A Novel and Efficient In Vitro Organogenesis Approach for Ajuga lupulina Maxim
Abstract
:1. Introduction
2. Results
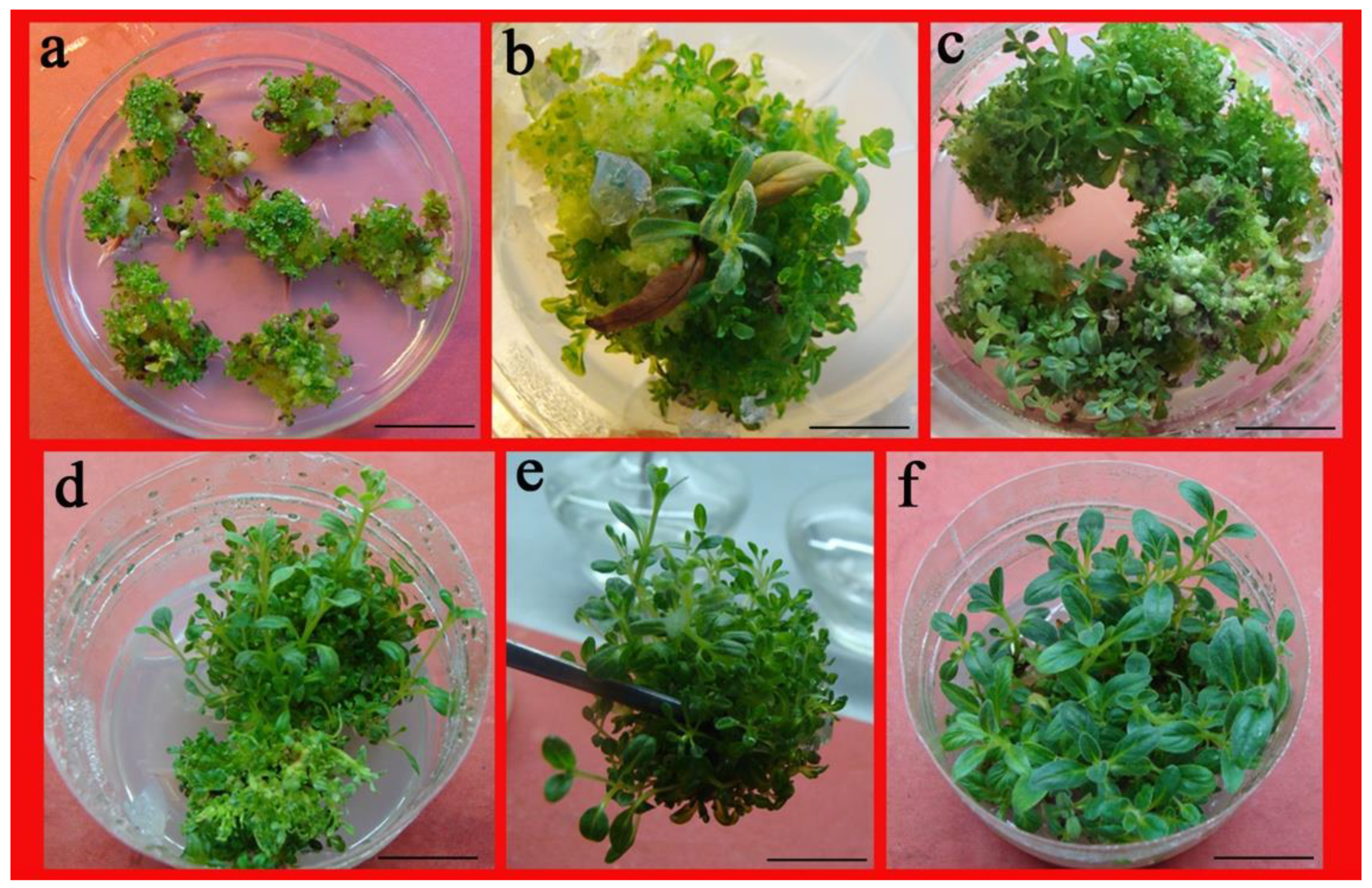
2.1. Impact of PGRs on Axillary Shoot Proliferation from Nodal Segments
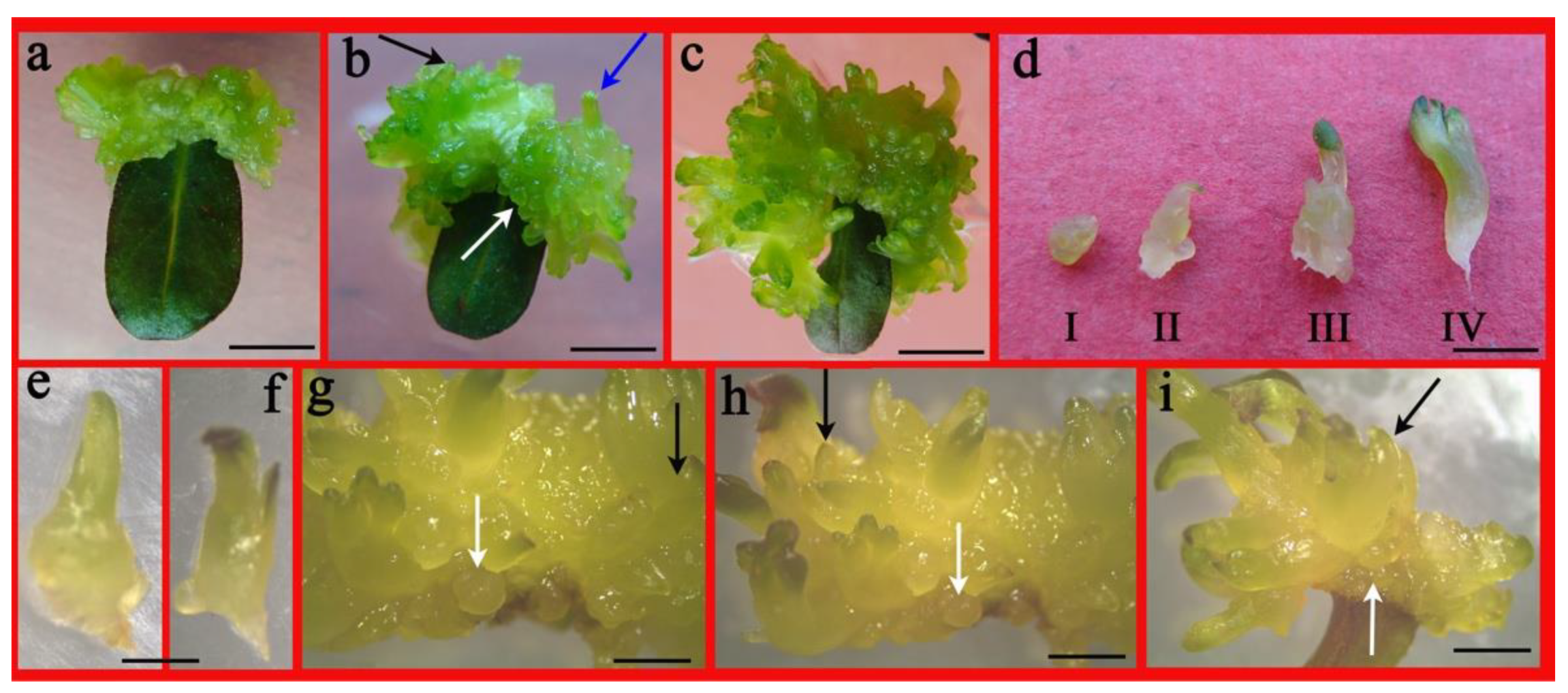
2.2. Impact of PGRs on Shoot Proliferation from Leaf
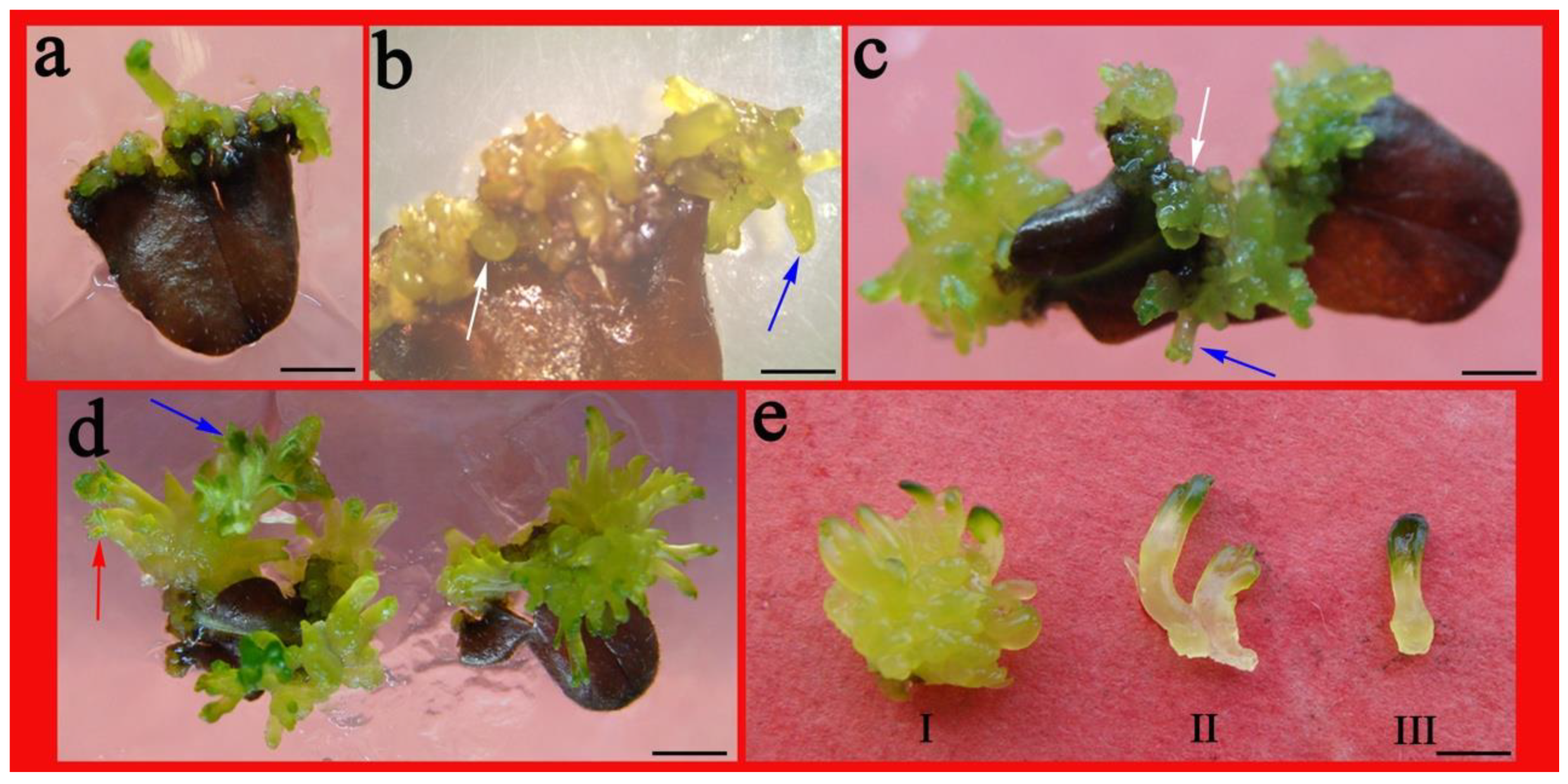
2.3. Impact of PGRs on Adventitious Shoot Regeneration from Shoot Tips
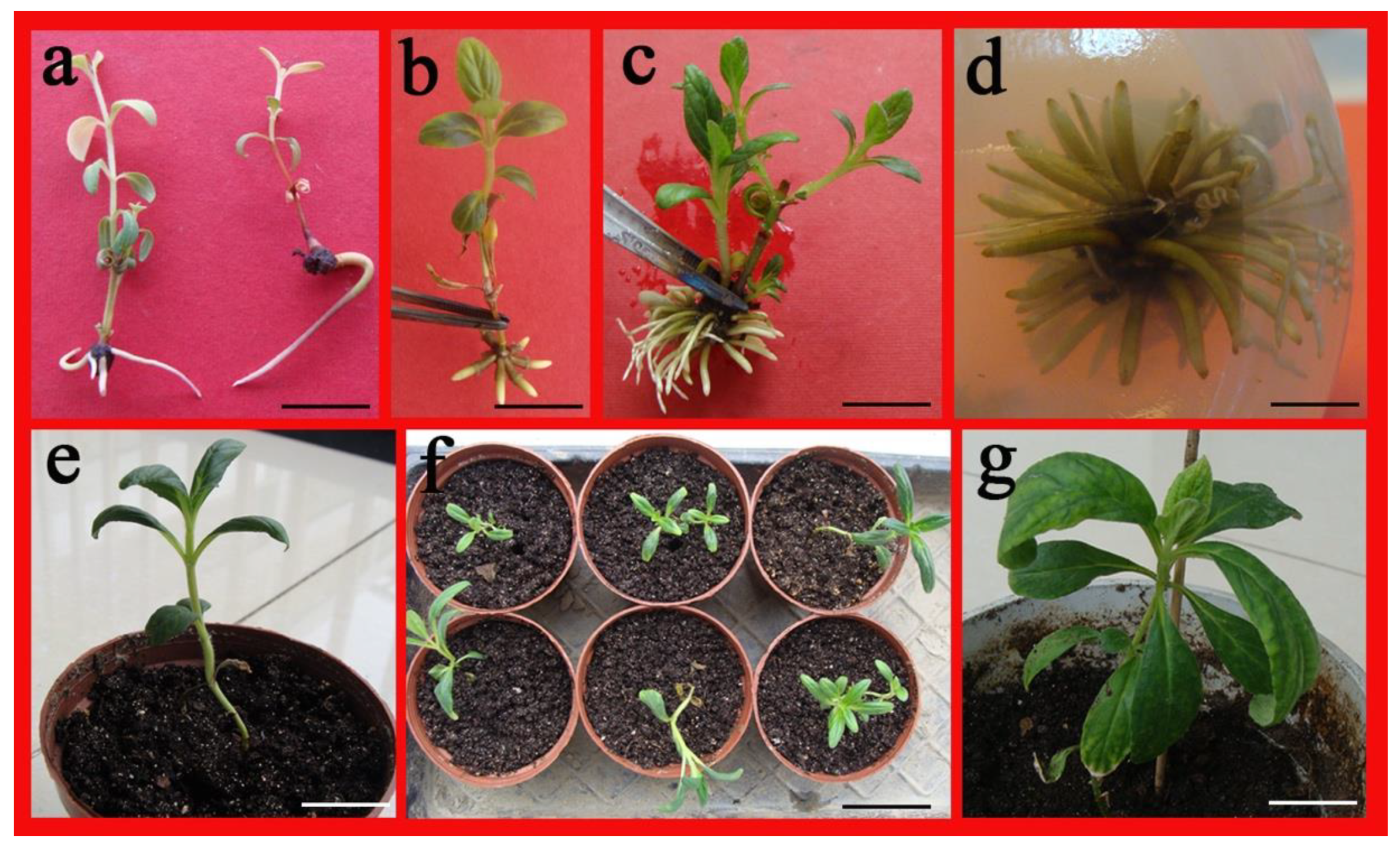
2.4. Root Formation in Regenerated Shoots
2.5. Acclimatization of Regenerated Plants
3. Discussion
3.1. Axillary Shoot Proliferation
3.2. Embryo-Like Structures (ELSs)
3.3. Rooting
3.4. Optimal Explants
4. Materials and Methods
4.1. Plant Source and Culture Details
4.2. Effect of PGRs on Leaf-Induced Adventitious Shoots and Somatic Embryo-Analogy like Structures (ELSs)
4.3. Effect of PGRs on Shoot Tip Adventitious Shoot Regeneration
4.4. Root Formation, Acclimation and Transplantation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atay, I.; Kirmizibekmez, H.; Kaiser, M.; Akaydin, G.; Yesilada, E. Evaluation of in vitro antiprotozoal activity of Ajuga laxmannii and its secondary metabolites. Pharm. Biol. 2016, 54, 1808–1814. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Kim, D.H.; Sivanesan, I. Micropropagation of Ajuga species: A mini review. Biotechnol. Lett. 2017, 39, 1291–1298. [Google Scholar] [CrossRef]
- Rukh, G.; Ahmad, N.; Rab, A.; Ahmad, N.; Fazal, H.; Akbar, F. Photo-dependent somatic embryogenesis from non-embryogenic calli and its polyphenolics content in high-valued medicinal plant of Ajuga bracteosa. J. Photochem. Photobiol. B. 2018, 190, 59–65. [Google Scholar] [CrossRef]
