Abstract
Soil salinization is one of the main abiotic stress factors impacting the growth of crops and the agricultural industry today. Thus, we aimed to investigate the effects of H2O2 pretreatment on seed germination in Tartary buckwheat (Fagopyrum tataricum) seeds under salt stress and to evaluate this species’ salt tolerance. Through the preliminary experiment, this study used 50 mmol L−1 NaCl solution to induce seed stress. After soaking for 12 h in different H2O2 concentrations, seeds were laid in Petri dishes with 50 mmol L−1 NaCl for seven days and the germination parameters and physiological indicators were measured to screen the optimal H2O2 pretreatment concentration and the salt tolerance index. Our results indicated that pretreatment with 5–10 mmol L−1 H2O2 was most effective in alleviating NaCl’s impacts on the seeds’ germination parameters. Furthermore, the growth and material accumulation of seedlings was promoted; catalase, superoxide dismutase activity, and proline content were enhanced; and malondialdehyde content was reduced. Principal component analysis and stepwise regression revealed six key indicators that had a significant impact on the salt tolerance characteristics of F. tataricum, namely, germination potential, shoot fresh weight, root surface area, root average diameter, catalase activity, and superoxide dismutase activity.
1. Introduction
Soil salinization is one of the main abiotic stress factors, which affects crop morphology, physiology, biochemistry, and gene expression. Furthermore, it restricts the planting and growth of certain varietals and limits the potential for agricultural development. The area impacted by soil salinization is expanding worldwide [1]. Saline soils in China represent approximately 3600 × 104 ha, which accounts for about 25% of the cultivated land area [2]. Studies have found that exposure to salt stress inhibits most plant growth parameters to varying degrees [3,4]. Therefore, screening salt-tolerant varieties and studying the tolerance of crops to salt stress are effective methods for improving their adaptability. Furthermore, it is crucial to examine the growth patterns of crops under suitable salt concentrations. Hydrogen peroxide (H2O2) is a relatively stable, free-diffusing, and fairly long-lived active oxygen compound [5,6]. When crops are under various types of stress, the application of exogenous H2O2 can alleviate the stress and reduce crop damage. For example, pretreatment with exogenous H2O2 can increase the active enzyme content of rice under drought stress [7], reduce salt-induced damage to wheat roots [8], and improve the cold resistance and cell viability of rape seedlings [9].
Buckwheat may refer to a variety of dicotyledonous plants in the genus Fagopyrum within the family Polygonaceae. They can be annual or perennial, and the plants are used for food and animal feed [10]. In China, buckwheat is divided into three major cultivated species: Tartary buckwheat (F. tataricum), sweet buckwheat (F. esculentum) and golden buckwheat (F. cymosum), and the rest are wild species [11]. F. tataricum is a cold-tolerant crop suitable for cultivation in high-altitude mountainous areas with short frost-free periods, such as southwestern China [12]. It has a high nutritional value, important health benefits, and contains starch, protein, vitamins, mineral elements and other nutrients. F. tataricum is also rich in flavonoids and other biologically active substances, which can help mitigate diabetes, prevent cardiovascular sclerosis, and regulate high blood pressure [13,14,15].
F. tataricum frequently grows in poor quality soil including soils with increased salinity and H2O2 can alleviate the negative impacts of salt stress on seed germination. At present, there are few studies that investigate the effect of H2O2 seed soaking on the germination characteristics of F. tataricum seeds under salt stress [16,17]. We soaked F. tataricum seeds in different concentrations of H2O2 to examine the germination characteristics, seedling growth, antioxidant enzyme activity, membrane lipid peroxidation, and osmotic adjustment substances present in seeds under salt stress. A principal component analysis, stepwise regression analysis, and other methods were used to evaluate the salt tolerance of F. tataricum. Our aim was to investigate the effects of H2O2 pretreatment on alleviating the inhibition of F. tataricum seeds under salt stress and test the relationship between salt tolerance indicators and pretreating seeds with H2O2. Through this study, we hope to provide the theoretical basis for a treatment to improve the salt tolerance of F. tataricum seeds.
2. Results
2.1. The Effects of Different NaCl Concentrations on the Seed Germination
The germination potential (GP), germination rate (GR), germination index (GI), vigor index (VI) and shoot length (SL) of F. tataricum seeds all decreased as the concentration of NaCl treatment increased (Table 1). VI and SL decreased significantly under 50 mmol L−1 NaCl compared to those under 0 mmol L−1 NaCl (p < 0.05); and GI, VI, and SL were significantly inhibited under the 100 mmol L−1 NaCl treatment (p < 0.05) (Table 1). However, all five germination indices further decreased under the 150 and 200 mmol L−1 NaCl treatments (p < 0.05) (Table 1). As the concentration of NaCl treatments was increased from 0 to 50, 100, 150 and 200 mmol L−1, VI decreased by 77.0%, 88.2%, 91.9%, and 95.2%, respectively; and SL decreased by 75.6%, 82.4%, 85.7%, and 89.0%, respectively (Table 1). In summary, 50 mmol L−1 NaCl significantly reduces GP, GR and GI, and significantly inhibits VI and SL by more than 75.0% (Table 1). Therefore, going forward, this study used 50 mmol L−1 NaCl solution to induce seed stress (Table 1).

Table 1.
Effects of different NaCl concentrations on germination parameters of F. tataricum seeds.
