Medicinal Properties and In Vitro Biological Activities of Selected Helichrysum Species from South Africa: A Review
Abstract
1. Introduction
2. Research Methodology
3. Helichrysum petiolare and Its Biological Activities
3.1. Cytotoxicity/Anti-Proliferative Activity of H. petiolare
3.2. Anti-Bacterial Activity of H. petiolare
3.3. Anti-Inflammatory Activity of H. petiolare
3.4. Anti-Fungal Activity of H. petiolare
3.5. Anti-Oxidant Activity of H. petiolare
3.6. Antigenotoxicity Activity of H. petiolare
3.7. Anti-Tyrosinase Activity of H. petiolare
4. Helichrysum cymosum and Its Biological Activities
4.1. Cytotoxicity of H. cymosum
4.2. Anti-Oxidant Activity of H. cymosum
4.3. Anti-Malarial Activity of H. cymocum
4.4. Anti-Fungal Activity of H. cymocum
4.5. Anti-Bacterial Activity of H. cymocum
4.6. Anti-Inflammatory Activity of H. cymocum
4.7. Anti-Viral Activity of H. cymocum
5. Helichrysum foetidum and Its Biological Activities
5.1. Cytotoxicity of H. foetidum
5.2. Anti-Ulcerogenic Properties of H. foetidum
5.3. Anti-Bacterial Activity of H. foetidum
5.4. Anti-Fungal Activity of H. foetidum
5.5. Anti-Oxidant Activity of H. foetidum
5.6. Anti-Viral Activities of H. foetidum
6. Helichrysum pandurifolium Schrank and Its Biological Activities
7. Common Phytochemicals Present in the Selected Helichrysum Species
8. Essential Oils Present in Selected Helichrysum Species
| Helichrysum Species | Plant Parts | Compounds | Method of Analysis | Pharmacological Activity | References |
|---|---|---|---|---|---|
H. petiolare A growing Helichrysum petiolare plant [70] SANBI available online http://pza.sanbi.org/helichrysum-petiolare (accessed on 19 June 2021) | Leaves | α-pinene (6.8%), 1, 8-cineole (22.4), p-cymene (9.8%) and β-caryophyllene (14%) | G.C.-M.S. | Anti-fungal, anti-inflammatory | [38] |
| Whole plant | (E)-Longipinane (11.79%), trans-Geranylgeraniol (11.68%), Phytol (11.28%) Geranyllinalool (11.13%) and α-Eicosane (12.07%) | G.C.-M.S. | Anti-microbial, anti-inflammatory | [71] | |
H. cymosum A growing Helichrysum cymosum plant [72] SANBI available online: http://pza.sanbi.org/helichrysum-cymosum-subsp-cymosum (accessed on 19 June 2021) | Leaves, Flowers | ∆-3-carene (16.1%), β-caryophyllene (12.0%) | G.C., G.C.-M.S. | Anti-fungal | [73] |
| Flowers | Monoterpenes (77.9%) | G.C.-M.S. G.C., G.C.-M.S. | Anti-inflammatory | [15] | |
| Leaves, Flowers | (Z) -β- ocimene | G.C.-M.S. | - | [74] | |
| Leaves | α-pinene (12.4%), 1, 8-cineole (20.4%), β-caryophyllene (10.8%) | Anti-bacterial | [38] | ||
H. foetidum A growing Helichrysum foetidum plant [39] SANBI available online http://pza.sanbi.org/helichrysum-foetidum (accessed on 19 June 2021) | Leaves, flower | Β-pinene (3.1%), Trans-Sabiene hydrate (1.8%), 4-terpineol (3.1%), β-caryophyllene (2.5%) | G.C.-M.S. | Anti-microbial Anti-inflammatory | [75] |
H. pandurifolium A growing Helichrysum pandurifolium plant [76] iNaturalist. Available online: https://www.inaturalist.org/observations/23571154 (accessed on 19 June 2021) | N/A | N/A | N/A | N/A |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hillard, O. Flora of southern Africa. In Asteraceae; Leistner, O.A., Ed.; Botanical Institute of South Africa: Pretoria, South Africa, 1983; Volume 33, pp. 61–310. [Google Scholar]
- Pooley, E. Mountain Flowers: A Field Guide to the Flora of the Drakensberg and Lesotho; Flora Publications Trust: Durban, South Africa, 2003. [Google Scholar]
- Viegas, D.A.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef]
- Rigano, D.; Formisano, C.; Pagano, E.; Senatore, F.; Piacente, S.; Masullo, M.; Capasso, R.; Izzo, A.A.; Borrelli, F. A new acetophenone derivative from flowers of Helichrysum italicum (roth) don ssp. Ital. Fitoter. 2014, 99, 198–203. [Google Scholar] [CrossRef]
- Czinner, E.; Lemberkovics, É.; Bihátsi-Karsai, E.; Vitányi, G.; Lelik, L. Composition of the essential oil from the inflorescence of Helichrysum arenarium (L.) Moench. J. Essent. Oil Res. 2000, 12, 728–730. [Google Scholar] [CrossRef]
- Eroğlu, H.E.; Hamzaoğlu, E.; Aksoy, A.; Budak, Ü.; Albayrak, S. Cytogenetic effects of Helichrysum arenarium in human lymphocytes cultures. Turk. J. Biol. 2010, 34, 253–256. [Google Scholar]
- Reidel, R.V.B.; Cioni, P.L.; Ruffoni, B.; Cervelli, C.; Pistelli, L. Aroma profile and essential oil composition of Helichrysum species. Nat. Prod. Commun. 2017, 12, 1934578X1701200931. [Google Scholar]
