Chemical Profile, Antioxidant, Antimicrobial, and Anticancer Activities of the Water-Ethanol Extract of Pulicaria undulata Growing in the Oasis of Central Saudi Arabian Desert
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Constituents from LC-MS Analysis of the Water-Ethanol Extract
2.2. Total Phenolics and Flavonoids Contents
2.3. Trace Elements Analysis
2.4. Antioxidant Potential of the P. undulata Water-Ethanol Extract
2.5. Antimicrobial Activity of the P. undulata Water-Ethanol Extract
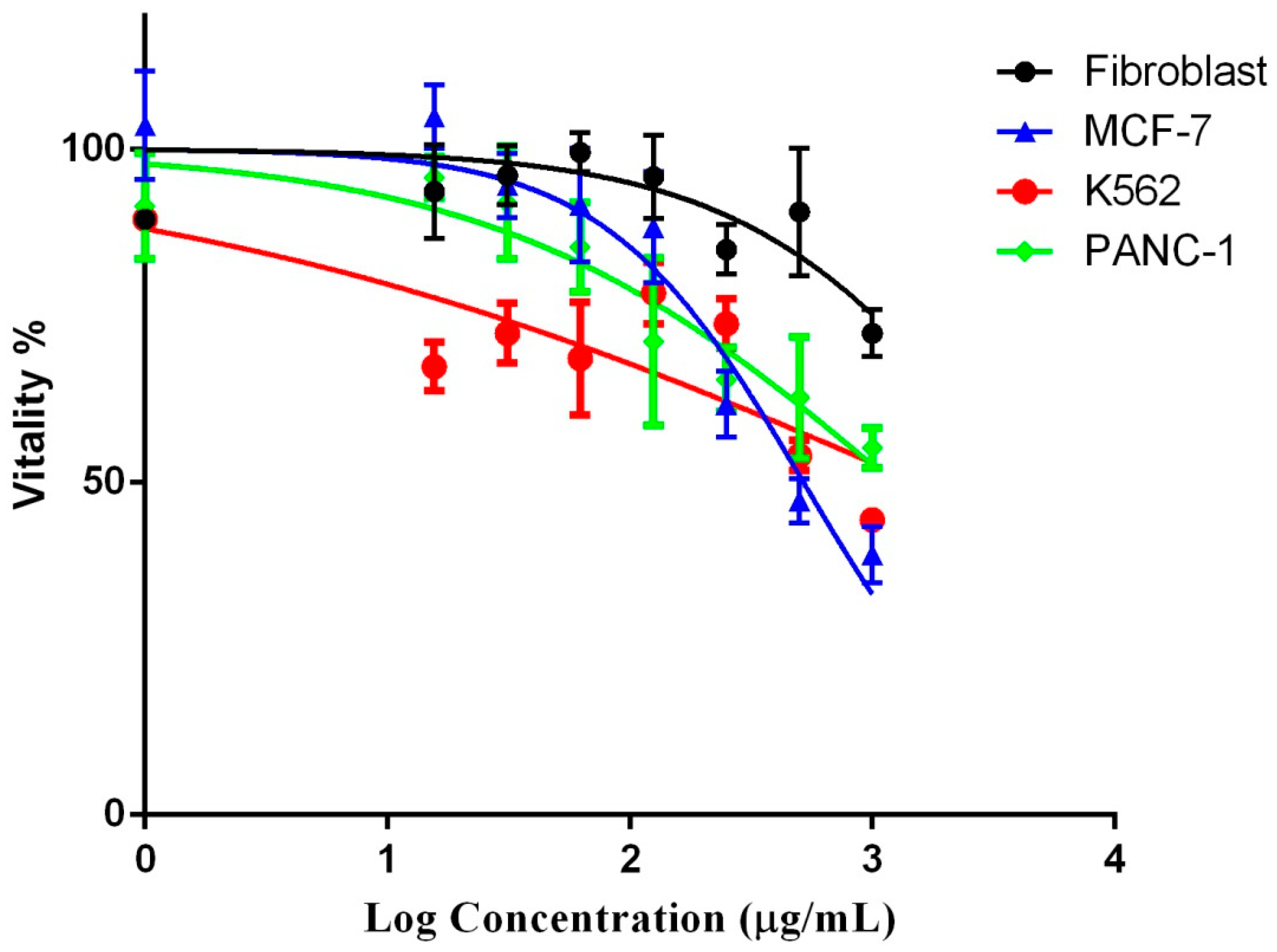
2.6. Anticancer Activity of the P. undulata Water-Ethanol Extract
3. Materials and Methods
3.1. Chemicals, Reagents and Materials
3.2. Plant Materials and Extraction
3.3. Liquid Chromatography-Mass Spectroscopy (LC-MS) Analysis
3.4. Quantitative Measurements of the Total Phenolics and Flavonoids Contents
3.5. Trace Elements Analysis of the P. undulata Water-Ethanol Extract
3.6. Antioxidant Activity of the P. undulata Water-Ethanol Extract
3.6.1. Total Antioxidant Capacity
3.6.2. DPPH Scavenging Activity
3.6.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6.4. Metal Chelating Activity
3.7. Antimicrobial Assay of the P. undulata Water-Ethanol Extract
3.7.1. Preliminary Antimicrobial Screenings
3.7.2. Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentrations (MBC)
3.8. Cytotoxic Assay of the P. undulata Water-Ethanol Extract
3.9. Annexin V Assay of the P. undulata Water-Ethanol Extract
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, T.; Gherib, M.; Bekhechi, C.; Atik-Bekkara, F.; Casabianca, H.; Tomi, F.; Casanova, J.; Bighelli, A. Thymyl esters derivatives and a new natural product modhephanone from Pulicaria mauritanica Coss. (Asteraceae) root oil. Flavour Fragr. J. 2015, 30, 83–90. [Google Scholar] [CrossRef]
- Emam, M.A.; Khattab, H.I.; Hegazy, M.G.A. Assessment of anticancer activity of Pulicaria undulata on hepatocellular carcinoma HepG2 cell line. Tumor Biol. 2019, 41, 1010428319880080. [Google Scholar] [CrossRef]
- Boumaraf, M.; Mekkiou, R.; Benyahia, S.; Chalchat, J.; Chalard, P.; Benayache, F.; Benayache, S. Essential oil composition of Pulicaria undulata (L.) DC. (Asteraceae) growing in Algeria. Int. J. Pharmacogn. Phytochem. Res 2016, 8, 746–749. [Google Scholar]
- Ali, N.A.A.; Sharopov, F.S.; Alhaj, M.; Hill, G.M.; Porzel, A.; Arnold, N.; Setzer, W.N.; Schmidt, J.; Wessjohann, L. Chemical composition and biological activity of essential oil from Pulicaria undulata from Yemen. Nat. Prod. Comm. 2012, 7, 1934578X1200700238. [Google Scholar] [CrossRef]