- Kayani, W.K.; Dilshad, E.; Ahmed, T.; Ismail, H.; Mirza, B. Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities. BMC Complement Altern. Med. 2016, 16, 375. [Google Scholar] [CrossRef] [Green Version]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.-M.; Moldovan, C.; Oniga, I. Phytochemical Composition, Antioxidant, Antimicrobial and in Vivo Anti-inflammatory Activity of Traditionally Used Romanian Ajuga laxmannii (Murray) Benth. (“Nobleman’s Beard”—Barba Împăratului). Front. Pharmacol. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin-Florian, R.; Vlase, L.; Casian, T.; Sesarman, A.; Gheldiu, A.; Toiu, A. Biologically active Ajuga species extracts modulate supportive processes for cancer cell development. Front. Pharmacol. 2019, 10, 334. [Google Scholar]
- China Pharmacopoeia Commission. Chinese Pharmacopeia; China Medical Science Press: Beijing, China, 2015; p. 346. [Google Scholar]
- Chen, X.; Ning, R. The crude medicine of Gansu Luqu is used as the ornamental plant II. South China Agric. 2011, 5, 7–10. [Google Scholar]
- Wang, G.Q. National Chinese Herbal Medicine Compilation; People’s Medical Publishing House: Beijing, China, 1996; Volume 2, p. 389. [Google Scholar]
- Chen, H.; Tan, R.X.; Liu, Z.L.; Zhang, Y.; Yang, L. Antibacterial neoclerodane diterpenoids from Ajuga lupulina. J. Nat. Prod. 1996, 59, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Ko, C.H.; Lee, J.P.; Jeong, B.R. Influence of cytokinins on adventitious shoot regeneration from leaf and petiole explants of Ajuga multiflora Bunge. Propag. Ornam. Plants 2011, 11, 156–158. [Google Scholar]
- Kaul, S.; Das, S.; Srivastava, P.S. Micropropagation of Ajuga bracteosa, a medicinal herb. Physiol. Mol. Biol. Plants 2013, 19, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyyakkannu, S.; Kumar, R.; Rafi, S.; Ahmad, N.; Zamany, J. In vitro propagation, carotenoid, fatty acid and tocopherol content of Ajuga multiflora Bunge. 3 Biotech 2016, 6, 1–10. [Google Scholar]
