Quinoa for the Brazilian Cerrado: Agronomic Characteristics of Elite Genotypes under Different Water Regimes
Abstract
:1. Introduction
2. Material and Methods
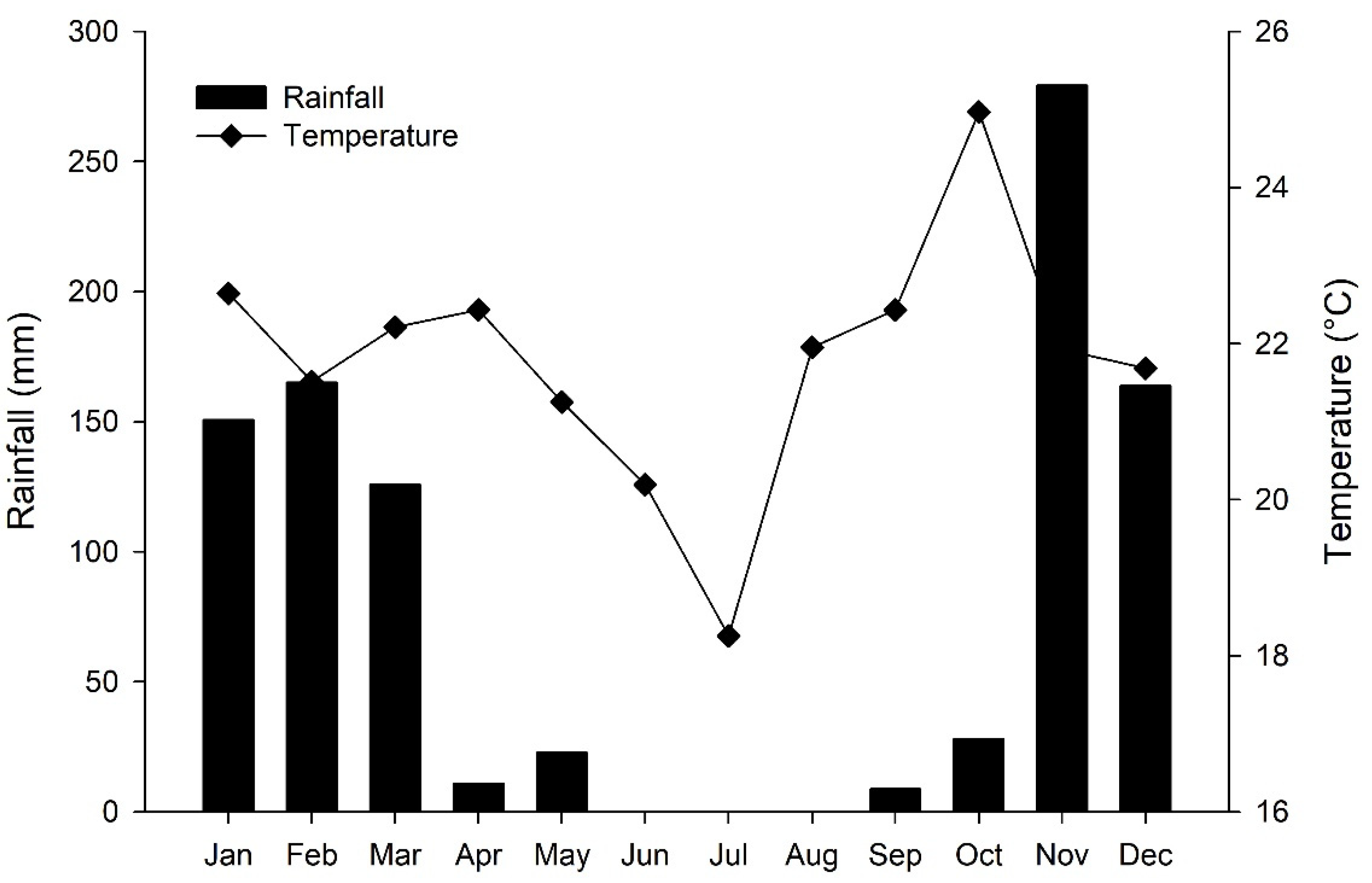
2.1. Experimental Area Characterization
2.2. Experimental Design
2.3. Variables Analyzed
2.3.1. Grain Analysis
2.3.2. Physiological Analysis
2.4. Statistical Analysis
3. Results
3.1. Productivity
3.2. Productivity Per Unit of Applied Water (PUAA)
3.3. Thousand Grain Weight
3.4. Functional Quality
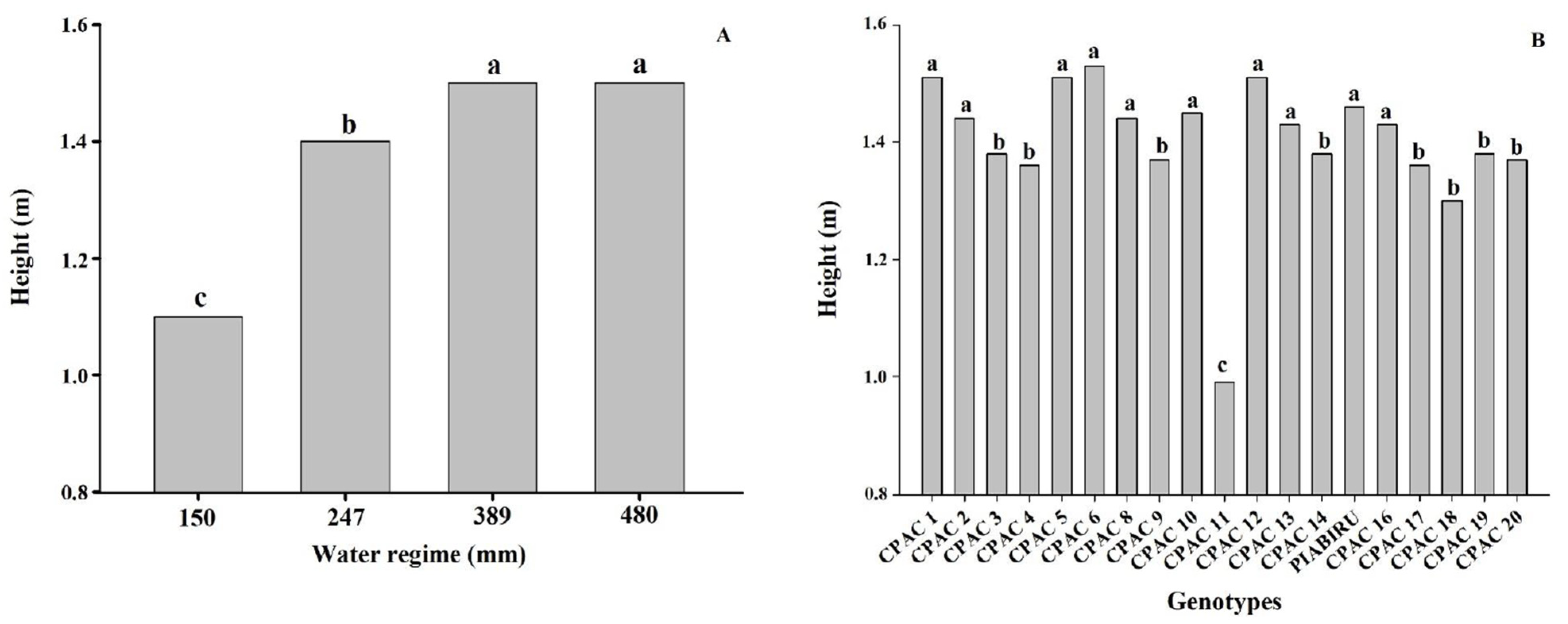
3.5. Plant Height
3.6. Canopy Temperature
3.7. Leaf Gas Exchange
3.8. Effective Quantum Yield of Photosystem II and Chlorophyll Index
3.9. Leaf Proline and Relative Water Content
4. Discussion
4.1. Productivity and Productivity Per Unit of Applied Water (PUAA)
4.2. The Effects of Water Regime and Genotype on Grain Quality Indicators
4.3. Low Water Availability Leads to Reduced Plant Height
4.4. Physiological Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheelbeek, P.F.; Bird, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.M.; Chalabi, Z.; Allemn, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Natl. Acad. Sci. USA 2018, 115, 6804–6809. [Google Scholar] [CrossRef] [Green Version]
- Soares, G.F.; Ribeiro, W.Q., Jr.; Pereira, L.F.; Lima, C.A.; Soares, D.D.S.; Muller, O.; Rascher, U.; Ramos, M.L.G. Characterization of wheat genotypes for drought tolerance and water use efficiency. Sci. Agric. 2021, 78, e20190304. [Google Scholar] [CrossRef]
