Metabolite Profiling of Dioscorea (Yam) Leaves to Identify Bioactive Compounds Reveals Their Potential as Renewable Resources
Abstract
:1. Introduction
2. Results
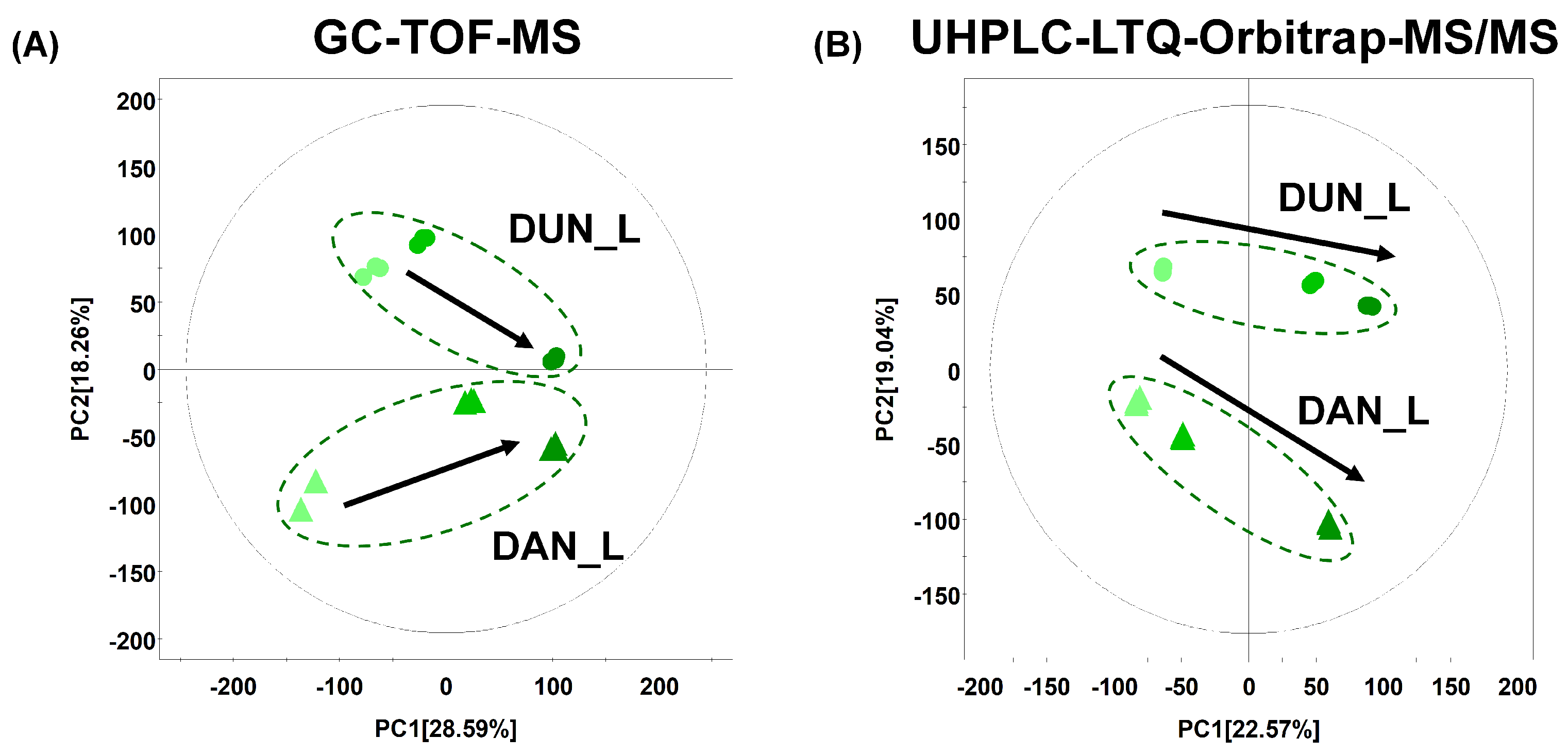
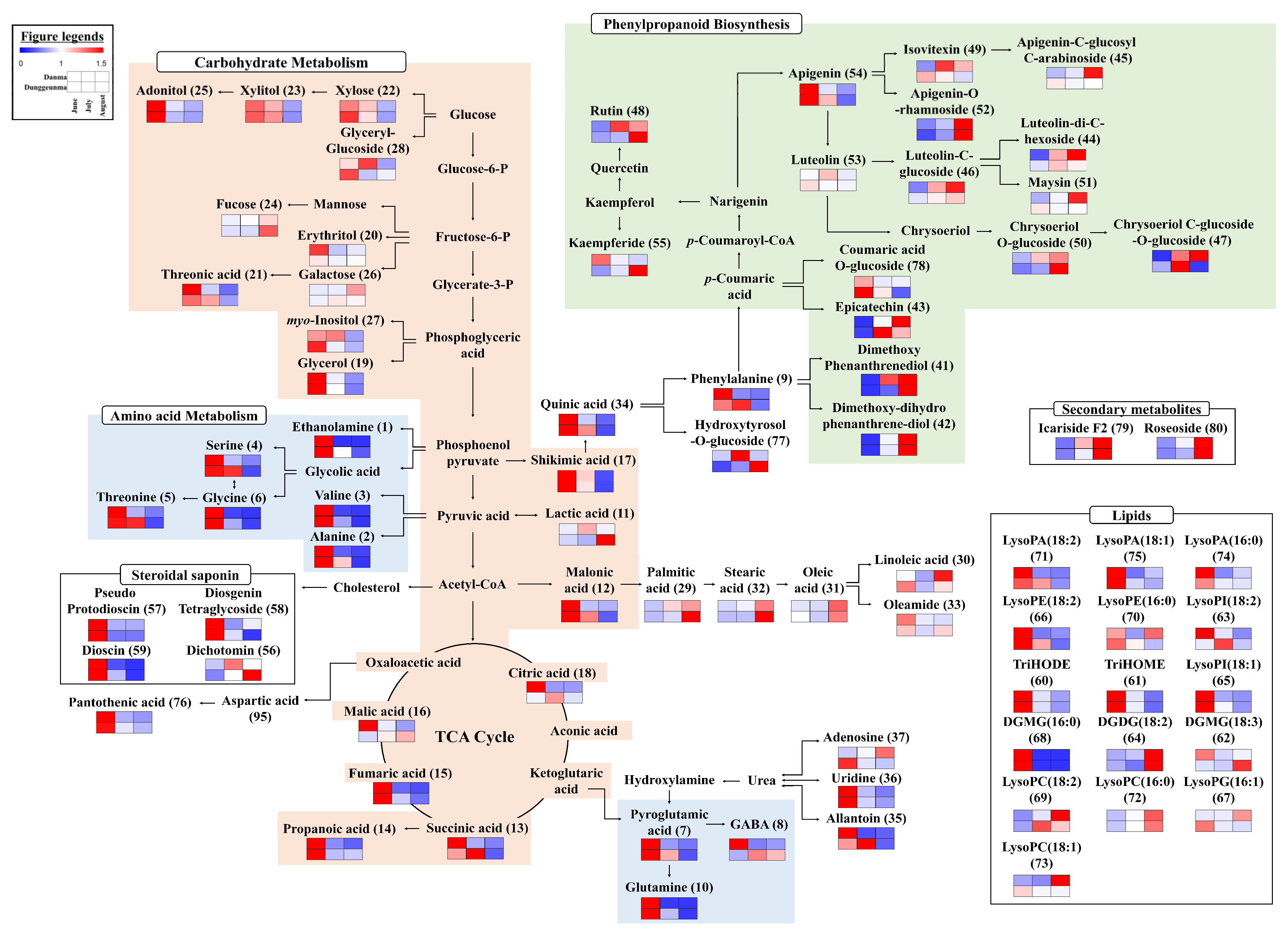
2.1. Differences in the Metabolite Content and Metabolic Pathways in Dioscorea Leaves According to Harvest Time
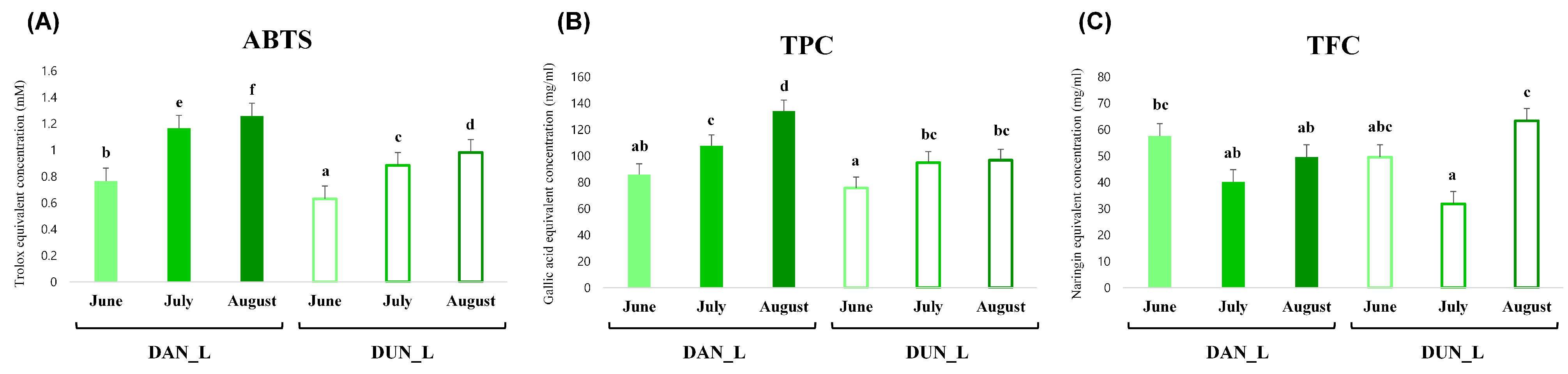
2.2. Correlation of the Metabolic Profiles and Antioxidant Activities of DAN and DUN Leaves
2.3. Differences between the Antioxidant Activity and Metabolite Composition of the Leaves and Roots
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Source and Preparation
4.3. Sample Extraction
4.4. GC-TOF-MS Analysis
4.5. UHPLC-LTQ-Orbitrap-MS/MS Analysis
4.6. Data Processing and Multivariate Statistical Analysis
4.7. Determination of Antioxidant Activity and Total Phenolic and Flavonoid Contents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khurana, N.; Kalsi, V.; Duggal, N.; Singh, A. Chromatographic Fingerprinting, Antioxidant, and Anti-inflammatory Potential of Dioscorea villosa (Wild Yam) Leaves. Int. J. Green Pharm. 2018, 12, 102–106. [Google Scholar]
- Kim, M.; Gu, M.J.; Lee, J.-G.; Chin, J.; Bae, J.-S.; Hahn, D. Quantitative Analysis of Bioactive Phenanthrenes in Dioscorea batatas Decne Peel, a Discarded Biomass from Postharvest Processing. Antioxidants 2019, 8, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam)—An Appraisal of Nutritional and Therapeutic Potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef]
- Dey, P.; Chowdhuri, S.R.; Sarkar, M.P.; Chaudhuri, T.K. Evaluation of anti-inflammatory activity and standardisation of hydro-methanol extract of underground tuber of Dioscorea alata. Pharm. Biol. 2016, 54, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Shi, X.; Ren, X.; Qin, Z. Chemical composition and antioxidant activity of phenolic compounds from Dioscorea (Yam) leaves. Pak. J. Pharm. Sci. 2018, 31, 1031–1038. [Google Scholar] [PubMed]
- Das, A.; Organisation, D.; Chaudhuri, D.; Ghate, N.; Chatterjee, A. Phytochemical analysis, antioxidant and anticancer potential of leaf extracts from edible greater yam, Dioscorea alata L., from north-east India. Int. J. Phytopharm. 2014, 5, 109–119. [Google Scholar]
- Omodamiro, O.D. Anti-inflammatory and Diuretic Activities of Ethanol Extract of Dioscorea bulbifera Leaf. Am. J. Drugs Deliv. Ther. 2015, 2, 29–38. [Google Scholar]