2.2. The Effects of H2O2 Pretreatment and NaCl Stress on the Seed Germination
The 50 mmol L−1 NaCl treatment had no significant inhibitory effect on GP, GR, and GI of F. tataricum seed (p < 0.05) (Figure 1A–C); however, it did significantly reduce their VI (p < 0.05), which was 76.6% lower than the CK (Figure 1D). Under NaCl stress, treatments of increasing H2O2 concentration caused an initial increase and then a decrease in the GP, GR, GI, and VI of F. tataricum seeds (Figure 1A–D). The GP, GR, and GI of seeds reached their maximum values under the treatment of NaCl + 5 mmol L−1 H2O2; increasing by 14.5%, 8.7%, and 18.4%, respectively, compared to those of seeds treated with NaCl alone (Figure 1A–C). VI reached its maximum value under NaCl + 10 mmol L−1 H2O2, which was 3.1 times higher than that under NaCl treatment alone (Figure 1D). Under the treatment of NaCl + 100 mmol L−1 H2O2, the GP, GR, GI, and VI all noticeably decreased compared to their values under the CK treatment, and NaCl alone (Figure 1A–D). These results suggest that pretreatment with H2O2 could alleviate the inhibitory effects that F. tataricum seeds experience under salt stress.
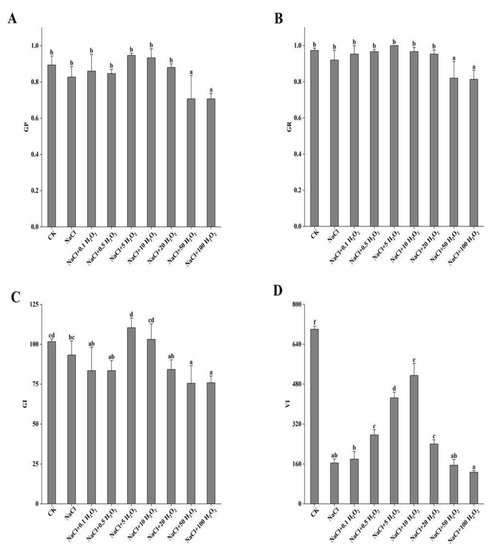
Figure 1.
Effect of H2O2 pretreatment on the GP (A), GR (B), GI (C), and VI (D) of F. tataricum seeds under NaCl stress. CK, control treatment (only water); NaCl, 50 mmol L−1 NaCl treatment; NaCl + xxx H2O2, treatments of standard NaCl + respective H2O2 concentration; GP, germination potential; GR, germination rate; GI, germination index; VI, vigor index. Different lowercase letters on each column indicate significant differences between treatments at p < 0.05.
2.3. The Effects of H2O2 Pretreatment and NaCl Stress on Shoot Length and Fresh Weight
Compared with the measurements in the CK treatment, shoot length (SL) and shoot fresh weight (SFW) decreased significantly under all other treatments (p < 0.05) (Figure 2A,B). In the NaCl treatment, SL and SFW of F. tataricum seedlings reduced significantly to 25.5% and 48.2% of the control values, respectively (Figure 2A,B). Excluding the control treatment, SL and SFW first increased and then decreased with increasing H2O2 concentration, reaching their highest values at 0.5–10 mmol L−1 H2O2 (Figure 2A,B). Under the 10 mmol L−1 H2O2 treatment, SL was 2.8 times higher than that of the NaCl treatment (Figure 2A); while SFW was 1.6 times higher (Figure 2B). However, they both reached their lowest values under the 100 mmol L−1 H2O2 treatment (Figure 2A,B).
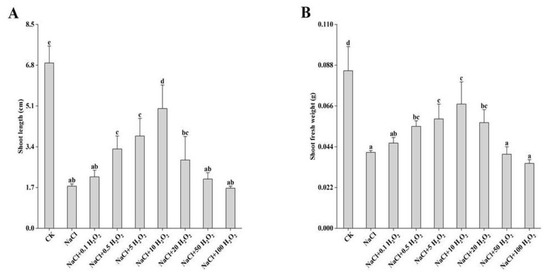
Figure 2.
Effect of H2O2 on SL (A) and SFW (B) of F. tataricum seedlings under NaCl stress. CK, control treatment (only water); NaCl, 50 mmol L−1 NaCl treatment; NaCl + xxx H2O2, treatments of standard NaCl + respective H2O2 concentration; SL, shoot length (cm); SFW, shoot fresh weight (g). Different lowercase letters on each column indicate significant differences between treatments at p < 0.05.
2.4. The Effects of H2O2 Pretreatment and NaCl Stress on Root Growth
The NaCl treatment had a significant effect on the root growth of F. tataricum seedlings compared to that of the control treatment (Table 2). Root length (RL) and Root surface area (RSA) were significantly reduced (p < 0.05) by 36.3% and 38.9%, respectively (Table 2); Root average diameter (RAD) and Root volume (RV) were significantly reduced by 4.1% and 35.7%, respectively (Table 2); whereas root fresh weight (RFW) increased significantly by 35.7% (Table 2). Excluding the control treatment, RL, RSA, RAD, RV, and RFW all increased and then decreased as H2O2 concentration increased (Table 2). Under the NaCl + 0.5 mmol L−1 H2O2 treatment, RL, RSA, and RFW reached their maximum values at 1.6, 1.9, and 1.4 times higher than those in the NaCl treatment (Table 2). RAD and RV reached their maximum values under the NaCl + 10 mmol L−1 H2O2 treatment, which were 7.3 and 3.4 times those of the NaCl treatment, respectively (Table 2). The RSA of F. tataricum seedlings decreased significantly at H2O2 concentrations of 5–100 mmol L−1, while the RAD increased significantly (Table 2). These results suggest that pretreatment with H2O2 can generally alleviate NaCl’s inhibitory effect on the root growth of F. tataricum seedlings; however, its effect on RSA was certainly different.

