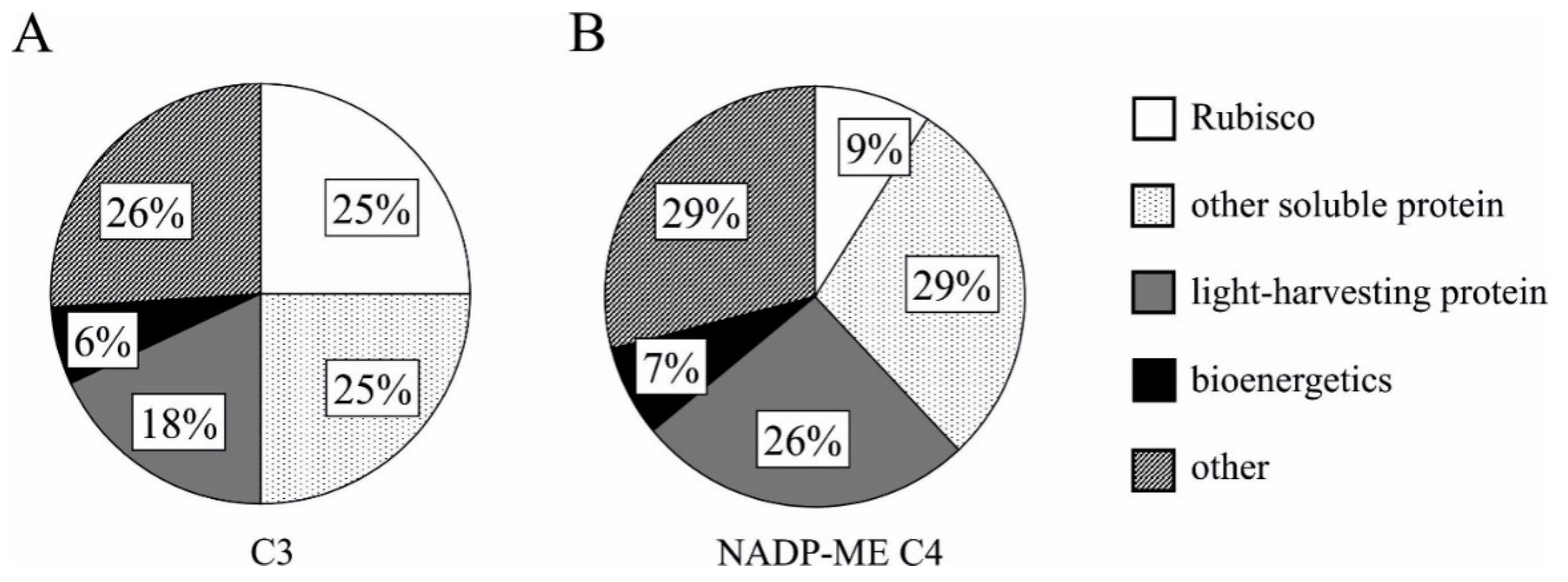
5. Nitrogen Investment in the PEPC and PPDK Enzymes and Rubisco
6. Nitrogen Level and Light Intensity Affect the Amount and Activity of Nitrate Reductase (NR) and Nitrite Reductase (NiR)
7. Main Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APAR | photosynthetically active radiation |
| BS | bundle sheath |
| HL | high light |
| LL | low light |
| M | mesophyll |
| N | nitrogen |
| NUE | nitrogen use efficiency |
| PPDK | pyruvate phosphate dikinase |
| PEPC | phosphoenolpyruvate carboxylase |
| PEPCK | phosphoenolpyruvate carboxykinase |
| Pn | photosynthesis |
| PNUE | photosynthetic nitrogen-use efficiency |
| SLA | specific leaf area |
| SLN | specific leaf nitrogen |
References
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F. Physiological functions of mineral nutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Ghannoum, O.; Evans, J.R.; Caemmerer, S.V. Nitrogen and water use efficiency of C4 plants. In C4 Photosynthesis and Related CO2 Concentrating Mechanisms; Raghavendra, A.S., Sage, R.F., Eds.; Springer Science and Business Media: Dordrecht, The Netherlands, 2011; pp. 129–146. [Google Scholar]
- Teixeira, E.I.; George, M.; Herreman, T.; Brown, H.; Fletcher, A.; Chakwizira, E.; de Ruiter, J.; Maley, S.; Noble, A. The impact of water and nitrogen limitation on maize biomass and resource-use efficiencies for radiation, water and nitrogen. Field Crops Res. 2014, 168, 109–118. [Google Scholar] [CrossRef]
- Szulc, P.; Bocianowski, J. Effects of application of different nitrogen fertilizer forms and magnesium on dynam-ics of dry matter accumulation in two maize (Zea mays L.) hybrids in their early growth stages. Pol. J. Agron. 2012, 11, 65–80. [Google Scholar]
- Subedi, K.D.; Ma, B.L. Nitrogen Uptake and Partitioning in Stay-Green and Leafy Maize Hybrids. Crop Sci. 2005, 45, 740–747. [Google Scholar] [CrossRef]
- Mueller, S.M.; Vyn, T.J. Maize plant resilience to N stress and post silking N capacity changes over time: A review. Front. Plant Sci. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Wright, I.; Evans, J.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef]
- Kopriva, S. Nitrogen and sulfur metabolism in C4 plants. In Photosynthesis and Related CO2 Concentrating Mecha-Nisms; Raghavendra, A.S., Sage, R.F., Eds.; Springer Science and Business Media: Dordrecht, The Netherlands, 2011; pp. 109–128. [Google Scholar]
- Yin, Z.H.; Johnson, G.N. Photosynthetic acclimation of higher plants to growth in fluctuating light environments. Photosynth. Res. 2000, 63, 97–107. [Google Scholar] [CrossRef]
- Björkman, O. Responses to different quantum flux densities. In Physiological Plant Ecology I, Responses to the Physical Environment; Lange, O.L., Nobel, S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 57–100. [Google Scholar]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Rogowski, P.; Wasilewska-Dębowska, W.; Krupnik, T.; Drozak, A.; Zienkiewicz, M.; Krysiak, M.; Romanowska, E. Photosynthesis and organization of maize mesophyll and bundle sheath thylakoids of plants grown in various light intensities. Environ. Exp. Bot. 2019, 162, 72–86. [Google Scholar] [CrossRef]