- Mohamed, E.A.A.; Muddathir, A.M.; Osman, M.A. Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.J.; Porter, R.B.R.; Rainford, L.; Dunbar, O.; Francis, S.; Laurieri, N.; Delgoda, R. Chemical composition and biological activities of the essential oil from Cleome rutidosperma DC. Fitoterapia 2018, 129, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Rav, M.; Valizadeh, J.; Noroozifar, M.; Khorasani-Motlagh, M. Screening of chemical composition of essential oil, mineral elements and antioxidant activity in Pulicaria undulata (L.) CA Mey from Iran. J. Med. Plants Res. 2011, 5, 2035–2040. [Google Scholar]
- Hussein, S.R.; Marzouk, M.M.; Soltan, M.M.; Ahmed, E.K.; Said, M.M.; Hamed, A.R. Phenolic constituents of Pulicaria undulata (L.) CA Mey. sub sp. undulata (Asteraceae): Antioxidant protective effects and chemosystematic significances. J. Food Drug Anal. 2017, 25, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Asfour, H.Z.; Khayat, M.T. Undulaterpene A: A new triterpene fatty acid ester from Pulicaria undulata. Pharmacogn. Mag. 2019, 15, 671. [Google Scholar] [CrossRef]
- Nasir, E.; Ali, S.I. Flora of West Pakistan; Fakhri Printing Press: Karachi, Pakistan, 1972; Volume 20, p. 770. [Google Scholar]
- Mey, C.A. Pulicaria Undulata (L.)|Species|India Biodiversity Portal. Available online: https://www.bing.com/search?q=Pulicaria+in+India&form=ANNTH1&refig=271c17426106471ca06a61466f9e9562. (accessed on 20 May 2021).
- Mossa, J.S.; Hifnawy, M.S.; Al-Yahya, M.A.; Al-Meshal, I.A.; Mekkawi, A.G. Aromatic Plants of Saudi Arabia-Part 8 GC/MS Analysis of Essential Oils of Pulicaria arabica and P. undulata. Int. J. Crude Drug Res. 1987, 25, 113–119. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Kuniyil, M.; Adil, S.F.; Al-Warthan, A.; Alkhathlan, H.Z.; Tremel, W.; Tahir, M.N.; Siddiqui, M.R.H. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalt. Trans. 2014, 43, 9026–9031. [Google Scholar] [CrossRef]
- WorldData. Available online: https://www.worlddata.info/asia/saudi-arabia/climate-al-qassim.php (accessed on 12 August 2021).
- Al-Ashrah, S.A. Radiation properties for red soil in Qassim province, Saudi Arabia. J. Rad. Res. App. Sci. 2016, 9, 363–369. [Google Scholar] [CrossRef]
- Nazzal, Y.; Hawari, F.M.; Jafri, M.K.; Naeem, M.; Gharefat, H. Risk assessment through evaluation of potentially toxic metals in the surface soils of the Qassim area, Central Saudi Arabia. Ital. J. Geosci. 2015, 134, 210–216. [Google Scholar] [CrossRef]
- Oregon State University. Environmental Factors Affecting Plant Growth. Available online: http://extension.oregonstate.edu/gardening/techniques/environmental-factors-affecting-plant-growth (accessed on 20 July 2021).