Table 2.
Effects of H2O2 on root growth of F. tataricum seedlings under NaCl stress.
2.5. The Effects of H2O2 Pretreatment and NaCl Stress on Antioxidant Enzyme Activity
Excluding the CK treatment, CAT and SOD first increased and then decreased in response to increasing H2O2 concentrations; whereas POD first decreased and then increased (Figure 3A–C). Under the NaCl + 10 mmol L−1 H2O2 treatment, the activity of CAT and SOD in F. tataricum seedlings reached maximums at 196.6 and 1.7 U g−1, respectively (Figure 3A,B). POD activity reached its minimum of 1177.0 U g−1 under the treatment of NaCl + 5 mmol L−1 H2O2 (Figure 3C).
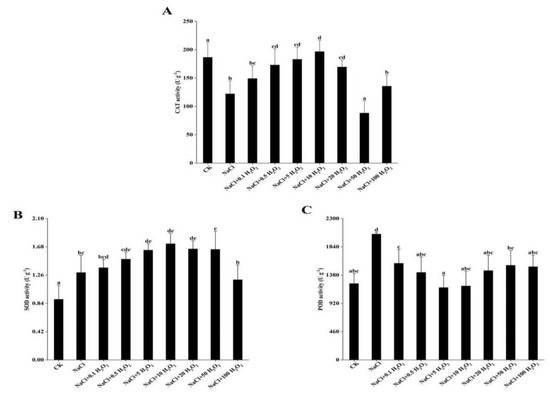
Figure 3.
Effect of H2O2 pretreatment on the activity of CAT (A), SOD (B), and POD (C) in F. tataricum seedlings under NaCl stress. CK, control treatment (only water); NaCl, 50 mmol L−1 NaCl treatment; NaCl + xxx H2O2, treatments of standard NaCl + respective H2O2 concentration; CAT, catalase (U g−1); SOD, superoxide dismutase (U g−1); POD, peroxidase (U g−1). Different lowercase letters on each column indicate significant differences between treatments at p < 0.05.
The SOD and POD activities increased significantly under NaCl stress in F. tataricum seedlings (p < 0.05) (Figure 3B, C). SOD activity increased significantly in all treatments when compared to that of the control; specifically, by 47.8% in the NaCl + 10 mmol L−1 H2O2 treatment (Figure 3B). POD activity in most treatments was higher than in the control, except for that of the NaCl + 5 mmol L−1 H2O2 and NaCl + 10 mmol L−1 H2O2 treatments (Figure 3C).
The CAT activity was significantly inhibited in F. tataricum seedlings under NaCl stress (p < 0.05) (Figure 3A). At low (0.1–0.5 mmol L−1) and high (50–100 mmol L−1) H2O2 concentrations, the CAT activity was significantly reduced (Figure 3A). It reached its lowest activity level, at 55.2% below the maximum, under the NaCl + 50 mmol L−1 H2O2 treatment (Figure 3A).
2.6. The Effects of H2O2 Pretreatment and NaCl Stress on MDA and Pro Contents
The MDA content of F. tataricum seedlings under the NaCl-only treatment increased significantly by 16.5% (p < 0.05) compared to that of the control treatment (Figure 4A). Under the treatments of increasing H2O2 concentration, MDA content generally decreased first and then increased (Figure 4A). Its minimum value was 8.2 nmol g−1 under the NaCl + 20 mmol L−1 H2O2 treatment, which was 68.7% of the MDA value in the NaCl treatment (Figure 4A). The MDA content increased significantly under the NaCl + 50 mmol L−1 and NaCl + 100 mmol L−1 H2O2 treatments, indicating that H2O2 pretreatment significantly reduced the effects of NaCl stress on F. tataricum seedlings (Figure 4A). These results indicate that soaking seeds in different H2O2 concentrations can have varying effects on MDA content.
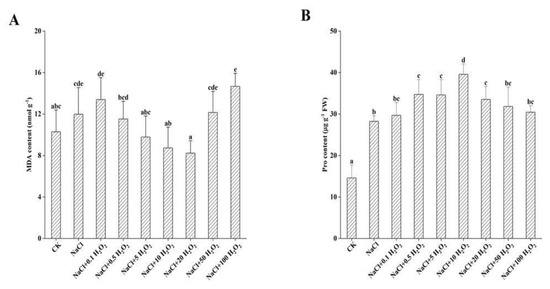
Figure 4.
Effect of H2O2 on MDA (A) and Pro (B) contents of F. tataricum seedlings under NaCl stress. CK, control treatment (only water); NaCl, 50 mmol L−1 NaCl treatment; NaCl + xxx H2O2, treatments of standard NaCl + respective H2O2 concentration; MDA, malondialdehyde (nmol g−1); Pro, proline (µg g−1 FW). Different lowercase letters on each column indicate significant differences between treatments at p < 0.05.
