An Apoplastic Defensin of Wheat Elicits the Production of Extracellular Polysaccharides in Snow Mold
Abstract
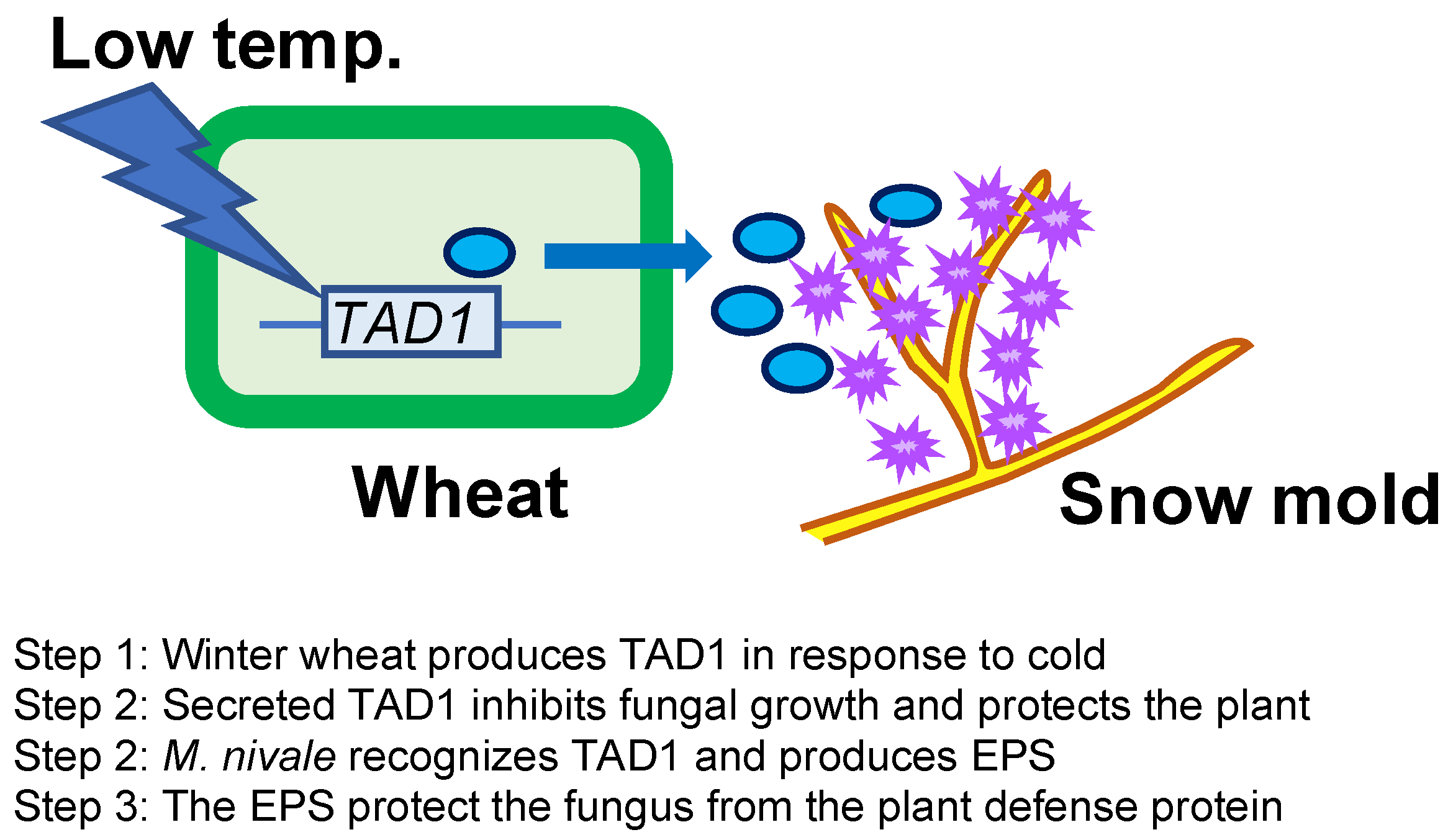
:1. Introduction
2. Results
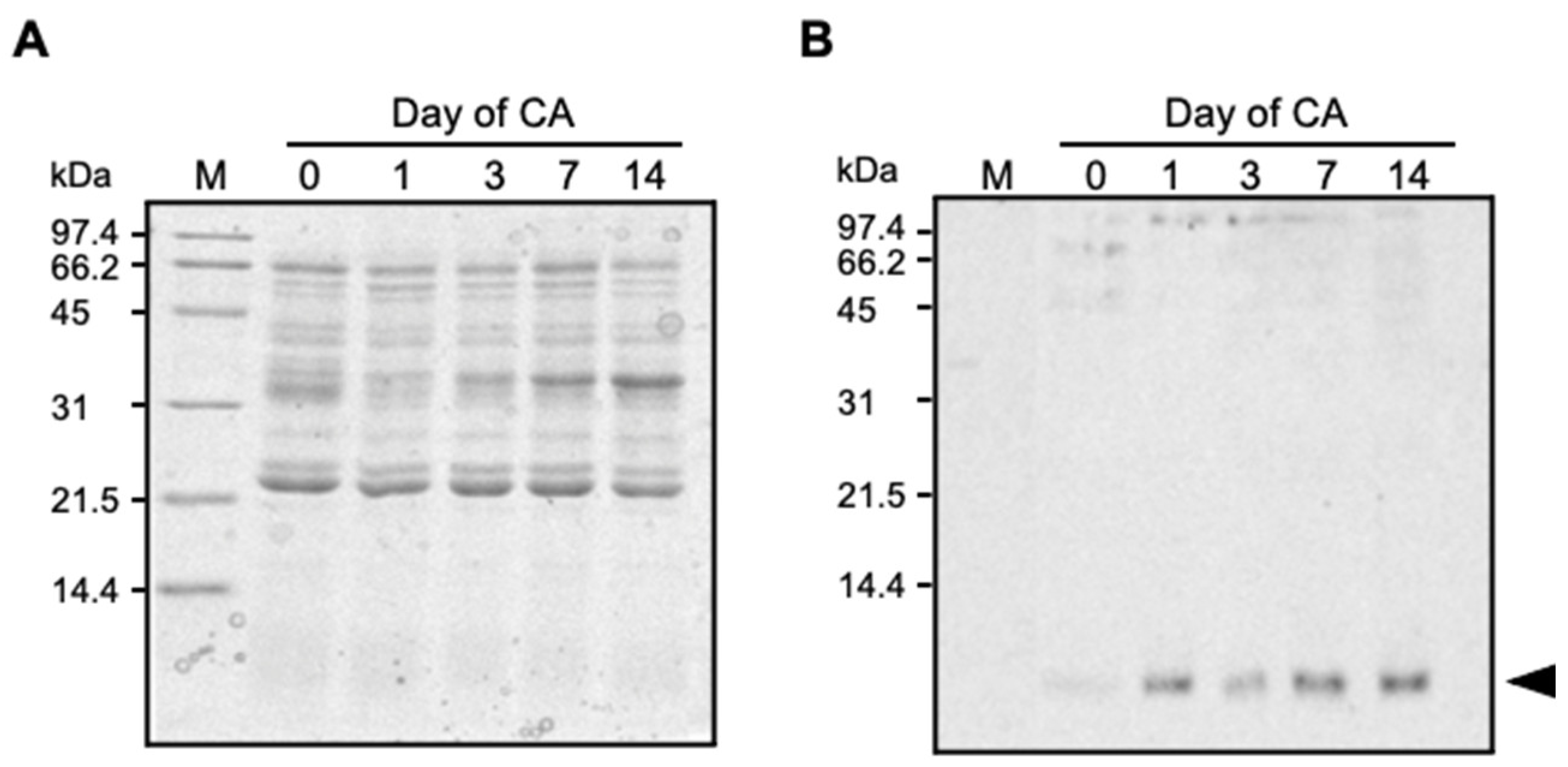
2.1. TAD1 Localized in Extracellular Space during Cold Acclimation
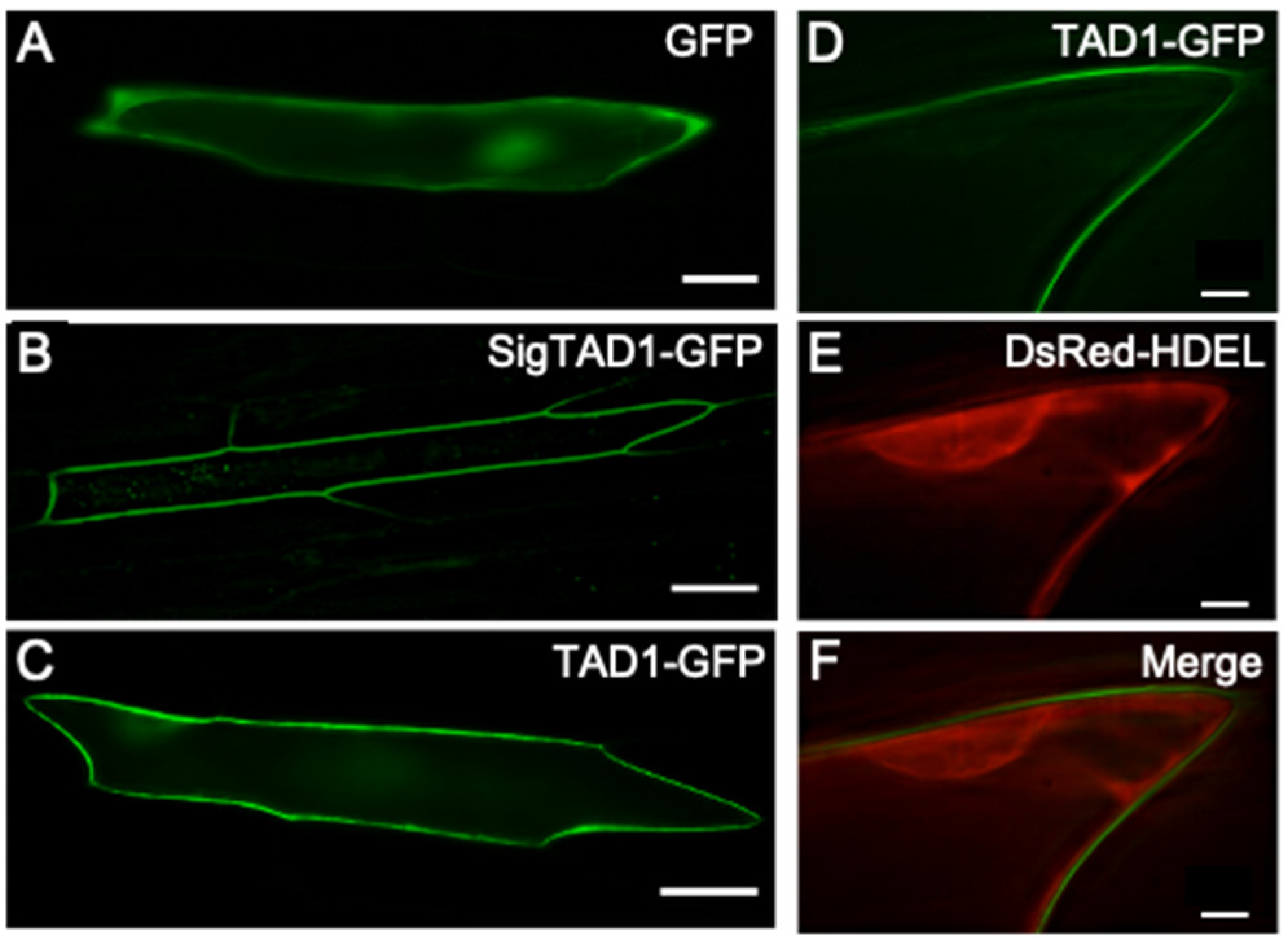
2.2. Subcellular Localization of TAD1-GFP Protein
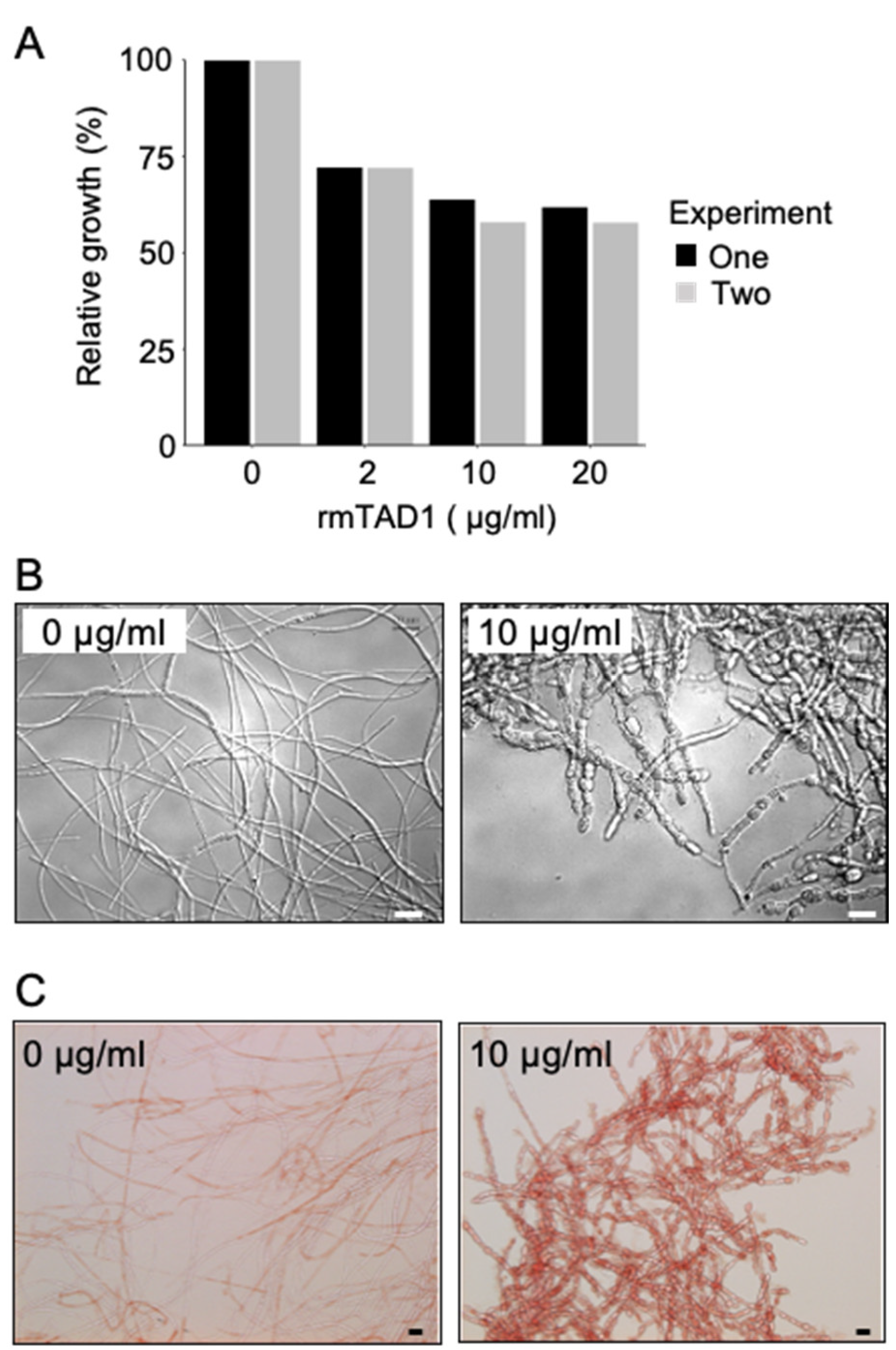
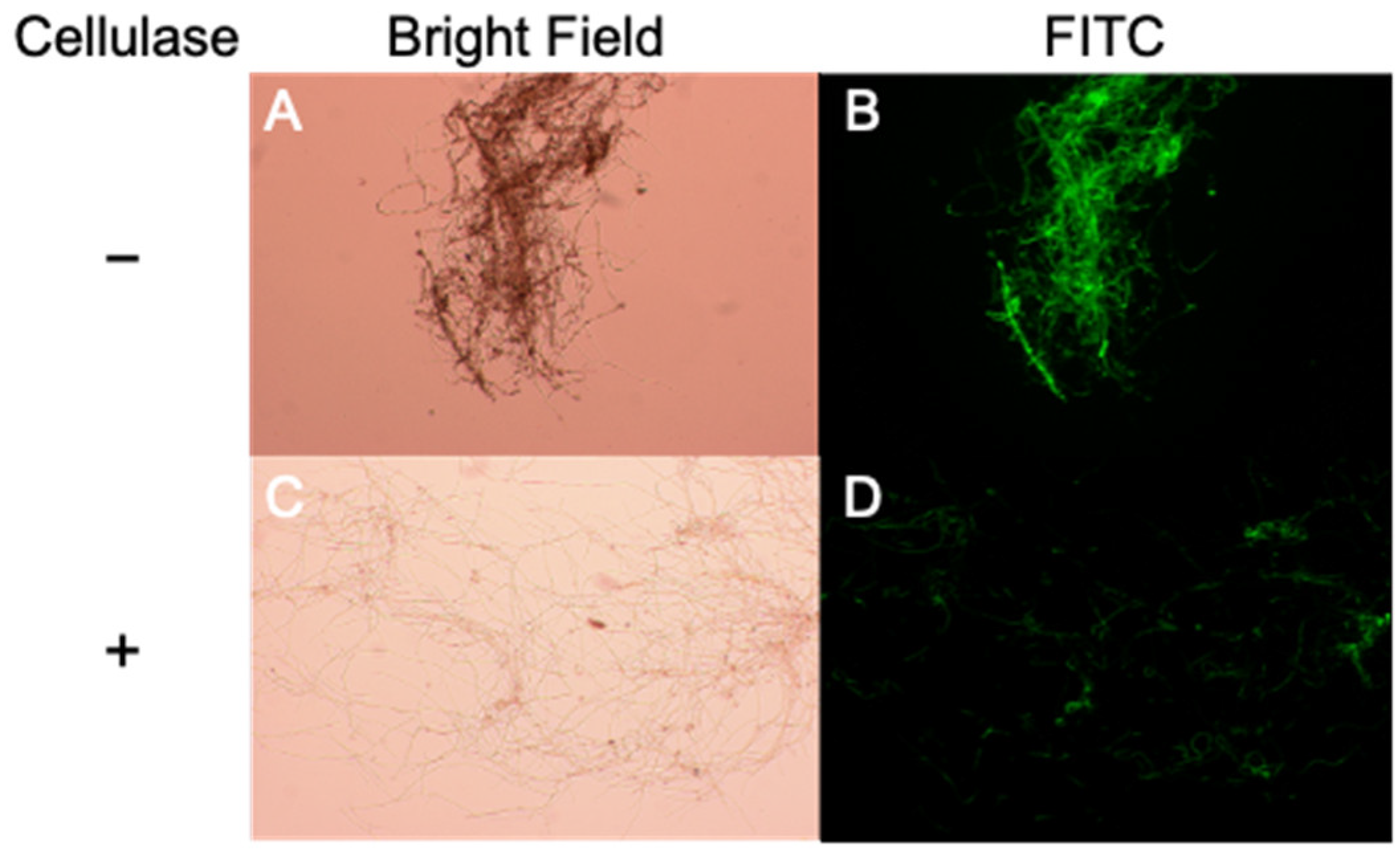
2.3. TAD1 Induced Production of Extracellular Polysaccharides in M. nivale
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Growth and Cold Acclimation Conditions
5.2. Extraction of Apoplastic Proteins
5.3. Western Blot Analysis
5.4. Localization of GFP-Fused Protein
5.5. Recombinant Protein Purification
5.6. Antifungal Activity of TAD1
5.7. FITC Labeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guy, C.L. Cold acclimation and freezing stress tolerance: Role of protein metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Nakajima, T.; Abe, J. Environmental factors affecting expression of resistance to pink snow mold caused by Microdochium nivale in winter wheat. Can. J. Bot. 1996, 74, 1783–1788. [Google Scholar] [CrossRef]