- Khan, R.A. Natural products chemistry: The emerging trends and prospective goals. Saudi Pharm. J. 2018, 26, 739–753. [Google Scholar] [CrossRef]
- Ceyhan, E.; Kahraman, A.; Onder, M. The Impacts of Environment on Plant Products. Inter. J. Biosci. Biochem. Bioinform. 2012, 2, 48–51. [Google Scholar] [CrossRef]
- Bansal, O.P. The Influence of Potentially Toxic Elements on Soil Biological and Chemical Properties. In Metals in Soil—Contamination and Remediation; Begum, Z.A., Rahman, I.M.M., Hasegawa, H., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Weber, D.J. Adaptive Mechanisms of Halophytes in Desert Regions. In Salinity and Water Stress. Tasks for Vegetation Sciences; Ashraf, M., Ozturk, M., Athar, H., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 44. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Kumar, N.; Lata, C.; Kumar, A.; Kumar, A. Halophytes-Miracle Plants to Survive in Extreme Saline Environment. OA J. Agri. Res. 2018, 3, 000176. [Google Scholar]
- Aslam, R.; Bostan, N.; Maria, M.; Safdar, W. A critical review on halophytes: Salt tolerant plants. J. Med. Plants Res. 2011, 5, 7108–7118. [Google Scholar] [CrossRef]
- Gonzalez, M.B. Adaptation of Halophytes to Different Habitats. Seed Dormancy Germination 2019, 1–23. [Google Scholar] [CrossRef]
- Mohammed, H.A. The Valuable Impacts of Halophytic Genus Suaeda; Nutritional, Chemical, and Biological Values. Med. Chem. 2020, 16, 1044–1057. [Google Scholar] [CrossRef]
- Mohammed, H.A. Behavioral Evaluation for Aqueous and Ethanol Extracts of Suaeda vermiculata Forssk. Cent. Nerv. Syst. Agents Med. Chem. 2020, 20, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.B.A.; Yagi, S.; Tzanova, T.; Schohn, H.; Abdelgadir, H.; Stefanucci, A.; Mollica, A.; Mahomoodally, M.F.; Adlan, T.A.; Zengin, G. Chemical profile, antiproliferative, antioxidant and enzyme inhibition activities of Ocimum basilicum L. and Pulicaria undulata (L.) CA Mey. grown in Sudan. S. Afr. J. Bot. 2020, 132, 403–409. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Dawood, M.; Mahmoud, N.; Elbadawi, M.; Sugimoto, Y.; Klauck, S.M.; Mohamed, N.; Efferth, T. 2α-Hydroxyalantolactone from Pulicaria undulata: Activity against multidrug-resistant tumor cells and modes of action. Phytomedicine 2021, 81, 153409. [Google Scholar] [CrossRef] [PubMed]
- Ajaib, M.; Mati-ur-Rehman, A.; Khan, K.M.; Perveen, S.; Shah, S. Pulicaria undulata: A Potential Phytochemical, Antimicrobial, and Antioxidant Source. J. Chem. Soc. Pak. 2015, 37, 559–566. [Google Scholar]
- Yasseen, B.T.; Al-Thani, R.F. Halophytes and associated properties of natural soils in the Doha area, Qatar. Aquat. Ecosys. Health Manag. 2007, 10, 320–326. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Botany 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Matinzadeh, Z.; Akhani, H.; Abedi, M.; Palacio, S. The elemental composition of halophytes correlates with key morphological adaptations and taxonomic groups. Plant Physiol Biochem. 2019, 141, 259–278. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Fahmi, A.A.; Abdur-Rahman, M.; Naser, A.F.A.; Hamed, M.A.; Abd-Alla, H.I.; Shalaby, N.M.M.; Nasr, M.I. Chemical composition and protective role of Pulicaria undulata (L.) C.A. Mey. subsp. undulata against gastric ulcer induced by ethanol in rats. Heliyon 2019, 5, e01359. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Shi, Y. Phytochemicals and biological activities of Pulicaria species. Chem. Biodiver. 2010, 7, 327–349. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, M.; Kumar, V.; Prakash, O.; Arya, R.; Rana, M.; Kumar, D. Traditional medicinal plants curing diabetes: A promise for today and tomorrow. Asian J. Trad. Med. 2012, 7, 178–188. [Google Scholar]