- Ahmad, N.; Anis, M. Rapid clonal multiplication of a woody tree, Vitex negundo L. through axillary shoots proliferation. Agrofor. Syst. 2007, 71, 195–200. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xiong, Y.P.; Yu, X.C.; Pang, J.H.; Ma, G. Adventitious shoot organogenesis from leaf explants of Portulaca pilosa L. Sci. Rep. 2020, 10, 3675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.G.; Yang, H.L.; Sun, Y.X.; Hu, J.Y.; Zou, L.J. Organogenesis and high-frequency plant regeneration in Caryopteris terniflora Maxim. using thidiazuron. In Vitro Cell Dev.-Plants 2021, 57, 39–47. [Google Scholar] [CrossRef]
- Makowczyńska, J.; Sliwinska, E.; Kalemba, D.; Piątczak, E.; Wysokińska, H. In vitro propagation, DNA content and essential oil composition of Teucrium scorodonia L. ssp. scorodonia. Plant Cell Tissue Organ Cult. 2016, 127, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sivanesan, I.; Park, S.W. Effect of plant growth regulators on axillary shoot multiplication from nodal explants of Ajuga multiflora bunge. Propag. Ornam. Plants 2015, 15, 42–44. [Google Scholar]
- Saeed, W.; Naseem, S.; Id, A.; Gohar, D. Efficient and reproducible somatic embryogenesis and micropropagation in tomato via novel structures -Rhizoid Tubers. PLoS ONE 2019, 14, e0215929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, P.; Singh, S.; Yadav, S.; Tran, L.S.P. Pretreatment of seeds with thidiazuron delimits its negative effects on explants and promotes regeneration in chickpea (Cicer arietinum L.). Plant Cell Tissue Oran Cult. 2018, 133, 103–114. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Y.L.; Lu, J.F.; Sliva, J.A.T.; Zhang, X.H.; Ma, G.H. Somatic embryogenesis and enhanced shoot organogenesis in Metabriggsia ovalifolia W. T. Wang. Sci. Rep. 2015, 6, 24662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solís, J.J.; Reyna, M.; Feria, M.D.; Cardona, M.A.; Rojas, D. In vitro propagation of Echeveria elegans, a species of the flora endangered Mexican. J. Environ. Sci. Eng. B 2013, 2, 555–558. [Google Scholar]
- Baskaran, P.; Moyo, M.; Staden, J. In vitro plant regeneration, phenolic compound production and pharmacological activities of Coleonema pulchellum. S. Afr. J. Bot. 2014, 90, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Lü, J.F.; Chen, R.; Zhang, M.H.; Da Silva Teixeira, J.A.; Ma, G.H. Plant regeneration via somatic embryogenesis and shoot organogenesis from immature cotyledons of Camellia nitidissima Chi. J. Plant Physiol. 2013, 170, 1202–1211. [Google Scholar] [CrossRef]
- Sivanesan, I.; Jeong, B.R. Direct shoot regeneration from nodal explants of Sida cordifolia Linn. In Vitro Cell Dev.-Plants 2007, 43, 436–441. [Google Scholar] [CrossRef]
- Karakas, F.P. Efficient plant regeneration and callus induction from nodal and hypocotyl explants of Goji berry (Lycium barbarum L.) and comparison of phenolic profiles in calli formed under different combinations of plant growth regulators. Plant Physiol. Biochem. 2020, 146, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Sherif, N.A.; Benjamin, J.F.; Kumar, T.S.; Rao, M.V. Somatic embryogenesis, acclimatization and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl. an endangered terrestrial jewel orchid. Plant Cell Tissue Organ. Cult. 2018, 132, 303–316. [Google Scholar] [CrossRef]