- Spehar, C.R.; Santos, R.L.B. Quinoa BRS Piabiru: Alternativa para diversificar os sistemas de produção de grãos. Pesq. Agropec. Bras. 2001, 37, 889–893. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.A.; Ceccon, G.; Sutier, G.A.D.; Santos, A.L.F.D. Soybean-corn succession according to seeding date. Pesq. Agropec. Bras. 2018, 53, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Präger, A.; Munz, S.; Nkebiwe, P.M.; Mast, B.; Graeff-Hönninger, S. Yield and quality characteristics of different quinoa (Chenopodium quinoa Willd.) cultivars grown under field conditions in Southwestern Germany. Agronomy. 2018, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Saad-Allah, K.M.; Youssef, M.S. Phytochemical and genetic characterization of five quinoa (Chenopodium quinoa Willd.) genotypes introduced to Egypt. Physiol. Mol. Biol. Plants 2018, 24, 617–629. [Google Scholar] [CrossRef]
- Wang, D.; Cao, D.; Yao, Y.; Wang, J.; Li, Z.; Liu, B. Understanding the checmial foundation and genetic mecanism of the black grain trait in quinoa by integrating metabolome and transcriptome analyses. Biotechnol. Biotechnol. Equip. 2020, 34, 1095–1103. [Google Scholar] [CrossRef]