- Harborne, J.B.; Turner, B.L. Plant Chemosystematics; Academic Press: London, UK, 1984. [Google Scholar]
- Mari, A.; Napolitano, A.; Masullo, M.; Pizza, C.; Piacente, S. Identification and quantitative determination of the polar constituents in Helichrysum italicum flowers and derived food supplements. J. Pharm. Biomed. Anal. 2014, 96, 249–255. [Google Scholar] [CrossRef]
- Akaberi, M.; Sahebkar, A.; Azizi, N.; Emami, S.A. Everlasting flowers: Phytochemistry and pharmacology of the genus Helichrysum. Ind. Crop. Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Aslan, M.; Orhan, D.D.; Orhan, N.; Sezik, E.; Yeşilada, E. A study of antidiabetic and anti-oxidant effects of Helichrysum graveolens capitulums in streptozotocin-induced diabetic rats. J. Med. Food 2007, 10, 396–400. [Google Scholar] [CrossRef]
- Hutchings, A.; van Staden, J. Plants used for stress-related ailments in traditional zulu, xhosa and sotho medicine. Part 1: Plants used for headaches. J. Ethnopharmacol. 1994, 43, 89–124. [Google Scholar] [CrossRef]
- Najar, B.; Nardi, V.; Cervelli, C.; Mecacci, G.; Mancianti, F.; Ebani, V.V.; Nardoni, S.; Pistelli, L. Volatilome analyses and In Vitro antimicrobial activity of the essential oils from five South African Helichrysum species. Molecules 2020, 25, 3196. [Google Scholar] [CrossRef]
- Giovanelli, S.; De Leo, M.; Cervelli, C.; Ruffoni, B.; Ciccarelli, D.; Pistelli, L. Essential oil composition and volatile profile of seven Helichrysum species grown in italy. Chem. Biodivers. 2018, 15, e1700545. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N. Are plants used for skin care in south africa fully explored? J. Ethnopharmacol. 2014, 153, 61–84. [Google Scholar] [CrossRef]
- Serabele, K.; Chen, W.; Tankeu, S.; Combrinck, S.; Veale, C.G.; van Vuuren, S.; Chaudhary, S.K.; Viljoen, A. Comparative chemical profiling and antimicrobial activity of two interchangeably used ‘imphepho’species (Helichrysum odoratissimum and Helichrysum petiolare). S. Afr. J. Bot. 2021, 137, 117–132. [Google Scholar] [CrossRef]
- Lourens, A.; Van Vuuren, S.; Viljoen, A.; Davids, H.; Van Heerden, F. Antimicrobial activity and in vitro cytotoxicity of selected South African Helichrysum species. S. Afr. J. Bot. 2011, 77, 229–235. [Google Scholar] [CrossRef]
- Sagbo, I.J.; Otang-Mbeng, W. Anti-proliferative and genotoxic activities of the Helichrysum petiolare Hilliard & B.L. Burtt. Sci. Pharm. 2020, 88, 49. [Google Scholar]
- Ajiboye, A.E.; Ameen, M.T.; Adedayo, M.R. Antimicrobial activity and phytochemical screening of the fruit pulp of Dialium guineense (velvet tamarind) on some microbial isolates. J. Microbiol. Antimicrob. 2015, 7, 33–41. [Google Scholar] [CrossRef]
- Di Mambro, V.M.; Azzolini, A.E.; Valim, Y.M.; Fonseca, M.J. Comparison of anti-oxidant activities of tocopherols alone and in pharmaceutical formulations. Int. J. Pharm. 2003, 262, 93–99. [Google Scholar] [CrossRef]
- Lourens, A.; Reddy, D.; Başer, K.; Viljoen, A.; Van Vuuren, S. In Vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J. Ethnopharmacol. 2004, 95, 253–258. [Google Scholar] [CrossRef]
- Makhuvele, R.; Matshoga, R.; Antonissen, R.; Pieters, L.; Verschaeve, L.; Elgorashi, E.E. Genotoxicity and antigenotoxicity of selected South African indigenous plants. S. Afr. J. Bot. 2018, 114, 89–99. [Google Scholar] [CrossRef]
- Słoczyńska, K.; Powroźnik, B.; Pękala, E.; Waszkielewicz, A.M. Antimutagenic compounds and their possible mechanisms of action. J. Appl. Genet. 2014, 55, 273–285. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Ameer, F.; Iwuoha, E.I.; Hussein, A.A. Inhibition of oxidative stress and skin aging-related enzymes by prenylated chalcones and other flavonoids from Helichrysum teretifolium. Molecules 2015, 20, 7143–7155. [Google Scholar] [CrossRef]
- Sonka, L. Exploring Anti-Tyrosinase Bioactive Compounds from the Cape Flora. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2018. [Google Scholar]
- Sagbo, I.J.; Otang-Mbeng, W. Evaluation of the efficacy of ethanol leaf extract of Helichrysum petiolare Hilliard and B.L. Burtt against skin aging. Trop. J. Pharm. Res. 2020, 19, 2631–2638. [Google Scholar] [CrossRef]
- Zenze, K. Helichrysum cymosum (L.) D. Don Subsp. cymosum (Asteraceae). 2012. Available online: http://pza.sanbi.org/helichrysum-cymosum-subsp-cymosum (accessed on 14 July 2021).