- Tronsmo, A.M.; Gregersen, P.; Hjeljord, L. Cold-Induced Disease Resistance. In Mechanisms of Plant Defense Responses; Springer: Berlin/Heidelberg, Germany, 1993; p. 369. [Google Scholar]
- Kuwabara, C.; Sasaki, K.; Umeki, N.; Hoshino, T.; Saburi, W.; Matsui, H.; Imai, R. A model system for studying plant–microbe interactions under snow. Plant Physiol. 2021, 185, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Okamoto, T.; Tsuda, S.; Imai, R. A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem. Biophys. Res. Commun. 2002, 298, 46–53. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kuwabara, C.; Umeki, N.; Fujioka, M.; Saburi, W.; Matsui, H.; Abe, F.; Imai, R. The cold-induced defensin TAD1 confers resistance against snow mold and Fusarium head blight in transgenic wheat. J. Biotechnol. 2016, 228, 3–7. [Google Scholar] [CrossRef]
- Schweiger-Hufnagel, U.; Ono, T.; Izumi, K.; Hufnagel, P.; Morita, N.; Kaga, H.; Morita, M.; Hoshino, T.; Yumoto, I.; Matsumoto, N.; et al. Identification of the extracellular polysaccharide produced by the snow mold fungus. Microdochium nivale. Biotechnol. Lett. 2000, 22, 183–187. [Google Scholar] [CrossRef]
- François, F.; Lombard, C.; Guigner, J.M.; Soreau, P.; Brian-Jaisson, F.; Martino, G.; Vandervennet, M.; Garcia, D.; Molinier, A.L.; Pignol, D.; et al. Isolation and characterization of environmental bacteria capable of extracellular biosorption of mercury. Appl. Environ. Microbiol. 2012, 78, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Aslam, S.N.; Newman, M.A.; Erbs, G.; Morrissey, K.L.; Chinchilla, D.; Boller, T.; Jensen, T.T.; De Castro, C.; Ierano, T.; Molinaro, A.; et al. Bacterial Polysaccharides Suppress Induced Innate Immunity by Calcium Chelation. Curr. Biol. 2008, 18, 1078–1083. [Google Scholar] [CrossRef]
- Doehlemann, G.; Hemetsberger, C. Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytol. 2013, 198, 1001–1016. [Google Scholar] [CrossRef]