- Khanam, M.N.; Anis, M. Organogenesis and efficient in vitro plantlet regeneration from nodal segments of Allamanda cathartica L. using TDZ and ultrasound assisted extraction of quercetin. Plant Cell Tissue Organ Cult. 2018, 134, 241–250. [Google Scholar] [CrossRef]
- Singh, C.; Raj, S.; Patil, V.; Jaiswal, P.; Subhash, N. Plant regenerationfrom leaf explants of mature sandalwood (Santalum album L.) trees under in vitro conditions. In Vitro Cell Dev. Biol. Plant 2013, 49, 216–222. [Google Scholar] [CrossRef]
- Kumari, A.; Baskaran, P.; Staden, J.V. In vitro propagation via organogenesis and embryogenesis of Cyrtanthus mackenii: A valuable threatened medicinal plant. Plant Cell Tissue Organ Cult. 2017, 131, 407–415. [Google Scholar] [CrossRef]
- Zeng, Q.; Han, Z.; Kang, X. Adventitious shoot regeneration from leaf, petiole and root explants in triploid (Populus alba × P. glandulosa) × P. tomentosa. Plant Cell Tissue Organ Cult. 2019, 138, 121–130. [Google Scholar] [CrossRef]
- Kumar, S.; Kumaria, S.; Tandon, P. Efficient in vitro plant regeneration protocol from leaf explant of Jatropha curcas L - a promising biofuel plant. J. Plant Biochem. Biotechnol. 2010, 19, 273–275. [Google Scholar] [CrossRef]
- Srinivasan, P.; Raja, H.D.; Tamilvanan, R. Effect of coconut water and cytokinins on rapid micropropagation of Ranunculus wallichianus Wight & Arnn-a rare and endemic medicinal plant of the Western Ghats, India. In Vitro Cell Dev. Biol. Plant 2021, 57, 365–371. [Google Scholar]
- Al-Qudah, T.; Shibli, R.A.; Alali, F.Q. In vitro propagation and secondary metabolites production in wild germander (Teucrium polium L.). In Vitro Cell Dev. Biol. Plant 2011, 47, 496–505. [Google Scholar] [CrossRef]
- Piatczak, E.; Wysokinska, H. In vitro regeneration of Centaurium erthraea Rafn from shoot tips and other seedling explants. Acta Soc. Bot. Pol. 2003, 72, 283–288. [Google Scholar] [CrossRef]