- Maroyi, A. Helichrysum cymosum (L.) D. Don (Asteraceae): Medicinal uses, chemistry, and biological activities. Asian J. Pharm. Clin. Res. 2019, 12, 19–26. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Viljoen, A.; Van Zyl, R.; Van Heerden, F.; Başer, K.H.C. The anti-microbial, anti-malarial and toxicity profiles of helihumulone, leaf essential oil and extracts of Helichrysum cymosum (L.) D. Don subsp. cymosum. S. Afr. J. Bot. 2006, 72, 287–290. [Google Scholar] [CrossRef]
- Heyman, H.M. Metabolomic Comparison of Selected Helichrysum Species to Predict Their Anti-Viral Properties. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2009. [Google Scholar]
- Reddy, D. The Phytochemistry and Microbial Activity of Selected Indigenous Helichrysum Species. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2008. [Google Scholar]
- Runyoro, D.; Ngassapa, O.; Kachali, L.; Obare, V.; Lyamuya, E. Biological activities of essential oils from plants growing in Tanzania. East Cent. Afr. J. Pharm. Sci. 2010, 13, 85–91. [Google Scholar]
- Bougatsos, C.; Ngassapa, O.; Runyoro, D.K.; Chinou, I.B. Chemical composition and In Vitro antimicrobial activity of the essential oils of two Helichrysum species from Tanzania. Z. Nat. C 2004, 59, 368–372. [Google Scholar] [CrossRef]
- Sindambiwe, J.; Calomme, M.; Cos, P.; Totte, J.; Pieters, L.; Vlietinck, A.; Berghe, D.V. Screening of seven selected Rwandan medicinal plants for anti-microbial and anti-viral activities. J. Ethnopharmacol. 1999, 65, 71–77. [Google Scholar] [CrossRef]
- Stafford, G.; Jäger, A.; Van Staden, J. Effect of storage on the chemical composition and biological activity of several popular South African medicinal plants. J. Ethnopharmacol. 2005, 97, 107–115. [Google Scholar] [CrossRef]
- Lourens, A.; Viljoen, A.M.; Van Heerden, F. South African Helichrysum species: A review of the traditional uses, biological activity and phytochemistry. J. Ethnopharmacol. 2008, 119, 630–652. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Anti-viral evaluation of herbal drugs. In Quality Control and Evaluation of Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 599–628. [Google Scholar]
- Swelankomo, N. Helichrysum foetidum (L.) Moench (Asteraceae). 2005. Available online: http://pza.sanbi.org/helichrysum-foetidum (accessed on 14 July 2021).
- Barcelos, L.; Heiden, G. First record of Helichrysum foetidum (L.) Moench (Asteraceae, Gnaphalieae) for South America. Check List 2017, 13, 331. [Google Scholar] [CrossRef]
- Kakam, A.M.Z.; Franke, K.; Ndom, J.C.; Dongo, E.; Mpondo, T.N.; Wessjohann, L.A. Secondary metabolites from Helichrysum foetidum and their chemotaxonomic significance. Biochem. Syst. Ecol. 2011, 2, 166–167. [Google Scholar] [CrossRef]
- Maroyi, A. Medicinal uses, biological and phytochemical properties of Helichrysum foetidum (L.) Moench (Asteraceae). Asian J. Pharm. Clin. Res. 2019, 12, 13–18. [Google Scholar] [CrossRef]
- Malolo, F.-A.E.; Nouga, A.B.; Kakam, A.; Franke, K.; Ngah, L.; Flausino, O.; Mpondo, E.M.; Ntie-Kang, F.; Ndom, J.C.; da Silva Bolzani, V. Protease-inhibiting, molecular modeling and antimicrobial activities of extracts and constituents from Helichrysum foetidum and Helichrysum mechowianum (compositae). Chem. Cent. J. 2015, 9, 1–11. [Google Scholar] [CrossRef][Green Version]
- Takagi, H.; Matsuzawa, H.; Ohta, T.; Yamasaki, M.; Inouye, M. Studies on the structure and function of subtilisin E by protein engineering. Ann. N. Y. Acad. Sci. 1992, 672, 52–59. [Google Scholar] [CrossRef]
- Steenkamp, V.; Mathivha, E.; Gouws, M.; Van Rensburg, C. Studies on anti-bacterial, anti-oxidant and fibroblast growth stimulation of wound healing remedies from South Africa. J. Ethnopharmacol. 2004, 95, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Tirillini, B.; Menghini, L.; Leporini, L.; Scanu, N.; Marino, S.; Pintore, G. Anti-oxidant activity of methanol extract of Helichrysum foetidum Moench. Nat. Prod. Res. 2013, 27, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Leonov, A.; Arlia-Ciommo, A.; Piano, A.; Svistkova, V.; Lutchman, V.; Medkour, Y.; Titorenko, V.I. Longevity extension by phytochemicals. Molecules 2015, 20, 6544–6572. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F. Natuerlich vorkommende terpenderivate. Xxii. Uber ein neues azulen aus Helichrysum bracteatum (vent.) willd. Chem. Ber. 1973, 106, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Jakupovic, J.; Zdero, C.; Grenz, M.; Tsichritzis, F.; Lehmann, L.; Hashemi-Nejad, S.; Bohlmann, F. Twenty-one acylphloroglucinol derivatives and further constituents from South African Helichrysum species. Phytochemistry 1989, 28, 1119–1131. [Google Scholar] [CrossRef]
- Heywood, V.H.; Harborne, J.B.; Turner, B.L. Biology and Chemistry of the Compositae; Academic Press: London, UK, 1977. [Google Scholar]