- An, L.; Yuan, Y.; Ma, J.; Wang, H.; Piao, X.; Ma, J.; Zhang, J.; Zhou, L.; Wu, X. NMR-based metabolomics approach to investigate the distribution characteristics of metabolites in Dioscorea opposita Thunb. cv. Tiegun. Food Chem. 2019, 298, 125063. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Feng, F.; Zhai, J.; Chen, F.; Liu, T.; Zhang, F.; Zhang, F. Development of an analytical method for twelve dioscorea saponins using liquid chromatography coupled to Q-Exactive high resolution mass spectrometry. Talanta 2019, 191, 11–20. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.L.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Guerrero-Muñoz, N.; Villegas-Aguilar, M.D.C.; Pimentel-Moral, S.; Ramos-Escudero, F.; Segura-Carretero, A. LC-MS and Spectrophotometric Approaches for Evaluation of Bioactive Compounds from Peru Cocoa By-Products for Commercial Applications. Molecules 2020, 25, 3177. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Plant By-Products and Food Industry Waste: A Source of Nutraceuticals and Biopolymers; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 279–315. [Google Scholar]
- Ku, K.M.; Kang, Y.H. Quinone Reductase Inductive Activity of Capsicum annuum Leaves and Isolation of the Active Compounds. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 709–715. [Google Scholar] [CrossRef]
- Aregheore, E.M. Nutritive Value and Inherent Anti-nutritive Factors in Four Indigenous Edible Leafy Vegetables in Human Nutrition in Nigeria: A Review. J. Food Resour. Sci. 2011, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ryu, R.; Jeong, T.-S.; Kim, Y.J.; Choi, J.-Y.; Cho, S.-J.; Kwon, E.-Y.; Jung, U.J.; Ji, H.-S.; Shin, D.-H.; Choi, M.-S. Beneficial Effects of Pterocarpan-High Soybean Leaf Extract on Metabolic Syndrome in Overweight and Obese Korean Subjects: Randomized Controlled Trial. Nutrients 2016, 8, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, G.R.; Lee, S.; Lee, S.; Do, S.-G.; Shin, E.; Lee, C.H. Maturity stage-specific metabolite profiling of Cudrania tricuspidata and its correlation with antioxidant activity. Ind. Crop. Prod. 2015, 70, 322–331. [Google Scholar] [CrossRef]
- Raterink, R.-J.; Lindenburg, P.W.; Vreeken, R.; Ramautar, R.; Hankemeier, T. Recent developments in sample-pretreatment techniques for mass spectrometry-based metabolomics. TrAC Trends Anal. Chem. 2014, 61, 157–167. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Metabolomics analysis of Camellia sinensis with respect to harvesting time. Food Res. Int. 2020, 128, 108814. [Google Scholar] [CrossRef]