- Razzaghi, F.; Bahadori-Ghasroldashti, M.R.; Henriksen, S.; Sepaskhah, A.R.; Jacobsen, S.E. Physiological characteristics and irrigation water productivity of quinoa (Chenopodium quinoa Willd.) in response to deficit irrigation imposed at different growing stages—A field study from Southern Iran. J. Agron. Crop Sci. 2020, 206, 390–404. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Jayme-Oliveira, A.; Ribeiro, W.Q.; Ramos, M.L.G.; Ziviani, A.C.; Jakelaitis, A. Amaranth, quinoa, and millet growth and development under different water regimes in the Brazilian Cerrado. Pesq. Agropec. Bras. 2017, 52, 561–571. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide evaluations of quinoa: Preliminary results from post international year of quinoa FAO projects in nine countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of Food Insecurity in the World 2014; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; Available online: http://www.fao.org/publications/sofi/en/ (accessed on 11 March 2021).
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Hanks, R.J.; Keller, J.; Rasmussen, V.P.; Wilson, G.D. Line source sprinkler for continuous variable irrigation crop production studies. Soil Sci. Soc. Am. J. 1976, 40, 426–429. [Google Scholar] [CrossRef]
- Embrapa—Empresa Brasileira de Pesquisa Agropecuária. Monitoramento de Irrigação no Cerrado. 2016. Available online: http://hidro.cpac.embrapa.br (accessed on 25 May 2017).