- Al-Hajj, N.Q.M.; Algabr, M.N.; Omar, K.A.; Wang, H. Anticancer, antimicrobial and antioxidant activities of the essential oils of some aromatic medicinal plants (Pulicaria inuloides-Asteraceae). J. Food Nutr. Res. 2017, 5, 490–495. [Google Scholar] [CrossRef]
- Vanhaelen, M.; Lejoly, J.; Hanocq, M.; Molle, L. Climatic and Geographical Aspects of Medicinal Plant Constituents. In The Medicinal Plant Industry; Routledge Press: London, UK, 1991; pp. 59–75. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A. Phytochemical Profiling, In Vitro and In Silico Anti-Microbial and Anti-Cancer Activity Evaluations and Staph GyraseB and h-TOP-IIβ Receptor-Docking Studies of Major Constituents of Zygophyllum coccineum L. Aqueous-Ethanolic Extract and Its Subsequent Fra. Molecules 2021, 26, 577. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, M.; Yadav, A.; Rohilla, P.; Yadav, J.P. Antiplasmodial potential and quantification of aloin and aloe-emodin in Aloe vera collected from different climatic regions of India. BMC Complement. Altern. Med. 2017, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Sleimi, N.; López-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Effects of combined abiotic stresses on growth, trace element accumulation, and phytohormone regulation in two halophytic species. J. Plant Growth Regul. 2014, 33, 632–643. [Google Scholar] [CrossRef]
- Sharma, A.; Gontia, I.; Agarwal, P.K.; Jha, B. Accumulation of heavy metals and its biochemical responses in Salicornia brachiata, an extreme halophyte. Mar. Biol. Res. 2010, 6, 511–518. [Google Scholar] [CrossRef]
- Gomes, M.P.; Carneiro, M.M.L.C.; Garcia, Q.S. Trace Elements Tolerance Modulated by Antioxidant System in Plants. In Oxidative Damage to Plants; Parvaiz, A., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 523–540. ISBN 9780127999630. [Google Scholar] [CrossRef]
- Bola, O.O.; Omowunmi, O.D.; Adelaja, O. Trace Elements and Antioxidants in Some Medicinal Plants. Res. Rev. Biosci. 2017, 12, 111. [Google Scholar]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- El-Sabagh, O.A.; El-Toumy, S.A.; Mounir, R.; Farag, M.A.; Mahrous, E.A. Metabolite profiles of Pulicaria crispa and P. incisa in relation to their in-vitro/in-vivo antioxidant activity and hepatoprotective effect: A comparative mass spectrometry-based metabolomics. J. Pharm. Biomed. Anal. 2021, 194, 113804. [Google Scholar] [CrossRef]
- Underwood, E. Trace Elements in Human and Animal Nutrition; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0323162185. [Google Scholar]
- Al-Omar, M.S.; Mohammed, H.A.; Mohammed, S.A.A.; Abd-Elmoniem, E.; Kandil, Y.I.; Eldeeb, H.M.; Chigurupati, S.; Sulaiman, G.M.; Al-Khurayyif, H.K.; Almansour, B.S. Anti-Microbial, Anti-Oxidant, and α-Amylase Inhibitory Activity of Traditionally-Used Medicinal Herbs: A Comparative Analyses of Pharmacology, and Phytoconstituents of Regional Halophytic Plants’ Diaspora. Molecules 2020, 25, 5457. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Standards for the contents of heavy metals in soils of some states. Ann. Agrar. Sci. 2016, 14, 257–263. [Google Scholar] [CrossRef]
- Bruand, A. Toward conditions favourable to mobility of trace elements in soils. Comptes Rendus Geosci. 2005, 337, 549–550. [Google Scholar] [CrossRef]