- Van Vuuren, S. Antimicrobial activity of South African medicinal plants. J. Ethnopharmacol. 2008, 119, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.N.; Rodrigues, K.A.; Carvalho, F.A.; Carneiro, S.M.; Maia, J.G.; Andrade, E.H.; Moraes, D.F. Molluscicidal and leishmanicidal activity of the leaf essential oil of Syzygium cumini (L.) Skeels from Brazil. Chem. Biodivers. 2013, 10, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Ganguly, U.; Chakrabarti, S.; Bhattacharjee, P. Is 1, 8-cineole-rich extract of small cardamom seeds more effective in preventing Alzheimer’s disease than 1, 8-cineole alone? Neuromol. Med. 2020, 22, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Vinholes, J.; Gonçalves, P.; Martel, F.; Coimbra, M.A.; Rocha, S.M. Assessment of the Anti-oxidant and anti-Proliferative effects of sesquiterpenic compounds in In Vitro caco-2 cell models. Food Chem. 2014, 156, 204–211. [Google Scholar] [CrossRef]
- Lomarat, P.; Sripha, K.; Phanthong, P.; Kitphati, W.; Thirapanmethee, K.; Bunyapraphatsara, N. In Vitro biological activities of black pepper essential oil and its major components relevant to the prevention of Alzheimer’s disease. Thai J. Pharm. Sci. (TJPS) 2015, 39, 94–101. [Google Scholar]
- Cavaleiro, C.; Pinto, E.; Gonçalves, M.; Salgueiro, L. Anti-fungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J. Appl. Microbiol. 2006, 100, 1333–1338. [Google Scholar] [CrossRef]
- Alam, M. Anti-hypertensive effect of cereal anti-oxidant ferulic acid and its mechanism of action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Müller, A.; Faubert, P.; Hagen, M.; Zu Castell, W.; Polle, A.; Schnitzler, J.-P.; Rosenkranz, M. Volatile profiles of fungi–chemotyping of species and ecological functions. Fungal Genet. Biol. 2013, 54, 25–33. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Shill, M.C.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Perigo, C.V.; Torres, R.B.; Bernacci, L.C.; Guimaraes, E.F.; Haber, L.L.; Facanali, R.; Vieira, M.A.; Quecini, V.; Marques, M.O.M. The chemical composition and anti-bacterial activity of eleven piper species from distinct rainforest areas in southeastern Brazil. Ind. Crop. Prod. 2016, 94, 528–539. [Google Scholar] [CrossRef]
- Moreira, C.M.; Fernandes, M.B.; Santos, K.T.; Schneider, L.A.; Da Silva, S.E.B.; Sant’Anna, L.S.; Paula, F.R. Effects of essential oil of Blepharocalyx salicifolius on cardiovascular function of rats. FASEB J. 2018, 32, 715–717. [Google Scholar] [CrossRef]
- Dos Santos, E.; Radai, J.A.S.; do Nascimento, K.F.; Formagio, A.S.N.; de Matos Balsalobre, N.; Ziff, E.B.; Castelon Konkiewitz, E.; Kassuya, C.A.L. Contribution of spathulenol to the anti-nociceptive effects of Psidium guineense. Nutr. Neurosci. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-C.; Yang, W.-C.; Tsai, W.-J.; Chen, C.-C.; Huang, H.-Y.; Tsai, Y.-C. A-bulnesene, a novel paf receptor antagonist isolated from Pogostemon cablin. Biochem. Biophys. Res. Commun. 2006, 345, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Benelli, G. Δ-cadinene, calarene and δ-4-carene from Kadsura heteroclita essential oil as novel larvicides against malaria, dengue and filariasis mosquitoes. Comb. Chem. High Throughput Screen. 2016, 19, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Mancianti, F. Use of essential oils in veterinary medicine to combat bacterial and fungal infections. Vet. Sci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Plant, R.M.; Dinh, L.; Argo, S.; Shah, M. The essentials of essential oils. Adv. Pediatrics 2019, 66, 111–122. [Google Scholar] [CrossRef]
- Helichrysum Petiolare. Available online: http://pza.sanbi.org/helichrysum-petiolare (accessed on 19 June 2021).
- Aladejana, A.E.; Bradey, G.; Afolayan, A.J. Comparative evaluation of essential oils of Helichrysum petiolare Hilliard & B.L. Burtt obtained from solvent-free microwave and hydrodistillation extraction methods. Asain J. Chem. 2020, 32, 1–13. [Google Scholar]
- Helichrysum cymosum (L.) D.Don Subsp. cymosum. Available online: https://keys.lucidcentral.org/keys/v3/helichrysum/key/Helichrysum/Media/Html/Helichrysum_cymosum_subsp._cymosum.htm (accessed on 14 July 2021).
- Franccedil, T.; Lambert, S.M.; Michel, J.D.P.; Gaby, N.M.E.; Fabrice, F.B.; Zaché, N.; Henri, A.Z.P.; Chantal, M. Composition, radical scavenging and anti-fungal activities of essential oils from 3 Helichrysum species growing in Cameroon against Penicillium oxalicum a yam rot fungi. Afr. J. Agric. Res. 2010, 5, 121–127. [Google Scholar]
- Sobhy, E.; El-Feky, S. Chemical constituents and antimicrobial activity of Helichrysum stoechas. Asian J. Plant Sci. 2007, 6, 692–695. [Google Scholar] [CrossRef]
- Najar, B.; Cervelli, C.; Ferri, B.; Cioni, P.; Pistelli, L. Essential oils and volatile emission of eight South African species of Helichrysum grown in uniform environmental conditions. S. Afr. J. Bot. 2019, 124, 178–187. [Google Scholar] [CrossRef]
- Fiddle Everlasting (Helichrysum pandurifolium). Available online: https://www.inaturalist.org/observations/23571154 (accessed on 19 June 2021).