- Naz, R.; Bano, A.; Wilson, N.L.; Guest, D.; Roberts, T.H. Pathogenesis-related protein expression in the apoplast of wheat leaves protected against leaf rust following application of plant extracts. Phytopathology 2014, 104, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Fujikawa, T.; Kuga, Y.; Yano, S.; Yoshimi, A.; Tachiki, T.; Abe, K.; Nishimura, M. Dynamics of cell wall components of Magnaporthe grisea during infectious structure development. Mol. Microbiol. 2009, 73, 553–570. [Google Scholar] [CrossRef]
- Benincasa, M.; Mattiuzzo, M.; Herasimenka, Y.; Cescutti, P.; Rizzo, R.; Gennaro, R. Activity of antimicrobial peptides in the presence of polysaccharides produced by pulmonary pathogens. J. Pept. Sci. 2009, 15, 595–600. [Google Scholar] [CrossRef]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 2016, 16, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Choi, J.; Cao, Y.; Stacey, G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant Sci. 2014, 5, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hon, W.-C.; Griffith, M.; Chong, P.; Yang, D.S.C. Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves. Plant Physiol. 1994, 104, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, Y.; Hirano, T.; Yoshimoto, K.; Shimizu, M.; Kobayashi, H. Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J. 1999, 18, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Shimada, T.; Hara-Nishimura, I.; Nishimura, M.; Minamikawa, T. C-terminal KDEL sequence of a KDEL-tailed cysteine proteinase (sulfhydryl-endopeptidase) is involved in formation of KDEL vesicle and in efficient vacuolar transport of sulfhydryl-endopeptidase. Plant Physiol. 2003, 132, 1892–1900. [Google Scholar] [CrossRef] [Green Version]
- Nakaminami, K.; Karlson, D.T.; Imai, R. Functional conservation of cold shock domains in bacteria and higher plants. Proc. Natl. Acad. Sci. USA 2006, 103, 10122–10127. [Google Scholar] [CrossRef] [Green Version]
- Spelbrink, R.G.; Dilmac, N.; Allen, A.; Smith, T.J.; Shah, D.M.; Hockerman, G.H. Differential Antifungal and Calcium Channel-Blocking Activity among Structurally Related Plant Defensins. Plant Physiol. 2004, 135, 2055–2067. [Google Scholar] [CrossRef] [Green Version]