- Bertheloot, J.; Martre, P.; Andrieu, B. Dynamics of Light and Nitrogen Distribution during Grain Filling within Wheat Canopy. Plant Physiol. 2008, 148, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Triboi, E.; Martre, P.; Triboi-Blondel, A.M. Environmentally induced changes of protein composition for developing grains of wheat are related to changes in total protein content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2020, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.; Baligar, V. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Lei, W.; Kong, L. Increased planting density combined with reduced nitrogen rate to achieve high yield in maize. Sci. Rep. 2021, 11, 358. [Google Scholar] [CrossRef]
- Bonelli, L.E.; Andrade, F.H. Maize radiation use-efficiency response to optimally distributed foliar-nitrogen-content depends on canopy leaf-area index. Field Crops Res. 2020, 247, 107557. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T. A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crops Res. 2011, 121, 2–18. [Google Scholar] [CrossRef]
- Jamal, Z.; Hamayun, M.; Ahmad, N.; Chaudhary, M.F. Effects of soil and foliar application of different concentrations of NPK and foliar application of (NH4)2SO4 on different yield parameters in wheat. J. Agron. 2006, 5, 251–256. [Google Scholar]
- Greef, J.M.; Ott, H.; Wulfes, R.; Taube, F. Growth analysis of dry matter accumulation and N uptake of forage maize cultivars affected by N supply. J. Agric. Sci. 1999, 132, 31–43. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G. N uptake and distribution in crops: An agronomical and ecophysiological perspective. J. Exp. Bot. 2002, 53, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Sebaa, E.D.; Prioul, J.L.; Brangeon, J. Acclimation of adult Lonummul tiflorum leaves to changes in irradiance: Effect on leaf photosynthesis and chloroplast ultrastructure. J. Plant Physiol. 1987, 127, 431–441. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, W.; Yang, Q. Quality changes in hydroponic lettuce grown under pre-harvest short-duration continuous light of different intensities. J. Hortic. Sci. Biotechnol. 2012, 87, 429–434. [Google Scholar] [CrossRef]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Fahnenstich, H.; Sonnewald, U. Maize Source Leaf Adaptation to Nitrogen Deficiency Affects Not Only Nitrogen and Carbon Metabolism But Also Control of Phosphate Homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef] [PubMed]
- Torney, F.; Moeller, L.; Scarpa, A.; Wang, K. Genetic engineering approaches to improve bioethanol production from maize. Curr. Opin. Biotechnol. 2007, 18, 193–199. [Google Scholar] [CrossRef]
- Lillo, C. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A world wide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef]
- Yamori, W.; Takahashi, S.; Makino, A.; Price, G.; Badger, M.; von Caemmerer, S. The Roles of ATP Synthase and the Cytochrome b 6/f Complexes in Limiting Chloroplast Electron Transport and Determining Photosynthetic Capacity. Plant Physiol. 2010, 155, 956–962. [Google Scholar] [CrossRef]
- Ye, Z.-P.; Robakowski, P.; Suggett, D.J. A mechanistic model for the light response of photosynthetic electron transport rate based on light harvesting properties of photosynthetic pigment molecules. Planta 2012, 237, 837–847. [Google Scholar] [CrossRef]