- Kassaye, Y.A.; Lindis, S.; Sondre, M.; Dadebo, E.; Einset, J.; Salbu, B. Trace element mobility and transfer to vegetation within the Ethiopian Rift Valley lake areas. J. Environ. Monitor. 2012, 14, 2698–2709. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabel, M.I.; Sallam, A.E.A.S.; Usman, A.R.A.; Ahmad, M.; El-Naggar, A.H.; El-Saeid, M.H.; Al-Faraj, A.; El-Enazi, K.; Al-Romian, F.A. Trace metal levels, sources, and ecological risk assessment in a densely agricultural area from Saudi Arabia. Environ. Monit. Assess. 2017, 189, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Bortey-Sam, N.; Nakayama, S.M.M.; Akoto, O.; Ikenaka, Y.; Baidoo, E.; Mizukawa, H.; Ishizuka, M. Ecological Risk of Heavy Metals and a Metalloid in Agricultural Soils in Tarkwa, Ghana. Inter. J. Environ. Res. Public Health 2015, 12, 11448–11465. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Lu, Y.; Khan, H.; Ishtiaq, M.; Khan, S.; Waqas, M.; Wei, L.; Wang, T. Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food Chem. Toxic. 2013, 58, 449–458. [Google Scholar] [CrossRef]
- Mendoza, M.P.; Lalopis, C.M.; Sanchez, J.; Bois, L.R. Heavy metal content of agricultural soils in a Mediterranean semiarid area, the Segura River Valley (Alicante, Spain). Span. J. Agri. Res. 2006, 4, 363–372. [Google Scholar]
- Hernandez, L.; Probst, A.; Probst, J.L.; Ulrich, E. Heavy metal distribution in some French forest soils: Evidence for atmospheric contamination. Sci. Total Environ. 2003, 312, 195–219. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Rakhmankulova, Z.F.; Shuyskaya, E.V.; Shcherbakov, A.V.; Fedyaev, V.V.; Biktimerova, G.Y.; Khafisova, R.R.; Usmanov, I.Y. Content of proline and flavonoids in the shoots of halophytes inhabiting the South Urals. Russ. J. Plant Physiol. 2015, 62, 71–79. [Google Scholar] [CrossRef]
- Ivan, M.A.; Lacra-Mioara, O. Study of polyphenols and flavonoids contents of some halophytes species collected from dobrogea region. Bull Transilvania Univ Braşov Series II: Forestry, Wood Industry. Agric. Food Eng. 2013, 6, 121–128. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics, and polyphenolics in foods, beverages, and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Helal, N.M.; Ibrahim, N.; Khattab, H. Phytochemical analysis and antifungal bioactivity of Pulicaria undulata (L.) methanolic extract and essential oil. Egypt. J. Bot. 2019, 59, 827–844. [Google Scholar] [CrossRef]
- Issa, M.Y.; Ezzat, M.I.; Sayed, R.H.; Elbaz, E.M.; Omar, F.A.; Mohsen, E. Neuroprotective effects of Pulicaria undulata essential oil in rotenone model of Parkinson’s disease in rats: Insights into its anti-inflammatory and anti-oxidant effects. S. Afr. J. Bot. 2020, 132, 289–298. [Google Scholar] [CrossRef]
- El-Kamali, H.H.; Mahjoub, S.A.-T. Antibacterial activity of Francoeuria crispa, Pulicaria undulata, Ziziphus spina-christi, and Cucurbita pepo against seven standard pathogenic bacteria. Ethnobot. Leafl. 2009, 2009, 6. [Google Scholar]
- Jiao, L.; Lin, F.; Cao, S.; Wang, C.; Wu, H.; Shu, M.; Hu, C. Preparation, characterization, antimicrobial and cytotoxicity studies of copper/zinc-loaded montmorillonite. J. Anim. Sci. Biotechnol. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In Vitro and In Vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G. Anticancer and apoptosis-inducing effects of quercetin In Vitro and In Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Hollósy, F.; Keri, G. Plant-derived protein tyrosine kinase inhibitors as anticancer agents. Curr. Med. Chem. Agents 2004, 4, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yu, M.; Qi, Z.; Cui, D.; Xin, G.; Wang, B.; Jia, W.; Chang, L. Study on apoptosis effect of human breast cancer cell MCF-7 induced by lycorine hydrochloride via death receptor pathway. Saudi Pharm. J. 2017, 25, 633–637. [Google Scholar] [CrossRef]