- QGIS Development Team; QGIS Geographic Information System. Open Source Geospatial Foundation Project; QGIS Development Team: Chur, Switzerland, 2021. [Google Scholar]
- Secretaria de Defesa Agropecuária, Brasil Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; Brasil Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2009. [Google Scholar]
- Lees, D.H.; Francis, F.J. Standardization of pigment analyses in cranberries. HortScience 1972, 7, 83–84. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- SAS-Statistical Analyses System. Statistical Analysis System User’s Guide; Institute, Statistical Analyses System: Cary, NC, USA, 2008. [Google Scholar]
- SYSTAT Software. Scientific Graphing Software: Sigma Plot, Versão 10.0; HearneScientific Software: San Rafael, CA, USA, 2006. [Google Scholar]
- Howell, T.A. Irrigation Efficiency. In Encyclopedia of Soil Science, 2nd ed.; CRC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Zhang, M.; Duan, L.; Tian, X.; He, Z.; Li, J.; Wang, B.; Li, Z. Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis, hormones and antioxidant system. J. Plant Physiol. 2007, 164, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Vacher, J.J. Responses of two main Andean crops, quinoa (Chenopodium quinoa Willd) and papa amarga (Solanum juzepczukii Buk.) to drought on the Bolivian Altiplano: Significance of local adaptation. Agric. Ecosyst. Environ. 1998, 68, 99–108. [Google Scholar] [CrossRef]
- Yousfi, S.; Serret, M.D.; Araus, J.L. Shoot δ(15)N gives a better indication than ion concentration or Δ(13)C of genotypic differences in the response of durum wheat to salinity. Funct. Plant Biol. 2009, 36, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Raes, D.; Garcia, M.; Condori, O.; Mamani, J.; Miranda, R.; Cusicanqui, J.; Taboada, C.; Yucra, E.; Vacher, J. Could deficit irrigation be a sustainable practice for quinoa (Chenopodium quinoa Willd.) in the Southern Bolivian Altiplano? Agric. Water Manag. 2008, 95, 909–917. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Vacher, J.; Mamani, R.; Mendoza, J.; Huanca, R.; Morales, B.; Miranda, R.; Cusicanqui, J.; et al. Introducing deficit irrigation to stabilize yields of quinoa (Chenopodium quinoa Willd.). Eur. J. Agron. 2008, 28, 427–436. [Google Scholar] [CrossRef]
- Martínez, E.A.; Veas, E.; Jorquera, C.; San Martín, R.; Jara, P. Re-introduction of quinoa into Arid Chile: Cultivation of two lowland races under extremely low irrigation. J. Agron. Crop Sci. 2009, 195, 1–10. [Google Scholar] [CrossRef]
- Delgado, A.I.; Palacios, J.H.; Betancourt, C. Evaluation of 16 genotypes of sweet quinoa (Chenopodium quinoa Willd.) in the municipality of Iles, Nariño (Colombia). Agron. Colomb. 2009, 27, 159–167. [Google Scholar]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Aninbon, C.; Jogloy, S.; Vorasoot, N.; Patanothai, A.; Nuchadomrong, S.; Senawong, T. Effect of end of season water deficit on phenolic compounds in peanut genotypes with different levels of resistance to drought. Food Chem. 2016, 196, 123–129. [Google Scholar] [CrossRef]