- Usuda, H. Variations in the Photosynthesis Rate and Activity of Photosynthetic Enzymes in Maize Leaf Tissue of Different Ages. Plant Cell Physiol. 1984, 25, 1297–1301. [Google Scholar] [CrossRef]
- Evans, J.R.; Seemann, J.R. The allocation of protein nitrogen in the photosynthetic apparatus: Costs, consequences, and control. In Photosynthesis; Briggs, W.R., Ed.; A.R. Liss: New York, NY, USA, 1989; pp. 183–205. [Google Scholar]
- Rotundo, J.L.; Cipriotti, P.A. Biological limits on nitrogen use for plant photosynthesis: A quantitative revision comparing cultivated and wild species. New Phytol. 2016, 214, 120–131. [Google Scholar] [CrossRef]
- Evans, J.R.; von Caemmerer, S. Would C4 rice produce more biomass than C3 rice? Stud. Plant Sci. 2000, 7, 53–71. [Google Scholar]
- Kelly, S. The Amount of Nitrogen Used for Photosynthesis Modulates Molecular Evolution in Plants. Mol. Biol. Evol. 2018, 35, 1616–1625. [Google Scholar] [CrossRef]
- Brown, R.H. A difference in N use efficiency in C3 and C4 plants and its implications in adaptation and evolution. Crop Sci. 1987, 18, 93–98. [Google Scholar] [CrossRef]
- Makino, A.; Sakuma, H.; Sudo, E.; Mae, T. Differences between maize and rice in N-use efficiency for photosynthesis and protein allocation. Plant Cell Physiol. 2003, 44, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.; Ferario-Mery, S.; Noctor, G. Interactions between carbon and nitrogen metabolism. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 237–254. [Google Scholar]
- Drożak, A.; Romanowska, E. Acclimation of mesophyll and bundle sheath chloroplasts of maize to different irradiances during growth. Biochim. Biophys. Acta 2006, 1757, 1539–1546. [Google Scholar] [CrossRef][Green Version]
- Romanowska, E.; Drożak, A. Comparison of photochemical activities in mesophyll and bundle sheath chloroplasts of C4 subtypes growing in moderate light. Acta Biochim. Pol. 2006, 53, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Tazoe, Y.; Noguchi, K.; Terashima, I. Effects of growth light and nitrogen nutrition on the organization of the photosynthetic apparatus in leaves of a C4 plant, Amaranthus cruentus. Plant Cell Environ. 2005, 29, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.D. C4 Photosynthesis: An Unlikely Process Full of Surprises. Plant Cell Physiol. 1992, 33, 333–342. [Google Scholar] [CrossRef]
- Romanowska, E.; Drożak, A.; Pokorska, B.; Shiell, B.J.; Michalski, W.P. Organization and activity of photosystems in the mesophyll and bundle sheath chloroplasts of maize. J. Plant Physiol. 2006, 163, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A.; Snyder, G.W.; Portis, A.R., Jr.; Orgen, W.L. Growth and photosynthesis under high and low irradiance of Arabidopsis thaliana antisense mutants with reduced ribulose-1,5-bisphosphate carboxylase/oxygenase activase content. Plant Physiol. 1997, 113, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Seemann, J.R. Differences between Wheat Genotypes in Specific Activity of Ribulose-1,5-bisphosphate Carboxylase and the Relationship to Photosynthesis. Plant Physiol. 1984, 74, 759–765. [Google Scholar] [CrossRef]