The Pro content of F. tataricum seedlings in each treatment was significantly higher than that of the control treatment (p < 0.05) (Figure 4B). It first increased and then decreased with increasing H2O2 concentration (Figure 4B); reaching its peak under the NaCl + 10 mmol L−1 H2O2 treatment which was 2.7 and 1.4 times that of the CK and NaCl treatments, respectively (Figure 4B). Thereafter, further increases in H2O2 concentration caused a significant decrease in seedling Pro content (Figure 4B); with Pro content of the NaCl + 100 mmol L−1 H2O2 treatment decreasing by 23.1% compared to that of the NaCl + 10 mmol L−1 H2O2 treatment (Figure 4B). These results indicate that H2O2 pretreatment increased the content of osmotic adjustment substances in F. tataricum seedlings under NaCl stress to a certain extent.
2.7. Hierarchical Clustering and Correlation Analysis
The clustering heat map indicates that the high values of all parameters, except POD and MDA, appeared in the 0.5–20 mmol L−1 H2O2 treatments (Figure 5). Under NaCl stress, the growth and material accumulation of F. tataricum seedlings above and below ground were reduced (the smaller value) (Figure 5). The germination characteristics and morphological characteristics of F. tataricum seedlings above and below ground were weakened (the smaller value) by treatments of 50 mmol L−1 H2O2 or higher. All the salt tolerance indices, except POD, MDA, and Pro, were reduced (the smaller value) under the NaCl + 100 mmol L−1 H2O2 treatment (Figure 5). The maximum values of the above parameters were all reflected in seedlings in the CK, NaCl + 5 mmol L−1 H2O2, and NaCl + 10 mmol L−1 H2O2 treatments (Figure 5).
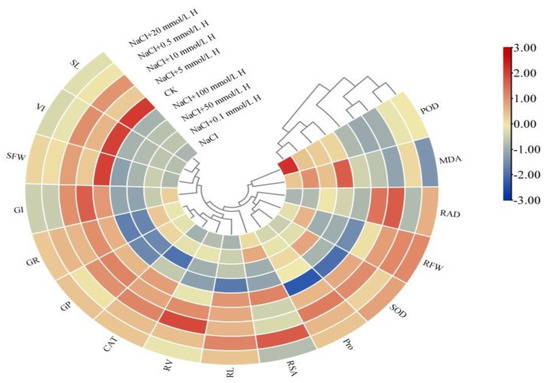
Figure 5.
Hierarchical cluster analysis among various salt tolerance indices under different treatments. CK, control treatment (only water); NaCl, 50 mmol L−1 NaCl treatment; NaCl + xxx H, treatments of standard NaCl + respective H2O2 concentration.
Correlation analysis indicates whether there is any dependency between the parameters; furthermore, it reveals the strength of the relationship, if any. As shown in Figure 6, SL, VI, and SFW shared a strong, positive correlation (p < 0.01). The strongest correlation was between SL and SFW (r = 0.997; p < 0.01) (Figure 6). GI was positively correlated with SL, VI, and SFW (p < 0.05) (Figure 6); GR shared a strong, positive correlation with GP, CAT, RV, and RL (p < 0.01) (Figure 6); and the maximum correlation coefficient was 0.964 (Figure 6). GP shared a strong, positive correlation with CAT and RV (p < 0.01); as well as RL (p < 0.05) (Figure 6). MDA and POD were negatively correlated with most indices, while they were positively correlated with one another (r = 0.492) (Figure 6). Through the cluster analysis and correlation analysis, the results of the two were consistent (Figure 5 and Figure 6).
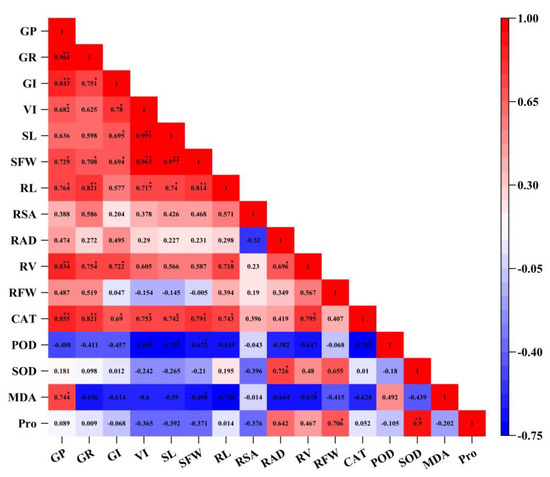
Figure 6.
Correlation matrix between all indices. GP, germination potential; GR, germination rate; GI, germination index; VI, vigor index; SL, shoot length; SFW, shoot fresh weight; RL, root length; RSA, root surface area; RAD, root average diameter; RV, root volume; RFW, root fresh weight; CAT, catalase; SOD, superoxide dismutase; POD, peroxidase; MDA, malondialdehyde; Pro, proline. Numbers represent the Pearson correlation statistics. ** denotes significance at p < 0.01; * denotes significance at p < 0.05.
2.8. Screening Biological Indicators of the H2O2 Pretreatment and NaCl Stress
2.8.1. Principal Component Analysis
The Origin 2019b Software was used to conduct a principal component analysis (PCA) of the 16 single indices of F. tataricum germination and salt tolerance. The eigenvalues of the first three components (PC1, PC2, PC3) were 8.625, 3.795, and 1.726, respectively (Table 3). Their contribution rates were 53.905%, 23.716%, and 10.790%, respectively, and their cumulative contribution was 88.411% (Table 3). As such, PC1, PC2, and PC3 accurately represent all the data contained in the original 16 indices (Table 3).