| Compounds | Biological Functions |
|---|---|
| Helihumulone | anti-bacterial and anti-mycotic [15,52] |
| (Z)-β-ocimene | Molluscidal and leshimanicidal agents [53] |
| Trans-caryophyllene | Anti-malarial [15,36] |
| 1, 8-cineole | Anti-inflammatory, antioxidant, anticancer, analgesic [54] |
| α-humulene | Anti-Proliferative [55] |
| (E)-β-ocimene | Molluscidal and leshimanicidal agents [53] |
| Caryophyllene oxide | Anti-malarial [15,36] |
| β-caryophyllene | Anti-malarial [15,36] |
| Δ-3-carene | AChE inhibition, anti-inflammatory Anti-fungal [56,57] |
| 5-hydroxy-8-methoxy-7-prenyloxyflavanone | Anti-viral [30] |
| Compounds | Biological Functions |
|---|---|
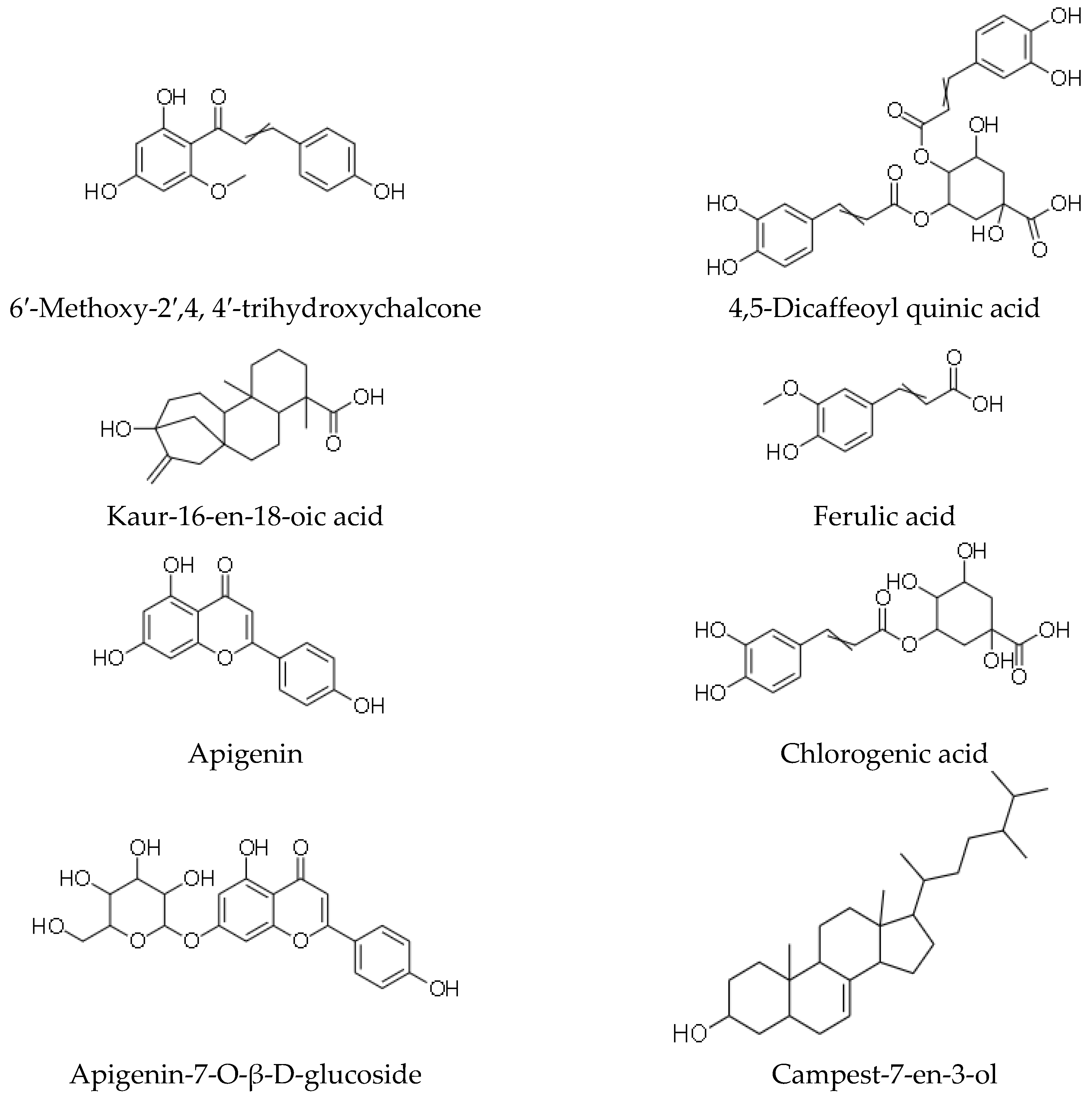
| 6′-methoxy-2′, 4, 4′-trihydroxychalcone | Anti-bacterial, anti-fungal, anti-ulcerogenic [43] |
| 6′-methoxy-2′,4-dihydroxychalcone- 4′-O-β-D-glucoside | Anti-bacterial, anti-fungal, anti-ulcerogenic [43] |
| Kaur-16-en-18-oic acid | Anti-bacterial, anti-fungal, anti-ulcerogenic [43] |