- Chen, X.W.; Shao, L.; Liang, L.; Pan, Z.T. Effect of activated charcoal on rooting in tissue culture seedling of Begonia fimbristipula on Dinghushan Mountain. J. Chin. Mater. Med. 2012, 35, 1369. [Google Scholar]
- Pan, M.; Staden, J.V.; Debergh, P. The effect of activated charcoal and auxins on root formation by hypocotyl segments of Daucus carota. S. Afr. J. Bot. 2002, 68, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.H.; Hung, Y.L.; Wu, H.W.; Pan, Z.J.; Hong, C.Y.; Wang, C.N. Shoot regeneration process and optimization of agrobacterium-mediated transformation in Sinningia speciosa. Plant Cell Tissue Organ Cult. 2018, 134, 317. [Google Scholar] [CrossRef] [Green Version]
- Satish, L.; Ceasar, S.A.; Ramesh, M. Improved agrobacterium-mediated transformation and direct plant regeneration in four cultivars of finger millet (eleusine coracana (L.) gaertn.). Plant Cell Tissue Organ Cult. 2017, 131, 547–565. [Google Scholar] [CrossRef]

| Cytokinin (mg L−1) | Axillary Shoot Proliferation (Per Explant) | Visible Appearance | ||
|---|---|---|---|---|
| The Color of the Leaves | Callus | Shoot Growth State | ||
| Control | 0 | Nil | Nil | Nil |
| BA 2.0 | 7.7 ± 0.5 d | Dark-green; | a small amount; | +++ |
| BA 3.0 | 10.2 ± 0.1 ab | Dark-green; | a small amount; | ++++ |
| BA 4.0 | 9.0 ± 0.3 b | Dark-green; | a small amount; | +++ |
| TDZ 2.0 | 1.5 ± 0.1 e | Dark-green; | a large amount; | +++ |
| TDZ 3.0 | 2.2 ± 0.2 e | Dark-green; | a large amount; | +++ |
| TDZ 4.0 | 1.8 ± 0.3 e | Dark-green; | a large amount; | +++ |
| ZT 2.0 | 8.3 ± 0.5 c | Light-green; | no callus; | ++ |
| ZT 3.0 | 9.6 ± 0.3 b | Light-green; | no callus; | ++ |
| ZT 4.0 | 9.4 ± 0.4 b | Light-green; | no callus; | |