- Furbank, R.T.; Taylor, W.C. Regulation of photosynthesis in C3 and C4 plants: A molecular approach. Plant Cell 1995, 7, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.D.; Agostino, A.; Jenkins, C. Measurement of the Leakage of CO2 from Bundle-Sheath Cells of Leaves during C4 Photosynthesis. Plant Physiol. 1995, 108, 173–181. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Furbank, R.T. Modeling C4 photosynthesis. In C4 Plant Biology; Sage, R.F., Monson, R.K., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 173–211. [Google Scholar]
- Bräutigam, A.; Gowik, U. Photorespiration connects C3 and C4 photosynthesis. J. Exp. Bot. 2016, 67, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Jolivet-Tournier, P.; Gerster, R. Incorporation of Oxygen into Glycolate, Glycine, and Serine during Photorespiration in Maize Leaves. Plant Physiol. 1984, 74, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Lea, P.J. The role of peroxisomes in the integration of metabolism and evolutionary diversity of photosynthetic organisms. Phytochemistry 2002, 60, 651–674. [Google Scholar] [CrossRef]
- Long, S.P. Environmental responses. In C4 Plant Biology; Sage, R.F., Monson, R.K., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 215–249. [Google Scholar]
- Kanai, R.; Edwards, G.E. The Biochemistry of C4 Photosynthesis. C4 Plant Biol. 1999, 49, 87. [Google Scholar] [CrossRef]
- Ghannoum, O.; Evans, J.R.; Chow, W.S.; Andrews, T.J.; Conroy, J.P.; von Caemmerer, S. Faster Rubisco is the Key to Superior Nitrogen-Use Efficiency in NADP-Malic Enzyme Relative to NAD-Malic Enzyme C4 Grasses. Plant Physiol. 2005, 137, 638–650. [Google Scholar] [CrossRef]
- Pittermann, J.; Sage, R.F. The response of the high altitude C4 grass Muhlenbergia montana (Nutt.) A.S. Hitchc. to long- and short-term chilling. J. Exp. Bot. 2001, 52, 829–838. [Google Scholar] [CrossRef]
- Sugiyama, T.; Mizuno, M.; Hayashi, M. Partitioning of nitrogen among ribulose-1,5-bisphosphate carboxylase/oxygenase, phosphoenolpyruvate carboxylase, and pyruvate orthophosphate dikinase as related to bio-mass productivity in maize seedlings. Plant Physiol. 1984, 75, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Martin, A.; Terce-Laforgue, T.; Gonzalez-Moro, M.B.; Estavillo, J.M. Physiology of maize I: A comprehensive and integrated view of nitrogen metabolism in a C4 plant. Physiol. Plant. 2005, 124, 167–177. [Google Scholar] [CrossRef]
- Paul, M.J.; Pellny, T.K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003, 54, 539–547. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Long, S.P.; Farage, P.K.; Rohrhofer, U. Separating the contribution of the upper and lower mesophyll to photosynthesis in Zea mays L. leaves. Planta 1989, 177, 207–216. [Google Scholar] [CrossRef]
- Zienkiewicz, M.; Drożak, A.; Wasilewska, W.; Bacławska, I.; Przedpełska-Wąsowicz, E.; Romanowska, E. The short-term response of Arabidopsis thaliana (C3) and Zea mays (C4) chloroplasts to red and far red light. Planta 2015, 242, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Romanowska, E.; Kargul, J.; Powikrowska, M.; Finazzi, G.; Nield, J.; Drożak, A.; Pokorska, B. Structural organization of photosynthetic apparatus in agranal chloroplasts of maize. J. Biol. Chem. 2008, 283, 26037–26046. [Google Scholar] [CrossRef]