- Martini, D.; Taddei, F.; Nicoletti, I.; Ciccoritti, R.; Corradini, D.; D’Egidio, M.G. Effects of genotype and environment on phenolic acids content and total antioxidant capacity in durum wheat. Cereal Chem. 2014, 91, 310–317. [Google Scholar] [CrossRef]
- Gámez, A.L.; Soba, D.; Zamarreño, Á.M.; García-Mina, J.M.; Aranjuelo, I.; Morales, F. Effect of water stress during grain filling on yield, quality and physiological traits of Illpa and Rainbow quinoa (Chenopodium quinoa Willd.) cultivars. Plants 2019, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Maliro, M.F.; Guwela, V.F.; Nyaika, J.; Murphy, K.M. Preliminary studies of the performance of quinoa (Chenopodium quinoa Willd.) genotypes under irrigated and rainfed conditions of central Malawi. Front. Plant Sci. 2017, 8, 227. [Google Scholar] [CrossRef] [Green Version]
- Talebnejad, R.; Sepaskhah, A.R. Effect of deficit irrigation and different saline groundwater depths on yield and water productivity of quinoa. Agric. Water Manag. 2015, 159, 225–238. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Amjad, M.; Iqbal, S.; Jacobsen, S.E. Growth and physiological responses of quinoa to drought and temperature stress. J. Agron. Crop Sci. 2016, 202, 445–453. [Google Scholar] [CrossRef]
- Endres, L. Photosynthesis and water relations in Brazilian sugarcane. Open Agric. J. 2010, 4, 31–37. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Shao, M.A.; Jaleel, C.A.; Hong-mei, M. Higher plant antioxidants and redox signaling under environmental stresses. Comptes Rendus Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.R. Whole Plant Response to Water Deficits: An Overview. Limit. Effic. Water Use Crop. Prod. 1983, 289–317. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Berninger, F. Picea seedlings show apparent acclimation to drought with increasing altitude in the eastern Himalaya. Trees Struct. Funct. 2004, 18, 277–283. [Google Scholar] [CrossRef]
- Balota, M.; Payne, W.A.; Evett, S.R.; Peters, T.R. Morphological and physiological traits associated with canopy temperature depression in three closely related wheat lines. Crop Sci. 2008, 48, 1897–1910. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef] [Green Version]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Waseem, M.; Ali, A.; Tahir, M.; Nadeem, M.A.; Ayub, M.; Tanveer, A.; Ahmad, R.; Hussain, M. Mechanism of drought tolerance in plant and its management through different methods. Cont. J. Agric. Sci. 2011, 5, 10–25. [Google Scholar]
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23. [Google Scholar] [CrossRef]
- Tatagiba, S.D.; Pezzopane, J.E.M.; Reis, E.F. Fotossíntese em Eucalyptus sob diferentes condições edafoclimáticas. Rev. Eng. Agric. RevEng 2015, 23, 336–345. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa Abiotic Stress Responses: A Review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in quinoa during drought and salt stress. Plants 2017, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussin, S.; Khalifa, W.; Geissler, N.; Koyro, H.W. Influence of the root endophyte Piriformospora indica on the plant water relations, gas exchange and growth of Chenopodium quinoa at limited water availability. J. Agron. Crop Sci. 2017, 203, 373–384. [Google Scholar] [CrossRef]