- Christova, P.K.; Christov, N.K.; Imai, R. A cold inducible multidomain cystatin from winter wheat inhibits growth of the snow mold fungus. Microdochium nivale. Planta 2006, 223, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isobe, A.; Kuwabara, C.; Koike, M.; Sutoh, K.; Sasaki, K.; Imai, R. An Apoplastic Defensin of Wheat Elicits the Production of Extracellular Polysaccharides in Snow Mold. Plants 2021, 10, 1607. https://doi.org/10.3390/plants10081607
Isobe A, Kuwabara C, Koike M, Sutoh K, Sasaki K, Imai R. An Apoplastic Defensin of Wheat Elicits the Production of Extracellular Polysaccharides in Snow Mold. Plants. 2021; 10(8):1607. https://doi.org/10.3390/plants10081607
Chicago/Turabian StyleIsobe, Ayako, Chikako Kuwabara, Michiya Koike, Keita Sutoh, Kentaro Sasaki, and Ryozo Imai. 2021. "An Apoplastic Defensin of Wheat Elicits the Production of Extracellular Polysaccharides in Snow Mold" Plants 10, no. 8: 1607. https://doi.org/10.3390/plants10081607
APA StyleIsobe, A., Kuwabara, C., Koike, M., Sutoh, K., Sasaki, K., & Imai, R. (2021). An Apoplastic Defensin of Wheat Elicits the Production of Extracellular Polysaccharides in Snow Mold. Plants, 10(8), 1607. https://doi.org/10.3390/plants10081607