- Feng, Z.; Gao, Z.; Jiao, X.; Shi, J.; Wang, R. Widely targeted metabolomic analysis of active compounds at different maturity stages of ‘Hupingzao’ jujube. J. Food Compos. Anal. 2020, 88, 103417. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Prabhu, P.R.; Hudson, A.O. Identification and Partial Characterization of an L-Tyrosine Aminotransferase (TAT) from Arabidopsis thaliana. Biochem. Res. Int. 2010, 2010, 549572. [Google Scholar] [CrossRef]
- Lee, M.Y.; Seo, H.S.; Singh, D.; Lee, S.J.; Lee, C.H. Unraveling dynamic metabolomes underlying different maturation stages of berries harvested from Panax ginseng. J. Ginseng Res. 2020, 44, 413–423. [Google Scholar] [CrossRef]
- du Toit, E.; Sithole, J.; Vorster, J. Leaf harvesting severity affects total phenolic and tannin content of fresh and dry leaves of Moringa oleifera Lam. trees growing in Gauteng, South Africa. S. Afr. J. Bot. 2020, 129, 336–340. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastore, C.; Santo, S.D.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole Plant Temperature Manipulation Affects Flavonoid Metabolism and the Transcriptome of Grapevine Berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef]
- Ha, J.; Kim, H. Changes in the association between summer temperature and mortality in Seoul, South Korea. Int. J. Biometeorol. 2012, 57, 535–544. [Google Scholar] [CrossRef]
- Hohtola, A.; Jaakola, L. Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 2003, 218, 721–728. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleifera Leaves in Two Stages of Maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Kho, K.; Sim, Y.Y.; Nyam, K.L. Antioxidant activities of tea prepared from kenaf (Hibiscus cannabinus L. KR9) leaves at different maturity stages. J. Food Meas. Charact. 2019, 13, 2009–2016. [Google Scholar] [CrossRef]
- Ziaei, M.; Sharifi, M.; Behmanesh, M.; Razavi, K. Gene expression and activity of phenyl alanine ammonilyase and essential oil composition of Ocimum basilicum L. at different growth stages. Iran. J. Biotechnol. 2012, 10, 32–39. [Google Scholar]
- Lee, J.; Dong, X.; Choi, K.; Song, H.; Yi, H.; Hur, Y. Identification of source-sink tissues in the leaf of Chinese cabbage (Brassica rapa ssp. pekinensis) by carbohydrate content and transcriptomic analysis. Genes Genom. 2019, 42, 13–24. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino Acid Transporters in Plants: Identification and Function. Plants 2020, 9, 972. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Zhang, M.; Fan, W.; Firon, N.; Pattanaik, S.; Yuan, L.; Zhang, P. Altered Phenylpropanoid Metabolism in the Maize Lc-Expressed Sweet Potato (Ipomoea batatas) Affects Storage Root Development. Sci. Rep. 2016, 6, 18645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logemann, E.; Tavernaro, A.; Schulz, W.; Somssich, I.; Hahlbrock, K. UV light selectively coinduces supply pathways from primary metabolism and flavonoid secondary product formation in parsley. Proc. Natl. Acad. Sci. USA 2000, 97, 1903–1907. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-M.; Kang, M.-K.; Kim, J.-S.; Kim, G.-C.; Choi, S.-Y. Physicochemical Composition and Antioxidant Activities of Korean Dioscorea Species. J. East Asian Soc. Diet. Life 2015, 25, 880. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, Y.J.; Chung, D.; Kim, D.-O. Antioxidant capacities of individual and combined phenolics in a model system. Food Chem. 2007, 104, 87–92. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Oh, D.-G.; Singh, D.; Lee, H.J.; Kim, G.R.; Lee, S.; Lee, J.S.; Lee, C.H. Untargeted Metabolomics Toward Systematic Characterization of Antioxidant Compounds in Betulaceae Family Plant Extracts. Metabolites 2019, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Allouche, N.; Allouche, N.; Feki, M.; Damak, M.; Sayadi, S. Isolation of Hydroxytyrosol 4-β-D-Glucoside and 3,4-Dihydroxyphenylglycol with Antioxidant Activity. J. Soc. Chim. Tunis. 2005, 7, 231–238. [Google Scholar]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, H.; Zhou, J.; Geng, Y.; Wang, X. Rapid Screening and Preparative Isolation of Antioxidants from Alpinia officinarum Hance Using HSCCC Coupled with DPPH-HPLC Assay and Evaluation of Their Antioxidant Activities. J. Anal. Methods Chem. 2018, 2018, 3158293. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 2014, 65, 70–75. [Google Scholar] [CrossRef]
- Bubols, G.B.; Vianna, D.D.R.; Medina-Remon, A.; Von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The Antioxidant Activity of Coumarins and Flavonoids. Mini-Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Lim, J.S.; Hahn, D.; Gu, M.J.; Oh, J.; Lee, J.S.; Kim, J.-S. Anti-inflammatory and antioxidant effects of 2, 7-dihydroxy-4, 6-dimethoxy phenanthrene isolated from Dioscorea batatas Decne. Appl. Biol. Chem. 2019, 62, 29. [Google Scholar] [CrossRef]
- Han, J.S.; Lee, S.; Kim, H.Y.; Lee, C.H. MS-Based Metabolite Profiling of Aboveground and Root Components of Zingiber mioga and Officinale. Molecules 2015, 20, 16170–16185. [Google Scholar] [CrossRef] [Green Version]