- González, J.A.; Gallardo, M.; Hilal, M.B.; Rosa, M.D.; Prado, F.E. Physiological responses of quinoa (Chenopodium quinoa) to drought and waterlogging stresses: Dry matter partitioning. Bot. Stud. 2009, 50, 35–42. [Google Scholar]
- Morales, A.; Zurita-Silva, A.; Maldonado, J.; Silva, H. Transcriptional responses of Chilean quinoa (Chenopodium quinoa Willd.) under water deficit conditions uncovers ABA-independent expression patterns. Front. Plant Sci. 2017, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, P.M.; Shanahan, J.F.; Schepers, J.S. Use of chlorophyll fluorescence assessments to differentiate corn hybrid response to variable water conditions. Crop Sci. 2006, 46, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Belous, O.; Klemeshova, K.; Malyarovskaya, V. Photosynthetic Pigments of Subtropical Plants. In Photosynthesis—From Its Evolution to Future Improvements in Photosynthetic Efficiency Using Nanomaterials; IntechOpen Limited: London, UK, 2018; pp. 31–52. [Google Scholar]
- Nounjan, N.; Chansongkrow, P.; Charoensawan, V.; Siangliw, J.L.; Toojinda, T.; Chadchawan, N.; Chansongkrow, P.; Charoensawan, V.; Siangliw, J.L.; Toojinda, T.; et al. High performance of photosynthesis and osmotic adjustment are associated with salt tolerance ability in rice carrying drought tolerance QTL: Physiological and co-expression network analysis. Front. Plant Sci. 2018, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Pintó-Marijuan, M.; Munné-Bosch, S. Ecophysiology of invasive plants: Osmotic adjustment and antioxidants. Trends Plant Sci. 2013, 18, 660–666. [Google Scholar] [CrossRef]
- Ben Rejeb, K.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. PPB 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed pretreatment and foliar application of proline regulate morphological, physio-biochemical processes and activity of antioxidant enzymes in plants of two cultivars of quinoa (Chenopodium quinoa Willd.). Plants 2019, 8, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. For. Biotechnol. 2011, 15, 173–177. [Google Scholar]
| Genotypes | Water Regime (mm) | |||
|---|---|---|---|---|
| 480 | 389 | 247 | 150 | |
| CPAC1 | 7.82 aA | 8.34 bA | 4.06 bB | 1.56 bC |
| CPAC2 | 8.32 aA | 7.89 bA | 3.94 cB | 1.83 bC |
| CPAC3 | 8.02 aA | 7.81 bB | 6.25 aC | 1.94 bD |
| CPAC4 | 8.40 aA | 8.23 bA | 5.21 aB | 1.95 bC |
| CPAC5 | 8.17 aA | 7.90 bA | 5.22 aB | 1.94 bC |
| CPAC6 | 8.50 aA | 8.84 aA | 5.68 aB | 2.46 aC |
| CPAC8 | 8.64 aA | 7.07 cB | 4.50 aC | 1.58 bD |
| CPAC9 | 8.21 aA | 7.01 cB | 5.75 aC | 2.11 bD |
| CPAC10 | 8.56 aA | 8.36 bA | 5.12 aB | 2.61 aC |
| CPAC11 | 5.66 bB | 6.80 cA | 5.40 aB | 2.38 aC |
| CPAC12 | 8.57 aA | 7.83 bA | 5.82 aB | 2.58 aC |
| CPAC13 | 8.85 aA | 9.73 aA | 4.17 bB | 2.60 aC |
| CPAC14 | 9.21 aA | 6.51 cB | 3.88 bB | 1.61 bB |
| BRS Piabiru | 7.58 aA | 8.14 bA | 5.40 aB | 1.84 bC |