- Mahassni, S.H.; Al-Reemi, R.M. Apoptosis and necrosis of human breast cancer cells by an aqueous extract of garden cress (Lepidium sativum) seeds. Saudi J. Biol. Sci. 2013, 20, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, L. Selenium uptake by plants as a function of soil type, organic matter content and pH. Plant Soil 1991, 133, 57–64. [Google Scholar] [CrossRef]
- Aroua, L.M.; Almuhaylan, H.R.; Alminderej, F.M.; Messaoudi, S.; Chigurupati, S.; Al-Mahmoud, S.; Mohammed, H.A. A facile approach synthesis of benzoylaryl benzimidazole as potential α-amylase and α-glucosidase inhibitor with antioxidant activity. Bioorg. Chem. 2021, 114, 105073. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of In Vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- López-Oviedo, E.; Aller, A.I.; Martin, C.; Castro, C.; Ramirez, M.; Pemán, J.M.; Cantón, E.; Almeida, C.; Martín-Mazuelos, E. Evaluation of disk diffusion method for determining posaconazole susceptibility of filamentous fungi: Comparison with CLSI broth microdilution method. Antimicrob. Agents Chemo. 2006, 50, 1108–1111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clinical and Laboratory Standards Institute. M44-A2: Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; Volume 29. [Google Scholar]
- EUCAST. Disk Diffusion Method for Antimicrobial Susceptibility Testing-Antimicrobial susceptibility testing EUCAST disk diffusion method. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2021_manuals/Manual_v_9.0_EUCAST_Disk_Test_2021.pdf (accessed on 20 July 2021).
- Qureshi, K.A.; Elhassan, G.O. Isolation, Purification and Characterization of Antimicrobial Agent Antagonistic to Escherichia coli ATCC 10536 Produced by Bacillus pumilus SAFR-032 Isolated from the Soil of Unaizah, Al Qassim Province of Saudi Arabia. Pak. J. Biol. Sci. 2016, 19, 191. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007; ISBN 1420014498. [Google Scholar]
- Mohammed, H.A.; Al-Omar, M.S.; El-Readi, M.Z.; Alhowail, A.H.; Aldubayan, M.A.; Abdellatif, A.A.H. Formulation of Ethyl Cellulose Microparticles Incorporated Pheophytin A Isolated from Suaeda vermiculata for Antioxidant and Cytotoxic Activities. Molecules 2019, 24, 1501. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, B.R.; Sterne, J.A.C. Essential Medical Statistics; John Wiley& Sons: Hoboken, NJ, USA, 2010; ISBN 9781444392845. [Google Scholar]


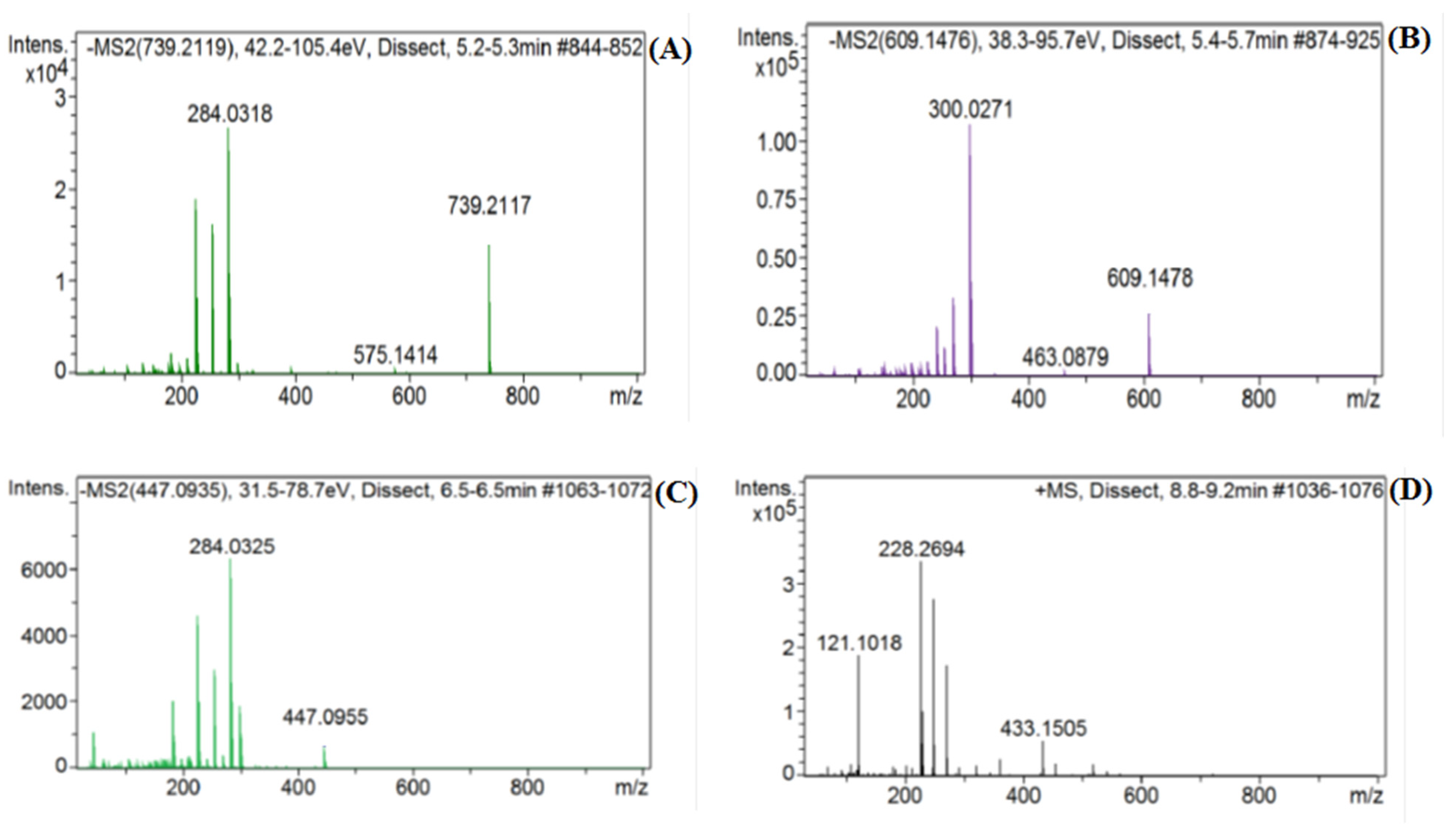


| Serial No. | RT (min) | Observed Mass (amu) | Calcd. Mass (amu) | Molecular Formula | Mass Fragments (m/z) | Compound Class | Compound’s Identity |
|---|---|---|---|---|---|---|---|
| 1. | 3.15 | 179.0725 [M−H]− | 179.0344 | C9H8O4 | 164, 149, 121, 93 | Phenolic acid | Caffeic acid * |
| 2. | 3.25 | 163.03775 [M−H]− | 163.0395 | C9H8O3 | 120,119, 93,85, 43 | Coumarin | Coumaric acid |
| 3. | 3.40 | 471.1875 [M−H]− | 471.1866 | C22H32O11 | 96, 231, 469 | Phenolic glycoside | Eugenol rutinoside |
| 4. | 4.50 | 449.1440 [M+H]+ | 449.1447 | C22H24O10 | 318, 421, 128 | Flavanone | Isosakuranin |
| 5. | 5.16 | 193.0551 [M−H]− | 193.0500 | C10H10O4 | Phenolic acid | Trans-Ferulic acid * | |
| 6. | 5.30 | 739.20966 [M−H]− | 739.2085 | C33H40O19 | 183, 211, 227, 255, 285 | Flavonol glycoside | Kaempferol 3-O-rutinoside |