- Rogowski, P.; Wasilewska-Dębowska, W.; Urban, A.; Romanowska, E. Maize bundle sheath chloroplasts—A unique model of permanent State 2. Environ. Exp. Bot. 2018, 155, 321–331. [Google Scholar] [CrossRef]
- Terashima, I.; Evans, J.R. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 1988, 29, 143–155. [Google Scholar]
- Hikosaka, K.; Terashima, I.; Katoh, S. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 1994, 97, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K. Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta 1996, 198, 144–150. [Google Scholar] [CrossRef]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2018, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Within-Leaf Nitrogen Allocation in Adaptation to Low Nitrogen Supply in Maize during Grain-Filling Stage. Front. Plant Sci. 2016, 7, 699. [Google Scholar] [CrossRef] [PubMed]
- Leegood, R.C.; Walker, R.P. Regulation and roles of phosphoenolpyruvate carboxykinase in plants. Arch. Biochem. Biophys. 2003, 414, 204–210. [Google Scholar] [CrossRef]
- Bellasio, C.; Griffiths, H. The Operation of Two Decarboxylases, Transamination, and Partitioning of C4 Metabolic Processes between Mesophyll and Bundle Sheath Cells Allows Light Capture To Be Balanced for the Maize C4 Pathway. Plant Physiol. 2013, 164, 466–480. [Google Scholar] [CrossRef]
- Sharwood, R.; Sonawane, B.V.; Ghannoum, O. Photosynthetic flexibility in maize exposed to salinity and shade. J. Exp. Bot. 2014, 65, 3715–3724. [Google Scholar] [CrossRef]
- Arrivault, S.; Obata, T.; Szecówka, M.; Mengin, V.; Guenther, M.; Hoehne, M.; Fernie, A.R.; Stitt, M. Metabolite pools and carbon flow during C4 photosynthesis in maize: 13CO2 labeling kinetics and cell type fractionation. J. Exp. Bot. 2016, 68, 283–298. [Google Scholar] [CrossRef]
- Sheen, J. C4 gene expression. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 187–217. [Google Scholar] [CrossRef]
- Hirel, B.; Bertin, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a Better Understanding of the Genetic and Physiological Basis for Nitrogen Use Efficiency in Maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; Wang, B.; Wang, X.; Lai, J.; Tian, F. Transcriptome sequencing reveals the roles of transcriptionfactors in modulating genotype by nitrogen interaction in maize. Plant Cell. Rep. 2015, 34, 1761–1771. [Google Scholar] [CrossRef]
- Patel, M.; Berry, J.O. Rubisco gene expression in C4 plants. J. Exp. Bot. 2007, 59, 1625–1634. [Google Scholar] [CrossRef]
- Usuda, H.; Ku, M.S.B.; Edwards, G.E. Influence of light intensity during growth on photosynthesis and activity of several key photosynthetic enzymes in a C4 plant (Zea mays). Physiol. Plant. 1985, 63, 65–70. [Google Scholar] [CrossRef]
- Gardeström, P.; Igamberdiev, A.U. The origin of cytosolic ATP in photosynthetic cells. Physiol. Plant. 2016, 157, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Wolt, J.D. Soil Solution Chemistry: Applications to Environmental Science and Agriculture; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Lillo, C. Light regulation of nitrate uptake, assimilation and metabolism. In Nitrogen Acquisition and Assimilation in Higher Plants; Plant Ecophysiology; Amancio, S., Stulen, I., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2004; pp. 149–184. [Google Scholar]