- Brecht, J. Underground Storage Organs. Postharvest Physiology and Pathology of Vegetables; CRC Press: Gainesville, FL, USA, 2002. [Google Scholar] [CrossRef]
- Preiss, J. Plant Starch Synthesis. In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–95. [Google Scholar]
- Lee, S.; Lee, S.; Singh, D.; Oh, J.Y.; Jeon, E.J.; Ryu, H.S.; Lee, D.W.; Kim, B.S.; Lee, C.H. Comparative evaluation of microbial diversity and metabolite profiles in doenjang, a fermented soybean paste, during the two different industrial manufacturing processes. Food Chem. 2017, 221, 1578–1586. [Google Scholar] [CrossRef]
- Godzien, J.; Alonso-Herranz, V.; Barbas, C.; Armitage, E.G. Controlling the quality of metabolomics data: New strategies to get the best out of the QC sample. Metabolomics 2014, 11, 518–528. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, X.; Zhang, J.; Chen, S.; Wu, Y. Chemical profiling and antioxidant evaluation of Yangxinshi Tablet by HPLC–ESI-Q-TOF-MS/MS combined with DPPH assay. J. Chromatogr. B 2017, 1060, 262–271. [Google Scholar] [CrossRef]
- Ibrahima, R.M.; El-Halawany, A.M.; Saleh, D.O.; El Naggar, E.M.B.; El-Shabrawy, A.E.-R.O.; El-Hawary, S.S. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev. Bras. Farmacogn. 2015, 25, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhu, J.; Cao, F.; Chen, F. Anti-inflammatory properties of extracts from Chimonanthus nitens Oliv. leaf. PLoS ONE 2017, 12, e0181094. [Google Scholar] [CrossRef]
- Karar, M.G.E.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 1, 012016. [Google Scholar] [CrossRef] [Green Version]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; Chaves, D.S.D.A.; Romaniuk-Drapała, A.; Rybczynska, M.; Gryszczyńska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Rev. Bras. Farm. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic Profiling of Portuguese Propolis by LC-MS Spectrometry: Uncommon Propolis Rich in Flavonoid Glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Fu, Z.; Zhang, Q.; Xu, L.; Liao, L. Identification and structural elucidation of steroidal saponins from the root of Paris polyphylla by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Res. 2015, 29, 1798–1803. [Google Scholar] [CrossRef]
- D’Urso, G.; Napolitano, A.; Cannavacciuolo, C.; Masullo, M.; Piacente, S. Okra fruit: LC-ESI/LTQOrbitrap/MS/MSnbased deep insight on polar lipids and specialized metabolites with evaluation of anti-oxidant and anti-hyperglycemic activity. Food Funct. 2020, 11, 7856–7865. [Google Scholar] [CrossRef] [PubMed]