| KT 2.0 | 9.1 ± 0.7 b | Light-green; | no callus; | +Hyperhydricity |
| KT 3.0 | 11.6 ± 0.3 a | Light-green; | no callus; | +Hyperhydricity |
| KT 4.0 | 10.4 ± 0.2 ab | Light-green; | no callus; | +Hyperhydricity |
| Treatment (mg L−1) | Browned (%) | Response (%) | Shoots Number | ELSs Number | In Vitro Morphogenesis |
|---|---|---|---|---|---|
| BA 3.0 | 75.4 ± 2.4 b | 43.1 ± 2.2 d | 5.5 ± 1.4 e | 3.3 ± 1.5 d | Leaf blade browned; adventitious shoot; globular somatic-analogy embryo (ELSs) buds |
| BA 4.0 | 77.2 ± 3.6 b | 47.5 ± 3.2 cd | 7.8 ± 2.3 e | 4.7 ± 1.4 d | Leaf blade browned; adventitious shoot; globular ELSs buds |
| BA 3.0 + NAA 0.1 | 72.5 ± 2.4 b | 54.2 ± 2.1 c | 12.4 ± 0.9 d | 7.6 ± 0.7 c | Leaf blade browned; adventitious shoot; globular ELSs buds |
| BA 4.0 + NAA 0.1 | 70.2 ± 3.1 b | 57.6 ± 1.9 c | 13.5 ± 3.2 d | 8.7 ± 1.4 b | Leaf blade browned; adventitious shoot; globular ELSs buds |
| TDZ 3.0 | 32.5 ± 2.5 c | 72.4 ± 2.5 b | 17.5 ± 5.6 c | 5.3 ± 1.2 c | Leaves darkened; adventitious shoot; globular ELSs buds |
| TDZ 4.0 | 35.7 ± 3.2 c | 79.3 ± 3.4 b | 20.8 ± 2.1 bc | 6.3 ± 0.8 c | Leaves darkened; adventitious shoot; globular ELSs buds |
| TDZ 3.0 + NAA 0.1 | 25.5 ± d | 83.5 ± 3.2 a | 22.5 ± 3.2 ab | 9.3 ± 1.4 b | Leaves darkened; adventitious shoot; globular ELSs buds |
| TDZ 4.0 + NAA 0.1 | 27.8 ± d | 81.6 ± 3.5 ab | 26.4 ± 2.1 a | 12.0 ± 2.2 a | Leaves darkened; adventitious shoot; globular ELSs buds |
| Treatment (mg L−1) | Callogenesis (%) | Shoot Proliferation Coefficient | Observed Results |
|---|---|---|---|
| Control | 0 b | 0 c | Nil |
| NAA 0.01 + TDZ 2.0 | 100 a | 25.4 ± 2.2 a | Light-green callus; Soft and fragile; Dwarf shoot |
| NAA 0.01 + 6-BA 2.0 | 100 a | 18.5 ± 3.7 b | Light-green callus; Soft and fragile; Robust shoot |