| 7. | 5.60 | 609.14274 [M−H]− | 609.1455 | C27H30O16 | 255, 271, 301, 302, 463 | Flavonol glycoside | Rutin * |
| 8. | 5.73 | 463.0861 [M−H]− | 463.0876 | C21H20O12 | 201. 215, 227, 255, 271, 300 | Flavonol glycoside | Hyperoside |
| 9. | 5.95 | 593.1527 [M−H]− | 593.1506 | C27H30O15 | 227, 255, 284, 285, 357 | Flavonol glycoside | Kaempferol-3-O-rutinoside |
| 10. | 6.50 | 593.14723 [M−H]− | 593.1506 | C27H30O15 | 301, 286, 271, 255, 227 | Flavonol glycoside | 3,7-Dirhamnosylquercetin |
| 11. | 6.55 | 447.0906 [M−H]− | 447.0927 | C21H20O11 | 185, 227, 255, 285 | Flavone glycoside | Luteolin 7-O-glucoside |
| 12. | 6.82 | 285.0402 [M−H]− | 285.0399 | C15H10O6 | 185, 227, 255 | Flavone | Luteolin |
| 13. | 7.25 | 301.1050 [M+H]+ | 301.1076 | C17H16O5 | 119, 135, 179 | Flavanone | Farrerol |
| 14. | 7.91 | 205.04807 [M−H]− | 205.0500 | C11H10O4 | 107, 135, 163, 191 | Coumarin | Scoparone |
| 15. | 8.59 | 301.0335 [M−H]− | 301.0339 | C15H10O7 | 271, 255, 243 | Flavonol | Quercetin * |
| 16. | 8.88 | 433.1134 [M+H]+ | 433.1134 | C21H20O10 | 271, 250, 231, 221 | Isoflavone glucoside | Genistin |
| 17. | 10.35 | 317.0657 [M+H]+ | 317.0661 | C16H12O7 | 137, 228, 274, 301 | Flavonol | 3-O-Methylquercetin |
| 18. | 14.25 | 285.1098 [M+H]+ | 285.1126 | C17H16O4 | 69, 122, 195 | Phenolic acid | Caffeic acid phenethyl ester * |
| 19. | 20.50 | 293.1737 [M−H]− | 293.1752 | C17H26O4 | 44, 149, 185, 253 | Phenolic | 6-Gingerol |
| 20. | 24.55 | 227.2008 [M−H]− | 227.2011 | C14H28O2 | 116, 136 | Fatty Acid | Myristic acid |
| 21. | 25.71 | 253.2149 [M−H]− | 253.2167 | C16H30O2 | 219, 185, 157, 116, 99, 45 | Fatty acid | Palmitoleic acid |
| 22. | 26.16 | 279.23045 [M−H]− | 279.2324 | C18H32O2 | 116, 61, 59, 34 | Fatty acid | Linoleic acid |
| 23. | 27.91 | 255.23089 [M−H]− | 255.2324 | C16H32O2 | 131, 117, 116, 99 | Fatty acid | 3-Hydroxy dodecanedioic acid |
| 24. | 28.23 | 281.2464 [M−H]− | 281.2480 | C18H34O2 | 116, 99, 61 | Fatty acid | Oleic acid |
| 25. | 29.96 | 283.2620 [M−H]− | 283.2637 | C18H36O2 | 265, 61, 44 | Fatty acid | Stearic acid |
| 26. | 30.01 | 353.34417 [M−H]− | 353.3419 | C23H46O2 | 255, 116, 89, 74, | Fatty acid | Methyl docosanoate |
| 27. | 30.30 | 423.42272 [M−H]− | 423.4202 | C28H56O2 | 250, 236, 84, 61 | Fatty acid | Octacosanoic acid |
| TPC | TFC | TAA | DPPH-SA | FRAP | MCA |
|---|---|---|---|---|---|
| 33.31 ± 0.46 | 10.83 ± 0.77 | 17.40 ± 1.60 | 19.13 ± 0.01 | 47.11 ± 4.09 | 8.79 ± 1.16 |
| Trace Elements Concentration in µg/kg * of the Dried Plant Powder | |||||
|---|---|---|---|---|---|
| Iron (Fe) | Copper (Cu) | Magnesium (Mg) | Cobalt (Co) | Manganese (Mn) | Zinc (Zn) |
| 1008 ± 3.60 | 20.13 ± 1.69 | 1150.67 ± 7.57 | 74.00 ± 3.43 | 99.66 ± 4.40 | 68.20 ±0.01 |
| Trace Elements Concentration in µg/kg of the Soil from Different Locations | |||||
|---|---|---|---|---|---|
| Location | Iron | Copper | Cobalt | Manganese | Zinc |
| Threshold value * | 4600 | 2–50 | 1.3 | 50 | 200 |
| KSA (Qassim, arid, high salt) | 9427 | 15.9 | 3.80 | 195 | 164 |
| Ghana (tropical green lands) | 6.2 | 1.8 | 39 | ||
| Pakistan (Swat area, temperate) | 0.44 | 7.56 | 0.59 | ||
| Spain (Alicante region, semi-arid) | 15,274 | 21.6 | 320 | 57.8 | |
| France (forest sites, temperate) | 23.77 | 1.56 | 45.01 | ||
| Egypt (Sinai desert lands) | 960 | 3.03 | 84 | 17 | |
| Microorganisms | Diameters of Zones of Inhibition (mm) * | |
|---|---|---|
| P. undulata Plant Extract (2 mg/disc) | Levofloxacin (5 µg/disc) | |