Table 3.
Eigenvalues, contribution rates, and eigenvectors for each principal component.
The eigenvector matrix reflects the load of each index on each principal component. In PC1, GP, CAT, RL, SFW, GR, RV, VI, SL and GI had higher loads and were the main factors of this principal component (Table 3). Thus, PC1 mainly represented the germination characteristics, morphological characteristics, and material synthesis and accumulation of F. tataricum seeds (Table 3). In PC2, Pro and SOD were the main factors and had relatively large loads of 0.962 and 0.942, respectively (Table 3). Thus, PC2 mainly represented the antioxidant enzyme activity and osmotic adjustment substances of the seedlings (Table 3). RSA and RAD were the main factors of PC3, indicating that this principle component mainly represented the morphological characteristics and material synthesis and accumulation of the seedlings underground. As such, GP, CAT, RL, SFW, GR, RV, VI, SL, and GI were used as first-level indicators for evaluating salt tolerance of F. tataricum seeds treated with different concentrations of H2O2; Pro and SOD were used as second-level indicators; RSA and RAD were used as third level indicators (Table 3). In summary, 13 indices could be used as comprehensive indicators for evaluating the effects of pretreatment with different H2O2 concentrations on the germination of F. tataricum seeds under NaCl stress (Table 3).
2.8.2. Stepwise Regression Analysis
A mathematical model for the evaluation of salt tolerance was established through stepwise regression. The comprehensive evaluation D value was the dependent variable, and each index value was used as an independent variable in the stepwise regression analysis. The optimal regression equation was set up with the coefficient of determination R2 = 1.000, and variance F = 118,710.98.
where Y is the predicted comprehensive evaluation value of F. tataricum seeds under salt stress with different H2O2 pretreatment concentrations; X1–X6 represent GP, SFW, RSA, RAD, CAT, and SOD, respectively. The correlation coefficient R = 1.000 (P = 0.002) between the predicted salt tolerance value, Y, and the salt tolerance evaluation value, D, of F. tataricum seeds, indicates that this equation is accurate and has a high predictive capability. Furthermore, it indicates that the indices used are key to evaluating the influence of different H2O2 concentrations on F. tataricum seeds under NaCl stress.
Y = −0.008X1 + 0.241X2 + 0.062X3 + 0.034X4 + 0.048X5 − 0.058X6 + 0.327,
3. Discussion
3.1. Salt Stress Adversely Affects Seed Germination of F. Tataricum
Soil salinization is just one of the negative environmental impacts experienced globally as industries strive to increase crop production and yields [18]. Seed germination is the initial and most critical stage in the crop life cycle, and its germination is severely affected by salt stress. As evidenced by the preliminary experiment of this study (Table 1), in which 50 mmol L−1 NaCl significantly reduced the GP, GR, and GI of F. tataricum seeds, and significantly inhibited their VI and SL, by more than 75.0% (Table 1).
F. tataricum seeds are clearly adversely affected by salt stress, as their GP, GR, GI, VI, SL, SFW, RL, RSA, RAD, and RV all decreased significantly under NaCl treatment alone (Figure 1, Figure 2, Figure 3 and Figure 4, Table 2). High extracellular NaCl concentrations create a water potential gradient that draws water from the inside of the cell to the outside. Plants frequently respond by closing their stomata to reduce cell transpiration and prevent cells from absorbing water from the outside [19]. Interestingly, this study found that the RFW of F. tataricum seeds increased slightly in a 50 mmol L−1 NaCl environment, which might be related to its adaptive strengths as a more tolerant variety (Table 2). F. tataricum is better adapted to unfavorable environments such as soils with high salinity and alkalinity and is able to better utilize the resources available in these adverse conditions. It can increase its material accumulation, transportation, and distribution to improve its survival capability. In nature, high salinity environments have many adverse effects on crops, causing the increase and accumulation of intracellular and intercellular antioxidant enzymes, membrane lipid peroxidation, and osmotic adjustment substances [20], protecting cell structure and improving self-resistance.
3.2. H2O2 Pretreatment Alleviates the Negative Effects of NaCl Stress on Seed Germination in F. Tataricum
Hydrogen peroxide (H2O2) is an exogenous signal substance involved in plant growth regulation and plays a crucial role in their physiological response to adversity. There were some reports on the use of H2O2 to improve seed germination. For example, H2O2 application at suitable concentrations alleviated the inhibition of pistachio seed germination under salt stress [21]; reduced the oxidative damage to rape seedlings under low-temperature conditions [9]; and enhanced the growth of wheat and the efficiency of its antioxidant defense systems under drought stress [22]. Due to differences in crop varieties and treatment methods, the optimal concentration at which exogenous H2O2 should be applied to alleviate NaCl stress is also diverse.