| Treatment (mg L−1) | Culture Time | Browned (%) | Rooting (%) | Mean Number of Shoots with Roots | Morphology | |
|---|---|---|---|---|---|---|
| 3 Weeks | 5 Weeks | |||||
| 0 | − | − | 88.4 ± 3.4 a | 0 e | 0 d | — |
| IBA 0.5 | − | − | 75.4 ± 4.1 b | 0 e | 0 e | White |
| IBA 1.0 | + | + | 52.8 ± 2.2 d | 32.5 ± 1.2 c | 3.1 ± 0.3 d | White |
| IBA 1.5 | + | + | 54.6 ± 2.3 d | 40.6 ± 1.4 c | 5.3 ± 0.5 c | White |
| IBA 2.0 | + | + | 58.8 ± 2.4 d | 35.3 ± 1.1 c | 3.3 ± 0.7 d | White |
| NAA 0.1 | − | − | 77.7 ± 2.6 b | 0 e | 0 d | Green |
| NAA 0.5 | − | + | 68.3 ± 3.2 c | 22.5 ± 1.8 d | 5.4 ± 0.1 c | Green |
| NAA1.0 | − | + | 65.2 ± 0.2 c | 17.4 ± 1.3 d | 3.5 ± 0.2 d | Green |
| IBA 1.0 + NAA 0.5 | + | + | 32.5 ± 1.7 e | 70.1 ± 2.1 b | 14.6 ± 0.2 b | White |
| IBA 1.5 + NAA 0.5 | + | + | 35.7 ± 2.3 e | 81.71 ± 2.4 a | 25.8 ± 0.6 a | White |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Yang, H.; Yang, Y.; He, J.; Aer, E.; Ma, Y.; Zou, L. A Novel and Efficient In Vitro Organogenesis Approach for Ajuga lupulina Maxim. Plants 2021, 10, 1918. https://doi.org/10.3390/plants10091918
Wu Q, Yang H, Yang Y, He J, Aer E, Ma Y, Zou L. A Novel and Efficient In Vitro Organogenesis Approach for Ajuga lupulina Maxim. Plants. 2021; 10(9):1918. https://doi.org/10.3390/plants10091918
Chicago/Turabian StyleWu, Qinggui, Honglin Yang, Yulin Yang, Jinyu He, Erga Aer, Yonghong Ma, and Lijuan Zou. 2021. "A Novel and Efficient In Vitro Organogenesis Approach for Ajuga lupulina Maxim" Plants 10, no. 9: 1918. https://doi.org/10.3390/plants10091918
APA StyleWu, Q., Yang, H., Yang, Y., He, J., Aer, E., Ma, Y., & Zou, L. (2021). A Novel and Efficient In Vitro Organogenesis Approach for Ajuga lupulina Maxim. Plants, 10(9), 1918. https://doi.org/10.3390/plants10091918