| S. aureus ATCC 29213 | 16.6 ± 0.2 | 31.7 ± 0.2 |
| MRSA-A ** | 11.5 ± 0.2 | 23.4 ± 0.3 |
| MRSA-B ** | 12.9 ± 0.2 | 6.2 ± 0.2 |
| S. saprophyticus ATCC 43867 | 21.6 ± 0.1 | 28.1 ± 0.2 |
| S. epidermidis ATCC 12228 | 11.6 ± 0.2 | 18.3 ± 0.3 |
| B. cereus ATCC 10876 | 14.9 ± 0.1 | 28.4 ± 0.2 |
| Microorganisms | P. undulata Plant Extract | Levofloxacin (5 µg/mL) | |
|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | ||
| S. aureus ATCC 29213 | 1563 | 3125 | Inhibition |
| MRSA-A | 1563 | 3125 | Inhibition |
| MRSA-B | 98 | 195 | No inhibition |
| S. saptophyticus ATCC 43867 | 49 | 49 | Inhibition |
| S. epidermidis ATCC 12228 | 196 | 195 | Inhibition |
| B. cereus ATCC 10876 | 49 | 49 | Inhibition |
| IC50 (µg/mL) | ||||
|---|---|---|---|---|
| Fibroblast | MCF-7 | K562 | PANC-1 | |
| P. undulata water-ethanol Extract | 4048 | 519.2 | 1212 | 1535 |
| Doxorubicin | 2.975 | 0.194 | 0.395 | 0.158 |
| Concentration (µg/mL) | Fibroblast | MCF-7 | PANC-1 | K562 |
|---|---|---|---|---|
| 1000 | 72.36 ± 2.05 | 39.09 ± 2.44 **** | 55.14 ± 1.75 ** | 44.31 ± 0.65 **** |
| 500 | 90.6 ± 5.53 | 47.19 ± 1.93 **** | 62.69 ± 5.28 **** | 53.98 ± 1.31 **** |
| 250 | 84.91 ± 2.14 | 61.66 ± 2.85 **** | 65.36 ± 2.78 ** | 73.8 ± 2.11 * |
| 125 | 95.88 ± 3.61 | 88.27 ± 4.79 ns | 71.12 ± 7.27 **** | 78.4 ± 2.69 ** |
| 62.5 | 99.52 ± 1.69 | 91.63 ± 4.93 ns | 85.32 ± 3.87 * | 68.59 ± 4.89 **** |
| 31.25 | 96.09 ± 2.58 | 94.58 ± 2.78 ns | 91.98 ± 4.88 ns | 72.36 ± 2.62 **** |
| 15.625 | 93.62 ± 4.03 | 104.87 ± 2.74 ns | 95.75 ± 1.8 ns | 67.35 ± 2.11 **** |
| (A) Untreated | (B) DMSO | (C) IC50 | (D) Twice of the IC50 | |
|---|---|---|---|---|
| Viable | 96.1% | 79.6% | 40.3% | 15.0% |
| Early apoptosis | 0.30% | 2.60% | 2.00% | 0.00% |
| Late apoptosis | 0.60% | 5.00% | 18.6% | 8.90% |
| Necrosis | 3.10% | 12.8% | 39.1% | 76.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, H.A.; Al-Omar, M.S.; Khan, R.A.; Mohammed, S.A.A.; Qureshi, K.A.; Abbas, M.M.; Al Rugaie, O.; Abd-Elmoniem, E.; Ahmad, A.M.; Kandil, Y.I. Chemical Profile, Antioxidant, Antimicrobial, and Anticancer Activities of the Water-Ethanol Extract of Pulicaria undulata Growing in the Oasis of Central Saudi Arabian Desert. Plants 2021, 10, 1811. https://doi.org/10.3390/plants10091811
Mohammed HA, Al-Omar MS, Khan RA, Mohammed SAA, Qureshi KA, Abbas MM, Al Rugaie O, Abd-Elmoniem E, Ahmad AM, Kandil YI. Chemical Profile, Antioxidant, Antimicrobial, and Anticancer Activities of the Water-Ethanol Extract of Pulicaria undulata Growing in the Oasis of Central Saudi Arabian Desert. Plants. 2021; 10(9):1811. https://doi.org/10.3390/plants10091811
Chicago/Turabian StyleMohammed, Hamdoon A., Mohsen S. Al-Omar, Riaz A. Khan, Salman A. A. Mohammed, Kamal A. Qureshi, Manal M. Abbas, Osamah Al Rugaie, Essam Abd-Elmoniem, Adel M. Ahmad, and Yasser I. Kandil. 2021. "Chemical Profile, Antioxidant, Antimicrobial, and Anticancer Activities of the Water-Ethanol Extract of Pulicaria undulata Growing in the Oasis of Central Saudi Arabian Desert" Plants 10, no. 9: 1811. https://doi.org/10.3390/plants10091811
APA StyleMohammed, H. A., Al-Omar, M. S., Khan, R. A., Mohammed, S. A. A., Qureshi, K. A., Abbas, M. M., Al Rugaie, O., Abd-Elmoniem, E., Ahmad, A. M., & Kandil, Y. I. (2021). Chemical Profile, Antioxidant, Antimicrobial, and Anticancer Activities of the Water-Ethanol Extract of Pulicaria undulata Growing in the Oasis of Central Saudi Arabian Desert. Plants, 10(9), 1811. https://doi.org/10.3390/plants10091811