- Rekik, O.; Ben Mansour, A.; Bouaziz, M. Evaluation of phenolic composition and antioxidant activity changes in olive flowers during development using HPLC/DAD and LC-MS/MS. Electrophoresis 2018, 39, 1663–1672. [Google Scholar] [CrossRef]
- Son, S.Y.; Kim, N.K.; Lee, S.; Singh, D.; Kim, G.R.; Lee, J.S.; Yang, H.-S.; Yeo, J.; Lee, S.; Lee, C.H. Metabolite fingerprinting, pathway analyses, and bioactivity correlations for plant species belonging to the Cornaceae, Fabaceae, and Rosaceae families. Plant Cell Rep. 2016, 35, 1917–1931. [Google Scholar] [CrossRef]
- Lee, S.; Oh, D.-G.; Lee, S.; Kim, G.R.; Lee, J.S.; Son, Y.K.; Bae, C.-H.; Yeo, J.; Lee, C.H. Chemotaxonomic Metabolite Profiling of 62 Indigenous Plant Species and Its Correlation with Bioactivities. Molecules 2015, 20, 19719–19734. [Google Scholar] [CrossRef] [Green Version]


| No. | Common Name | Scientific Name | Gene a | Organ Type | Abbreviation | Collection Date |
|---|---|---|---|---|---|---|
| 1 | Danma | Dioscorea ploystchya Turcz | DC17010 | leaf | DAN_L | 4 June 2020 |
| 2 | 7 July 2020 | |||||
| 3 | 21 August 2020 | |||||
| 4 | Dunggeunma | Dioscorea bulbifera | DS001 | leaf | DUN_L | 4 June 2020 |
| 5 | 7 July 2020 | |||||
| 6 | 21 August 2020 | |||||
| 7 | Danma | Dioscorea ploystchya Turcz | DC17010 | root | DAN_R | 21 August 2020 |
| 8 | Dunggeunma | Dioscorea bulbifera | DS001 | root | DUN_R | 21 August 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Son, S.-Y.; Jeon, S.-G.; Kim, J.-G.; Lee, C.-H. Metabolite Profiling of Dioscorea (Yam) Leaves to Identify Bioactive Compounds Reveals Their Potential as Renewable Resources. Plants 2021, 10, 1751. https://doi.org/10.3390/plants10081751
Kim M-J, Son S-Y, Jeon S-G, Kim J-G, Lee C-H. Metabolite Profiling of Dioscorea (Yam) Leaves to Identify Bioactive Compounds Reveals Their Potential as Renewable Resources. Plants. 2021; 10(8):1751. https://doi.org/10.3390/plants10081751
Chicago/Turabian StyleKim, Min-Ji, Su-Young Son, Su-Gyeong Jeon, Jeong-Gu Kim, and Choong-Hwan Lee. 2021. "Metabolite Profiling of Dioscorea (Yam) Leaves to Identify Bioactive Compounds Reveals Their Potential as Renewable Resources" Plants 10, no. 8: 1751. https://doi.org/10.3390/plants10081751
APA StyleKim, M.-J., Son, S.-Y., Jeon, S.-G., Kim, J.-G., & Lee, C.-H. (2021). Metabolite Profiling of Dioscorea (Yam) Leaves to Identify Bioactive Compounds Reveals Their Potential as Renewable Resources. Plants, 10(8), 1751. https://doi.org/10.3390/plants10081751