In this study, the germination of F. tataricum seeds under NaCl stress was distinctly improved by treatments of different H2O2 concentrations (Figure 1). Furthermore, the growth of F. tataricum seedlings was enhanced significantly (Figure 2, Table 2), the amount of active oxygen was increased, and the accumulation of osmotic adjustment substances was promoted (Figure 3 and Figure 4). Under NaCl stress and increasing H2O2 concentrations, all indices (except for POD and MDA) increased first and then decreased (Figure 1A–D, Figure 2A,B, Table 2, Figure 3A,B and Figure 4B). SL and RL increased as the H2O2 concentration increased from 0.1–50 mmol L−1, indicating that concentrations of H2O2 in that range promoted the growth of F. tataricum seedlings aboveground and underground (Figure 2A, Table 2). In treatments of 0.1–20 mmol L−1 H2O2, GP, GR, and VI of F. tataricum seeds were alleviated (Figure 1A–D); SFW, RV, RFW, and CAT were increased (Figure 2B, Table 2, Figure 3A); and the content of MDA was reduced (Figure 4A). These results indicate that seed vigor was enhanced by treatment within this concentration range. Furthermore, the material accumulation of seedlings was promoted, and the oxidative damage and membrane damage caused by NaCl stress was reduced. POD activity first decreased and then increased with increasing H2O2 treatment concentration (Figure 3C). One explanation may be that under NaCl stress, peroxisomes were inhibited through redox reactions in cell metabolism during the germination of F. tataricum seeds. This subsequently limits the mitigation of NaCl-associated toxic effects by hydrogen peroxide, oxidized phenols and other substances. Another explanation may be that during the germination period of F. tataricum, POD activity in its tissues is weak, and the impact of 50 mmol L−1 NaCl was not strong enough to cause a significant increase in this enzyme’s activity (Figure 3C).
Furthermore, pretreatment with H2O2 can alleviate the negative effects of NaCl on the germination characteristics of F. tataricum (Figure 1A–D); promote the growth and material accumulation of above-ground and underground parts of the germinated seeds (Figure 2A,B, Table 2); increase the antioxidant enzyme activity and osmotic adjustment substance content in the seedlings (Figure 3A,B and Figure 4B); and reduce the peroxidation of the cell membrane (Figure 4A). However, although low H2O2 concentrations promote seed germination, high H2O2 concentrations may poison seeds. As the concentration of H2O2 is increased, its mitigation effect on NaCl stress is first enhanced and then weakened. Our results suggest that pretreatment with H2O2 of concentration 5–10 mmol L−1 has the best mitigation effect.
3.3. Analyses Reveal Six Key Indices to Evaluate the Salt Tolerance of F. Tataricum
Salt tolerance in F. tataricum is a complex trait. Researchers have used a variety of methods to evaluate and screen traits that indicate salt tolerance in crop varieties. In this study, a hierarchical cluster analysis and correlation analysis were used to investigate the correlation between various indicators of NaCl stress and the effects of all treatments (Figure 5 and Figure 6). The maximum values of most indicators resulted from the CK, NaCl + 5 mmol L−1 H2O2, and NaCl + 10 mmol L−1 H2O2 treatments (Figure 5). Correlation analysis showed that there was a significant positive correlation among germination parameters, among above-ground indices, and underground indices; and that enzymatic activity was correlated positively or negatively among themselves (Figure 6). When looking at the results of both the cluster analysis and the correlation analysis, the significant relationship between treatments, agronomic traits, and physiological traits can be seen clearly (Figure 5 and Figure 6). Through PCA, this study obtained three new independent principal components with a cumulative contribution rate of 88.411% (Table 3). Concurrently, stepwise regression was used to establish the optimal regression equation for predicting the salt tolerance between the comprehensive evaluation D value and various salt tolerance indicators under H2O2 seed soaking and NaCl stress. PCA and correlation analysis revealed six key indicators that had a significant impact on the salt tolerance characteristics of F. tataricum. These were GP, SFW, RSA, RAD, CAT, and SOD, respectively. Our results suggest that treatment of 5–10 mmol L−1 H2O2 could enhance seed vigor, promote seedling growth, and increase enzymatic activity to effectively alleviate the toxic effects, oxidative damage, and osmotic imbalance caused by NaCl stress (Figure 5 and Figure 6, Table 3).
4. Material and Methods
4.1. Plant Material
Seeds from the salt-sensitive, “Chuanqiao No. 2” variety of F. tataricum were used in this study. They were provided by the Alpine Crops Research Station (102°20′ E and 27°96′ N) of the Xichang Institute of Agricultural Sciences, Liangshan Prefecture, Sichuan Province, China.
4.2. Preliminary Seed Sensitivity Experiments Using NaCl
There were five NaCl treatments in this preliminary experiment with 50 F. tataricum seeds used in each. The treatments were replicated three times. Healthy F. tataricum seeds were carefully selected for uniform size and full grain. The seeds were soaked in distilled water for 12 h; and thereafter, 50 seeds were laid evenly on two layers of quantitative filter paper (9 9 cm) in each sterilized petri dish (90 90 mm) with different concentrations of NaCl solution (0, 50, 100, 150, and 200 mmol L−1). All Petri dishes were placed in a dark incubator room at 22 ± 3 °C for seven days. During this period the germination rate was recorded at 24-h intervals and the NaCl solution was replenished regularly. After seven days, the germination potential, germination rate, germination index, and vigor index were calculated, and the shoot length was determined. The NaCl solution of 50 mmol L−1 was selected to induce seed stress in this study because this concentration has a significant inhibitory effect on the vigor index and shoot length of seeds.
4.3. Seed Soaking Treatments
This experiment was conducted in the laboratory of the College of Agriculture, Guizhou University, Guiyang, China (26°46′ N and 106°67′ E) in April 2021. Disease-free F. tataricum seeds were carefully selected for uniform size and full grain. They were washed with distilled water, disinfected by soaking in 1% NaClO solution for 10 min, rinsed again with distilled water, and then gently blotted to remove any surface moisture. The Hydrogen peroxide (H2O2) used was analytically pure.