- Shi-Wei, G.; Yi, Z.; Ying-Xu, G.; Yong, L.; Qi-Rong, S. New insights into the nitrogen form effect on photosynthesis and photorespiration. Pedosphere 2007, 17, 601–610. [Google Scholar]
- Glass, A.D.M.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Okamoto, M.; Rawat, S.; Sid-Diqi, M.Y.; Unkles, S.E.; et al. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 2002, 53, 855–864. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Huber, S.C. Post translational regulation of nitrate reductase in higher plants. Plant Physiol. 1994, 106, 817–821. [Google Scholar] [CrossRef]
- Kopriva, S.; Koprivova, A. Sulfate assimilation and glutathione synthesis in C4 plants. Photosynth. Res. 2005, 86, 363–372. [Google Scholar] [CrossRef]
- de Bruijn, F. Biological Nitrogen Fixation. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Sugiura, M.; Georgescu, M.N.; Takahashi, M. A Nitrite Transporter Associated with Nitrite Uptake by Higher Plant Chloroplasts. Plant Cell Physiol. 2007, 48, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Friso, G.; Majeran, W.; Huang, M.; Sun, Q.; van Wijk, K.J. Reconstruction of metabolic pathways, protein expression, and homeostasis machineries across maize bundle sheath and mesophyll chloroplasts: Large-scale quantitative proteomics using the first maize genome assembly. Plant Physiol. 2010, 152, 1219–1250. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Ireland, R.J. Nitrogen metabolism in higher plants. In Plant Amino Acids Biochemistry and Biotechnology; Singh, B.K., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 1–47. [Google Scholar]
- Feng, Z.; Rütting, T.; Pleijel, H.; Wallin, G.; Reich, P.; Kammann, C.I.; Newton, P.C.; Kobayashi, K.; Luo, Y.; Uddling, J. Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Glob. Chang. Biol. 2015, 21, 3152–3168. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, eaaq1012. [Google Scholar] [CrossRef]


| Protein-N (%) | ||
|---|---|---|
| Maize (C4) | Rice (C3) | |
| Soluble protein | 33 | 50 |
| Insoluble protein | 53 | 37 |
| Rubisco | 8.5 | 27 |
| Thylakoid complexes (PSI, PSII, LHCII, cyt b6f, CF1/CF0) | 34 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urban, A.; Rogowski, P.; Wasilewska-Dębowska, W.; Romanowska, E. Understanding Maize Response to Nitrogen Limitation in Different Light Conditions for the Improvement of Photosynthesis. Plants 2021, 10, 1932. https://doi.org/10.3390/plants10091932
Urban A, Rogowski P, Wasilewska-Dębowska W, Romanowska E. Understanding Maize Response to Nitrogen Limitation in Different Light Conditions for the Improvement of Photosynthesis. Plants. 2021; 10(9):1932. https://doi.org/10.3390/plants10091932
Chicago/Turabian StyleUrban, Aleksandra, Paweł Rogowski, Wioleta Wasilewska-Dębowska, and Elżbieta Romanowska. 2021. "Understanding Maize Response to Nitrogen Limitation in Different Light Conditions for the Improvement of Photosynthesis" Plants 10, no. 9: 1932. https://doi.org/10.3390/plants10091932
APA StyleUrban, A., Rogowski, P., Wasilewska-Dębowska, W., & Romanowska, E. (2021). Understanding Maize Response to Nitrogen Limitation in Different Light Conditions for the Improvement of Photosynthesis. Plants, 10(9), 1932. https://doi.org/10.3390/plants10091932