| Apigenin | Anti-bacterial, anti-fungal, anti-ulcerogenic [43] |
| Apigenin-7-O-β-D-glucoside | Anti-bacterial, anti-fungal, anti-ulcerogenic [43] |
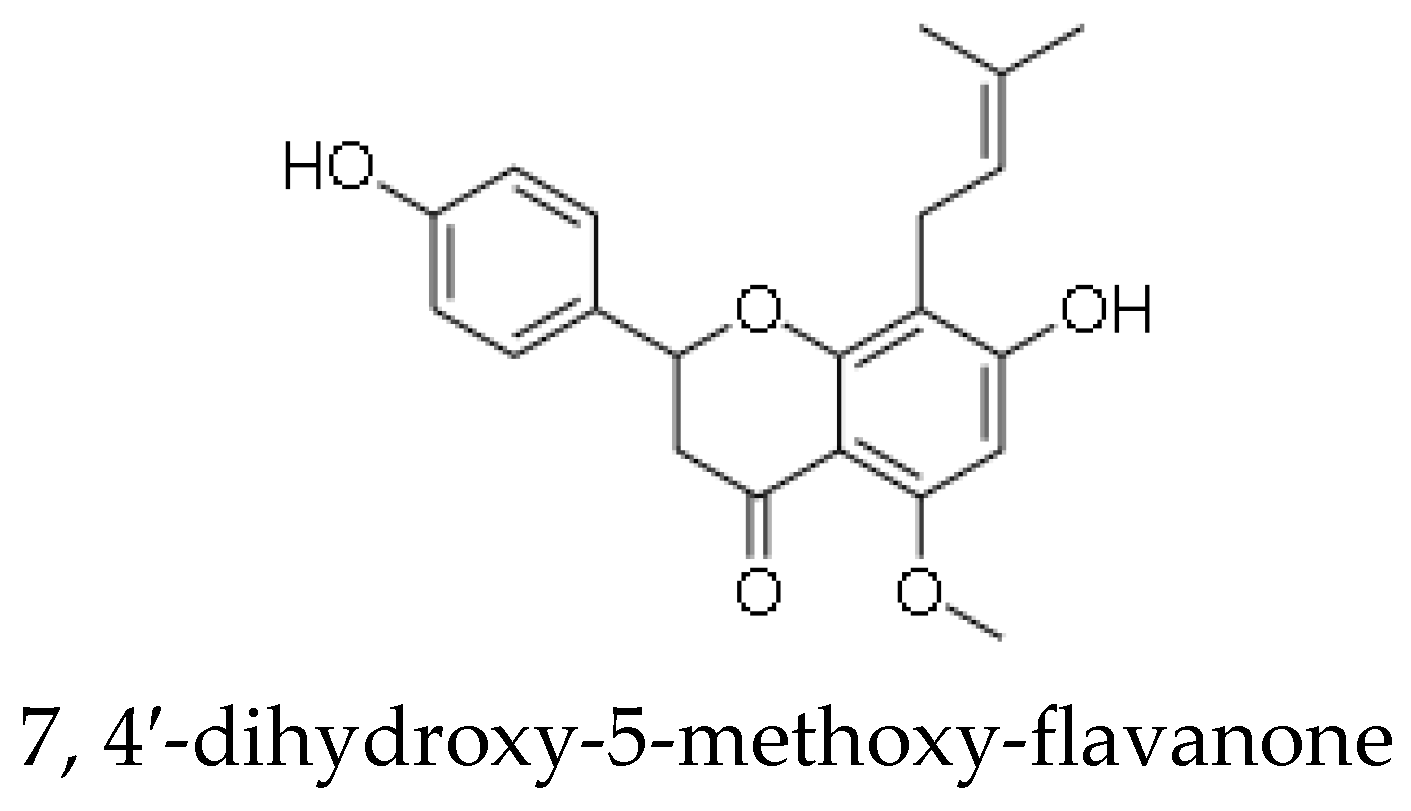
| 7,4′-dihydroxy-5-methoxy-flavanone | Anti-bacterial, anti-fungal, anti-ulcerogenic [43] |
| 4,5 –diacaffeoyl quinic acid | Anti-bacterial, anti-fungal, anti-ulcerogenic [43] |
| Ferulic acid | Anti-inflammatory, anti-oxidant, anti-diabetic, anti-hypertensive [58] |
| Chlorogenic acid | Anti-oxidant, anti-inflammatory, Anti-bacterial, anti-mutagenic and anti-cancer [59] |
| Campest-7-en-3-ol | Anti-bacterial, antifungal, anti-ulcerogenic [43] |
| Compounds | Biological Functions |
|---|---|
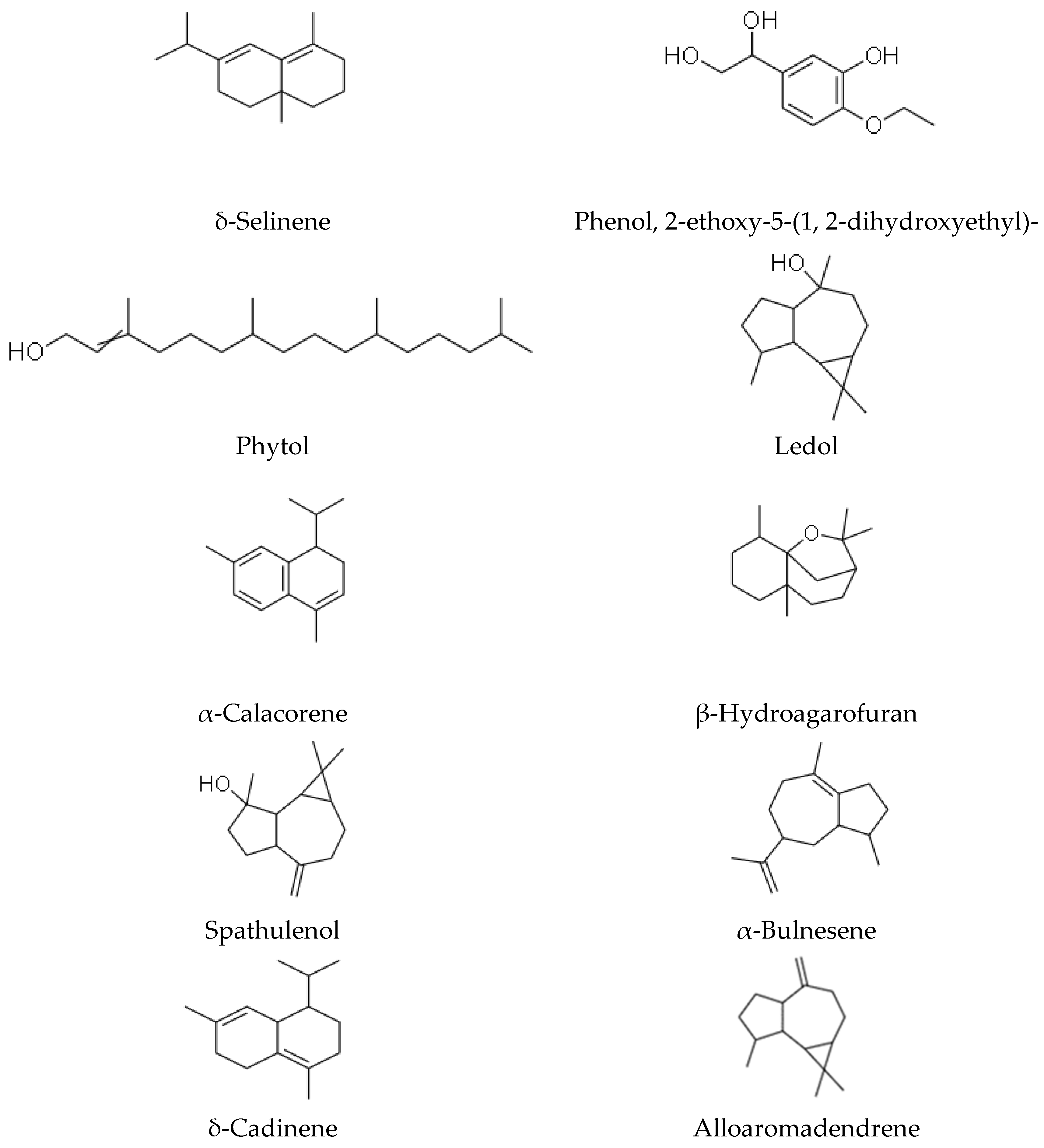
| Alloaromadendrene | Anti-microbial, anti-viral, anti-diabetic, anti-inflammatory [60] |
| δ-Selinene | anti-fungal [61] |
| Phenol, 2-ethoxy-5- (1, 2-dihydroxyethyl) - | N/A |