There were nine treatments in this experiment, comprising one control, one NaCl treatment, and seven H2O2 + NaCl treatments, with 50 F. tataricum seeds used in each. These treatments were replicated three times and the germination parameters of the seeds were evaluated. A further four replications were conducted to investigate the effect on physiological indicators. Distilled water was used as the control (CK). NaCl of 50 mmol L−1 was used in the NaCl treatment. For the H2O2 treatments, seeds were soaked in solutions of 0.1, 0.5, 5, 10, 20, 50, and 100 mmol L−1 H2O2 for 12 h under normal temperature and darkness conditions. Thereafter, the soaked seeds were rinsed 3–5 times with distilled water and any moisture remaining on their surface was absorbed by the filter paper. The seeds from each treatment were laid evenly between two layers of quantitative filter paper (9 9 cm) in sterilized Petri dishes (90 90 mm), and 5 mL of 50 mmol L−1 NaCl solution were added to each. Petri dishes were placed in a dark incubator room at 22 ± 3 °C for seven days. During this period, the germination number of each treatment was recorded once a day and the NaCl solution was replenished regularly. After seven days, the germination potential, germination rate, germination index, and vigor index were calculated; as were the root and shoot growth indices of F. tataricum seedlings.
4.4. Calculation of Germination Parameters, Seedling Traits, and Physiological Indices
4.4.1. Seed Germination Parameters
The seed germination parameters were determined adopting the procedure given by Agami et al. [23]. The germination standard was considered as the radicle length reaching half of the seed length. Germination potential (GP), germination rate (GR), germination index (GI) and vigor index (VI) were calculated using the following equations:
where G3 is the number of germinated seeds on the 3rd day of cultivation; G7 is the number of germinated seeds on the 7th day of cultivation; N is the total number of seeds in each treatment (50); Gt is the number of seeds germinated at time t; Dt is the number of seeds placed in the seedling bed; SL is the average shoot length.
GP = G3/N × 100%
GR = G7/N × 100%
GI = ∑(Gt/Dt)
VI = GI × SL
4.4.2. Seedling Traits
After the germination experiment, five seedlings that met the germination standard were randomly selected from each treatment, and their shoots and roots were removed using scissors. Shoot lengths (SL) were measured, averaged, and expressed in cm. Shoot fresh weight (SFW) and root fresh weight (RFW) were measured with an electronic balance; the average values were expressed in g. WinRHIZO Root Analyzer (where it is produced by Guangzhou Hangxin Scientific Instrument Co., Ltd., Guangzhou, China) was used to measure root traits, including root length (RL), root surface area (RSA), root average diameter (RAD), and root volume (RV).
4.4.3. Physiological Indices
Physiological indices were measured by rapid methods as suggested by Lu et al. [24] and the instructions of the BOXBIO kit. Catalase (CAT) and proline (Pro) were run through an ultraviolet spectrophotometer (where it is produced by Unico Instrument Co., Ltd., Shanghai, China) to determine the absorbance at 240 nm and 520 nm, respectively. Superoxide dismutase (SOD) and peroxidase (POD) were measured using a microplate reader (where it is produced by Chengdu Baile Technology Co., Ltd., Chengdu, China) at 560 nm and 470 nm, respectively; and malondialdehyde (MDA) was measured at 450, 532, and 600 nm, respectively.
4.5. Statistical Analysis
Microsoft Excel 2010 was used for data input, processing of the original data, and basic statistical analysis. SPSS software version 26 was used to perform single-factor analysis of variance, Pearson correlation analysis, and stepwise regression analysis to screen the salt tolerance index of germination of F. tataricum seeds for each germination parameter and physiological indicator. Duncan’s multiple range test (p < 0.05) was used to measure the significance of differences. The results are expressed as means ± standard deviations. Variance analysis charts, correlation heat map, and principal component analysis were created using the Origin 2019b Software. The cluster heat map was created using Tbtools.
5. Conclusions
In this study, 5–10 mmol L−1 H2O2 effectively enhanced the vigor of “Chuanqiao-2” F. tataricum seeds; promoted their germination and growth; increased their enzymatic activity; and efficiently alleviated the toxic effects, oxidative damage, and osmotic imbalances caused by salt stress. Germination potential, shoot fresh weight, root surface area, root average diameter, catalase activity, and superoxide dismutase activity were the six key indicators that can be used to assess the impact of salt stress in F. tataricum. In this study, only the germination of F. tataricum seeds, the phenotype estimate of the shoots and roots of the seedlings, and the determination of related enzyme activities were involved, but the study of gene expression and molecular mechanisms was not researched. Therefore, it is necessary to study the effect of H2O2 on F. tataricum under salt stress.
Author Contributions
X.Y. designed the study. X.Y. and Y.P. performed most of the experiments. A.G., Y.T. and H.Y. performed some experiments. X.Y. and M.Z. analyzed the results and wrote the first draft. J.R. and J.C. edited the manuscript. All the authors read, revised, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R & D Project of China (2017YFE0117600, SQ2020YFF0402959), the National Natural Science Foundation of China (31660531) and the Guizhou Science and Technology Support Program (Qianke He Support [2020]1Y051).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the present study.