| CPAC16 | 8.51 aA | 7.46 cA | 4.74 aB | 2.33 aC |
| CPAC17 | 8.92 aA | 8.44 bA | 4.80 aB | 3.64 aC |
| CPAC18 | 8.96 Aa | 9.16 aA | 4.75 aB | 2.21 bC |
| CPAC19 | 7.71 aA | 6.42 cB | 2.53 cC | 1.79 bC |
| CPAC20 | 7.97 aA | 7.08 cA | 3.93 bB | 2.09 bC |
| Genotypes | Water Regime (mm) | |||
|---|---|---|---|---|
| 480 | 389 | 247 | 150 | |
| CPAC1 | 17.18 aB | 22.2 aA | 16.43 cB | 10.41 cC |
| CPAC2 | 15.89 aB | 21.0 aA | 13.02 dB | 13.0 cB |
| CPAC3 | 18.59 aB | 20.8 aB | 25.29 aA | 13.5 cD |
| CPAC4 | 16.89 aB | 21.15 aA | 21.1 bA | 13.03 cC |
| CPAC5 | 16.9 aB | 21.0 aA | 23.9 bA | 12.1 cC |
| CPAC6 | 17.7 aB | 22.72 aA | 23.0 aA | 16.4 bB |
| CPAC8 | 18.01 aA | 18.18 bA | 18.23 cA | 10.52 bB |
| CPAC9 | 17.73 aB | 18.01 bB | 23.3 aA | 14.10 cC |
| CPAC10 | 17.83 aB | 21.49 aA | 20.6 bA | 15.38 cB |
| CPAC11 | 11.1 bC | 18.0 bB | 21.89 bA | 15.85 bA |
| CPAC12 | 18.0 aB | 20.1 aB | 23.56 aA | 17.24 bD |
| CPAC13 | 19.83 aB | 25.9 aA | 19.1 bB | 17.6 bB |
| CPAC14 | 12.69 aB0 | 16.3 bA | 15.71 cA | 10.1 cC |
| BRS Piabiru | 15.79 aB | 20.92 aA | 21.88 bA | 14.37 cB |
| CPAC16 | 17.74 aA | 19.8 bA | 19.18 bA | 15.57 bA |
| CPAC17 | 18.58 aB | 21.70 aA | 19.44 bB | 24.25 aA |
| CPAC18 | 18.66 aB | 23.56 aA | 16.89 cB | 14.71 cC |
| CPAC19 | 16.08 aA | 16.50 bA | 11.6 dB | 10.25 cB |
| CPAC20 | 17.82 aA | 18.82 bA | 15.94 cA | 11.89 cB |
| Genotypes | Water Regime (mm) | |||
|---|---|---|---|---|
| 480 | 389 | 247 | 150 | |
| CPAC1 | 81.59 cB | 101.62 dA | 66.21 dC | 88.34 dA |
| CPAC2 | 95.97 bA | 97.23 dA | 85.71 dA | 92.21 dA |
| CPAC3 | 98.83 bA | 51.54 eB | 97.12 cA | 113.25 cA |
| CPAC4 | 80.02 cB | 98.31 dA | 91.64 cA | 96.23 dA |
| CPAC5 | 84.07 cB | 103.0 dA | 74.58 dB | 86.80 dB |
| CPAC6 | 83.65 cC | 115.23 cA | 82.81 dC | 100.05 dB |
| CPAC8 | 96.95 bB | 110.72 dA | 114.25 bA | 93.58 dB |
| CPAC9 | 215.22 aB | 171.32 aC | 205.11 aB | 226.02 aA |
| CPAC10 | 85.48 cC | 110.0 dB | 96.32 cC | 210.81 bA |
| CPAC11 | 89.00 cB | 118.59 cA | 100.97 cB | 113.25 cA |
| CPAC12 | 96.17 bB | 120.98 cA | 84.82 dB | 112.51 cA |
| CPAC13 | 99.47 bA | 111.33 dA | 84.73 dB | 102.33 dA |
| CPAC14 | 115.19 bA | 119.74 cA | 84.94 dB | 104.71 cA |
| BRS Piabiru | 104.15 bA | 105.89 dA | 101.98 cA | 104.56 cA |
| CPAC16 | 105.09 bB | 124.80 cA | 99.48 cB | 110.93 cB |
| CPAC17 | 115.73 bC | 145.76 bA | 97.05 cC | 118.86 cB |
| CPAC18 | 88.27 cB | 62.76 eC | 94.25 cB | 113.57 cA |
| CPAC19 | 109.63 bB | 119.74 cA | 102.59 cB | 100.01 dB |
| CPAC20 | 119.93 bB | 139.03 bA | 114.40 bB | 115.41 cB |
| Genotypes | Water Regime (mm) | |||
|---|---|---|---|---|
| 480 | 389 | 247 | 150 | |
| CPAC1 | 0.80 cA | 0.76 dA | 0.76 cA | 0.80 dA |
| CPAC2 | 0.63 dA | 0.68 dA | 0.62 dA | 0.40 cB |
| CPAC3 | 0.63 dD | 1.0 bB | 0.83 cC | 1.16 cA |
| CPAC4 | 0.59 dB | 0.65 eB | 0.59 dB | 0.76 dA |
| CPAC5 | 0.74 cA | 0.59 eB | 0.54 dB | 0.70 dA |
| CPAC6 | 0.60 dB | 0.73 dA | 0.65 dB | 0.84 dA |
| CPAC8 | 0.58 dB | 1.07 bA | 0.55 dB | 0.57 eB |
| CPAC9 | 1.72 aC | 1.89 aB | 1.44 aD | 2.05 aA |
| CPAC10 | 1.16 bA | 0.73 dB | 0.76 cB | 0.91 dB |