| Phytol | Anti-oxidant, anti-microbial, anti-convulsant, cytotoxic, anti-inflammatory [62] |
| Ledol | N/A |
| α-Calacorene | Anti-microbial, anti-oxidant [63] |
| β-Hydroagarofuran | Anti-bacterial and anti-mycotic [15] |
| Spathulenol | Anti-inflammatory, anti-nociceptive [64,65] |
| α-Bulnesene | Anti-platelet aggregation agent [66] |
| δ-Cadinene | Anti-malarial [67] |
| Compounds | Biological Functions |
|---|---|
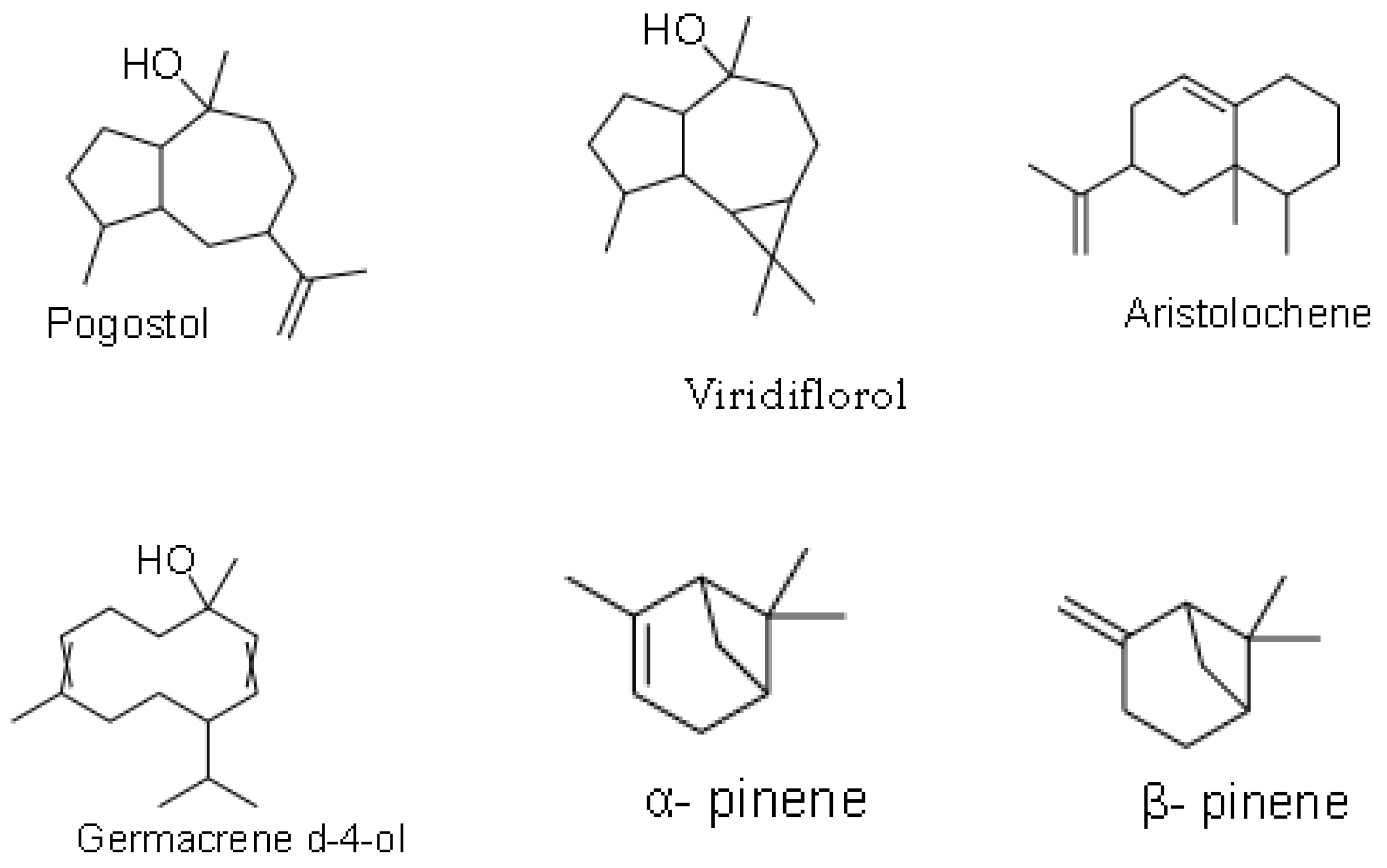
| Viridiflorol | Anti-fungal, anti-bacterial [14] |
| Pogostol | N/A [14] |
| α-pinene | N/A [14] |
| β-pinene | N/A [14] |
| Aristolochene | N/A [14] |
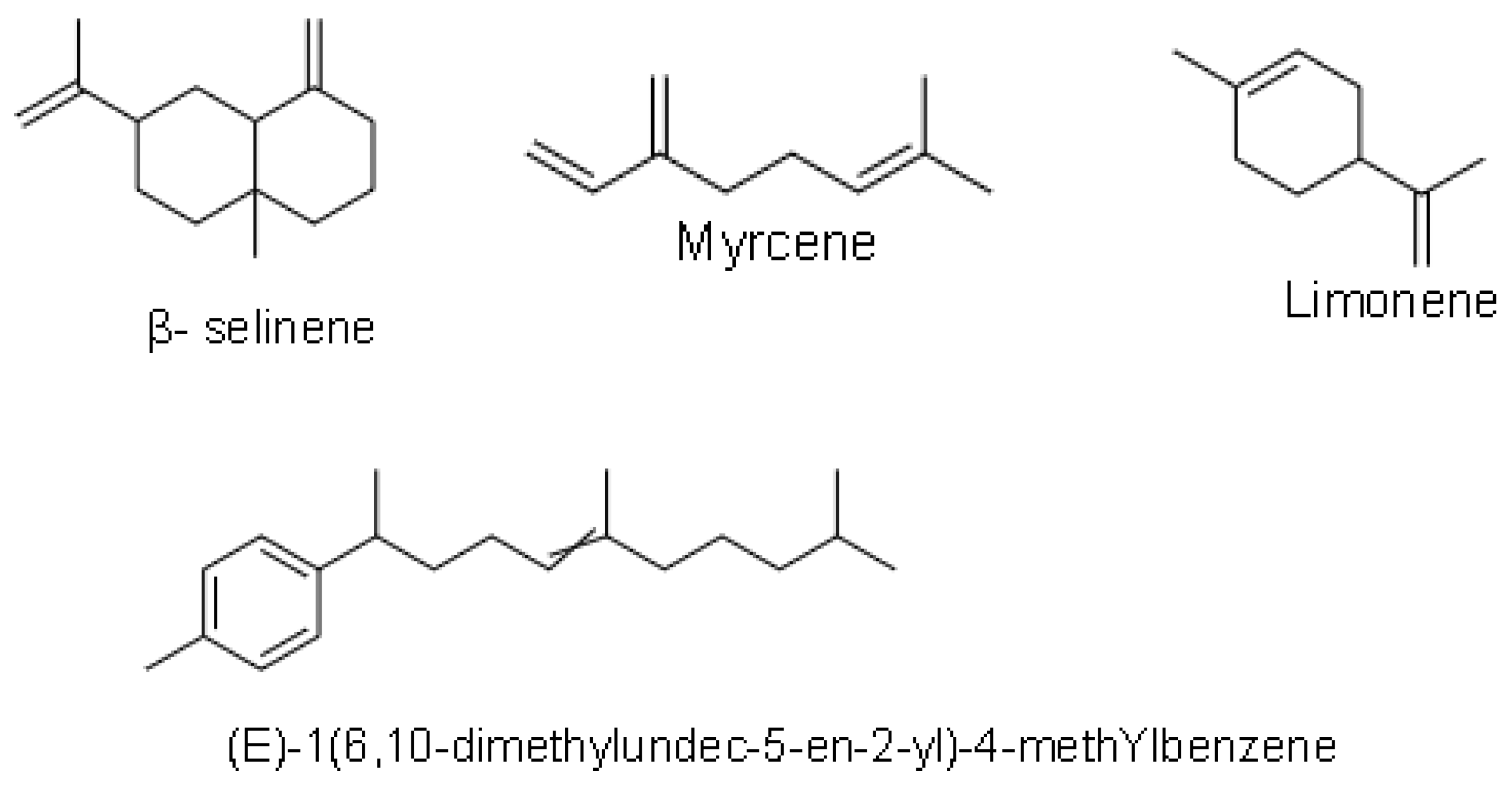
| (E)-1(6,10-dimethylundec-5-en-2-yl)-4-methylbenzene | N/A [14] |
| Z-β-ocimene | N/A [14] |
| δ-cadinene | N/A [14] |
| Germacrene d-4-ol | N/A [14] |
| 1,8-cineole | N/A [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinyede, K.A.; Cupido, C.N.; Hughes, G.D.; Oguntibeju, O.O.; Ekpo, O.E. Medicinal Properties and In Vitro Biological Activities of Selected Helichrysum Species from South Africa: A Review. Plants 2021, 10, 1566. https://doi.org/10.3390/plants10081566
Akinyede KA, Cupido CN, Hughes GD, Oguntibeju OO, Ekpo OE. Medicinal Properties and In Vitro Biological Activities of Selected Helichrysum Species from South Africa: A Review. Plants. 2021; 10(8):1566. https://doi.org/10.3390/plants10081566
Chicago/Turabian StyleAkinyede, Kolajo Adedamola, Christopher Nelson Cupido, Gail Denise Hughes, Oluwafemi Omoniyi Oguntibeju, and Okobi Eko Ekpo. 2021. "Medicinal Properties and In Vitro Biological Activities of Selected Helichrysum Species from South Africa: A Review" Plants 10, no. 8: 1566. https://doi.org/10.3390/plants10081566
APA StyleAkinyede, K. A., Cupido, C. N., Hughes, G. D., Oguntibeju, O. O., & Ekpo, O. E. (2021). Medicinal Properties and In Vitro Biological Activities of Selected Helichrysum Species from South Africa: A Review. Plants, 10(8), 1566. https://doi.org/10.3390/plants10081566