Acknowledgments
We acknowledge the College of Agronomy, Guizhou University, Guiyang, China, for providing the laboratory facilities and other necessary materials for the conductance and analysis of the study. We are also thankful to F. tataricum breeders for provided by the Alpine Crops Research Station of the Xichang Institute of Agricultural Sciences, Liangshan Prefecture, Sichuan Province, and China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karle, S.B.; Guru, A.; Dwivedi, P.; Kumar, K. Insights into the Role of Gasotransmitters Mediating Salt Stress Responses in Plants. J. Plant Growth Regul. 2021, 1–17. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B. Protection of Halophytes and Their Uses for Cultivation of Saline-Alkali Soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Pour-Aboughadareh, A.; Mehrvar, M.R.; Sanjani, S.; Amini, A.; Nikkhah-Chamanabad, H.; Asadi, A. Effects of salinity stress on seedling biomass, physiochemical properties, and grain yield in different breeding wheat genotypes. Acta Physiol. Plant. 2021, 43, 98. [Google Scholar] [CrossRef]
- Tao, R.; Ding, J.; Li, C.; Zhu, X.; Guo, W.; Zhu, M. Evaluating and Screening of Agro-Physiological Indices for Salinity Stress Tolerance in Wheat at the Seedling Stage. Front. Plant Sci. 2021, 12, 646175. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Prakash, P.; Singh, A.K. Salicylic Acid and Hydrogen Peroxide Improve Antioxidant Response and Compatible Osmolytes in Wheat (Triticum aestivum L.) Under Water Deficit. Agric. Res. 2020, 10, 175–186. [Google Scholar] [CrossRef]
- Bhardwaj, R.D.; Singh, N.; Sharma, A.; Joshi, R.; Srivastava, P. Hydrogen peroxide regulates antioxidant responses and redox related proteins in drought stressed wheat seedlings. Physiol. Mol. Biol. Plants 2021, 27, 151–163. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Lin, X.; Sun, C. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Sun, Y.; Zheng, S.; Wang, J.; Zhang, T. Hydrogen peroxide is involved in strigolactone induced low temperature stress tolerance in rape seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef]
- Li, C.; Xie, Z.; Wang, Y.; Lu, W.; Yin, G.; Sun, D.; Ren, C.; Wang, L. Correlation and genetic analysis of seed shell thickness and yield factors in Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.). Breed. Sci. 2019, 69, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; He, Y.J.; Li, H.M.; Hu, J.J.; Cheng, Z. Diversity of Endophytes in Fagopyrum esculentum Moench. Seeds from Different Locations in China. Russ. J. Plant Physiol. 2021, 68, 413–420. [Google Scholar] [CrossRef]
- Zheng, C.; Hu, C.; Ma, X.; Peng, C.; Zhang, H.; Qin, L. Cytotoxic phenylpropanoid glycosides from Fagopyrum tataricum (L.) Gaertn. Food Chem. 2011, 132, 433–438. [Google Scholar] [CrossRef]
- Ma, W.; Kim, J.K.; Jia, C.; Yin, F.; Kim, H.J.; Akram, W.; Hu, X.; Li, X. Comparative Transcriptome and Metabolic Profiling Analysis of Buckwheat (Fagopyrum tataricum (L.) Gaertn.) under Salinity Stress. Metabolites 2019, 9, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, J.; Wang, J.; Zhao, G.; Zou, L.; Lei, Y. Buckwheat derived nitrogen-rich porous carbon material with a high-performance Na-storage. J. Porous Mater. 2020, 27, 1139–1147. [Google Scholar] [CrossRef]
- Luthar, Z.; Golob, A.; Germ, M.; Vombergar, B.; Kreft, I. Tartary Buckwheat in Human Nutrition. Plants 2021, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.-X.; Wang, J.-F.; Ma, H.; Wang, S.-M.; Luo, L.; Wang, S.-M. Effect of microwave radiation on antioxidant capacities of Tartary buckwheat sprouts. J. Food Sci. Technol. 2020, 57, 3913–3919. [Google Scholar] [CrossRef]
- Aubert, L.; Konrádová, D.; Barris, S.; Quinet, M. Different drought resistance mechanisms between two buckwheat species Fagopyrum esculentum and Fagopyrum tataricum. Physiol. Plant. 2020, 172, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.; Daliakopoulos, I.N.; Del Moral, F.; Hueso, J.J.; Tsanis, I.K. A Review of Soil-Improving Cropping Systems for Soil Salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, J.-L.; Liu, L.; Xie, Q.; Sui, N. Photosynthetic Regulation Under Salt Stress and Salt-Tolerance Mechanism of Sweet Sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef]
- Hossain, S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous Melatonin Modulates the Physiological and Biochemical Mechanisms of Drought Tolerance in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef]
- Bagheri, M.; Gholami, M.; Baninasab, B. Role of hydrogen peroxide pre-treatment on the acclimation of pistachio seedlings to salt stress. Acta Physiol. Plant. 2021, 43, 51. [Google Scholar] [CrossRef]
- Sehar, Z.; Jahan, B.; Masood, A.; Anjum, N.A.; Khan, N.A. Hydrogen peroxide potentiates defense system in presence of sulfur to protect chloroplast damage and photosynthesis of wheat under drought stress. Physiol. Plant. 2020, 172, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Q.-F.; Li, J.; Xiong, J.; Zhou, L.-N.; He, S.-L.; Zhang, J.-Q.; Chen, Z.-A.; He, S.-G.; Liu, H. Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci. Rep. 2019, 9, 7397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).