| CPAC11 | 0.61 dA | 0.48 fA | 0.54 dA | 0.61 eA |
| CPAC12 | 0.55 dB | 0.68 dA | 0.50 dB | 0.65 eB |
| CPAC13 | 0.66 dB | 0.63 eB | 0.50 dC | 0.73 dA |
| CPAC14 | 0.83 cB | 0.82 cB | 0.68 cC | 0.94 cA |
| BRS Piabiru | 0.89 cB | 1.08 bA | 0.61 dD | 0.74 dC |
| CPAC16 | 0.72 cA | 0.65 eA | 0.81 cA | 0.80 dA |
| CPAC17 | 1.03 bB | 0.86 cC | 0.79 cC | 1.18 bA |
| CPAC18 | 0.68 dA | 0.61 eA | 0.72 cA | 0.60 eA |
| CPAC19 | 0.64 dA | 0.63 eA | 0.71 cA | 0.66 eA |
| CPAC20 | 1.13 bA | 0.99 bA | 1.06 bA | 1.05 cA |
| Genotypes | Water Regime (mm) | ||||
|---|---|---|---|---|---|
| 150 | 247 | 389 | 480 | ||
| gs | CPAC4 | 0.034 cD | 0.235 bC | 0.470 bB | 0.583 bA |
| CPAC11 | 0.040 cC | 0.232 bB | 0.358 cA | 0.342 cA | |
| BRS Piabiru | 0.058 aD | 0.218 bC | 0.516 abB | 0.629 aA | |
| CPAC19 | 0.050 bC | 0.329 aB | 0.552 aA | 0.594 abA | |
| E | CPAC4 | 1.45 bcD | 6.15 aC | 8.96 aB | 9.88 aA |
| CPAC11 | 1.20 cD | 4.87 bC | 6.43 cA | 5.67 cB | |
| BRS Piabiru | 3.11 aD | 4.76 bC | 8.20 aB | 9.46 aA | |
| CPAC19 | 1.71 bD | 5.83 aC | 7.27 bB | 8.38 bA | |
| Ci | CPAC 4 | 144.0 aC | 195.3 aB | 239.9 bA | 241.2 bA |
| CPAC11 | 112.6 bC | 198.6 aB | 253.6 aA | 259.2 aA | |
| BRS Piabiru | 129.0 abD | 191.3 aC | 257.0 aB | 270.3 aA | |
| CPAC19 | 82.5 cC | 198.4 aB | 255.9 aA | 256.9 abA | |
| A | CPAC4 | 4.9 cD | 19.0 cC | 22.0 bB | 28.1 bA |
| CPAC11 | 5.9 bC | 20.5 bcB | 22.9 bA | 20.0 cB | |
| BRS Piabiru | 9.0 aD | 22.4 bC | 32.9 aB | 35.4 aA | |
| CPAC19 | 8.9 aC | 27.0 aB | 32.9 aA | 34.3 aA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, P.C.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Celestino, S.M.C.; Silva, A.d.N.; Casari, R.A.d.C.N.; Santana, C.C.; de Lima, C.A.; Williams, T.C.R.; Vinson, C.C. Quinoa for the Brazilian Cerrado: Agronomic Characteristics of Elite Genotypes under Different Water Regimes. Plants 2021, 10, 1591. https://doi.org/10.3390/plants10081591
da Silva PC, Ribeiro Junior WQ, Ramos MLG, Celestino SMC, Silva AdN, Casari RAdCN, Santana CC, de Lima CA, Williams TCR, Vinson CC. Quinoa for the Brazilian Cerrado: Agronomic Characteristics of Elite Genotypes under Different Water Regimes. Plants. 2021; 10(8):1591. https://doi.org/10.3390/plants10081591
Chicago/Turabian Styleda Silva, Patrícia Carvalho, Walter Quadros Ribeiro Junior, Maria Lucrecia Gerosa Ramos, Sonia Maria Costa Celestino, Alberto do Nascimento Silva, Raphael Augusto das Chagas Noqueli Casari, Charles Cardoso Santana, Cristiane Andrea de Lima, Thomas Christopher Rhys Williams, and Christina Cleo Vinson. 2021. "Quinoa for the Brazilian Cerrado: Agronomic Characteristics of Elite Genotypes under Different Water Regimes" Plants 10, no. 8: 1591. https://doi.org/10.3390/plants10081591
APA Styleda Silva, P. C., Ribeiro Junior, W. Q., Ramos, M. L. G., Celestino, S. M. C., Silva, A. d. N., Casari, R. A. d. C. N., Santana, C. C., de Lima, C. A., Williams, T. C. R., & Vinson, C. C. (2021). Quinoa for the Brazilian Cerrado: Agronomic Characteristics of Elite Genotypes under Different Water Regimes. Plants, 10(8), 1591. https://doi.org/10.3390/plants10081591






