Polygonum multiflorum Thunb. Hot Water Extract Reverses High-Fat Diet-Induced Lipid Metabolism of White and Brown Adipose Tissues in Obese Mice
Abstract
1. Introduction
2. Results
2.1. Effects of PW on Body Weight and Food Intake
2.2. Effects of PW on Relative Adipose Tissue Weight and Adipocyte Size
2.3. Effects of PW on Lipid Metabolism and Inflammatory Gene Expression in Epididymal WAT
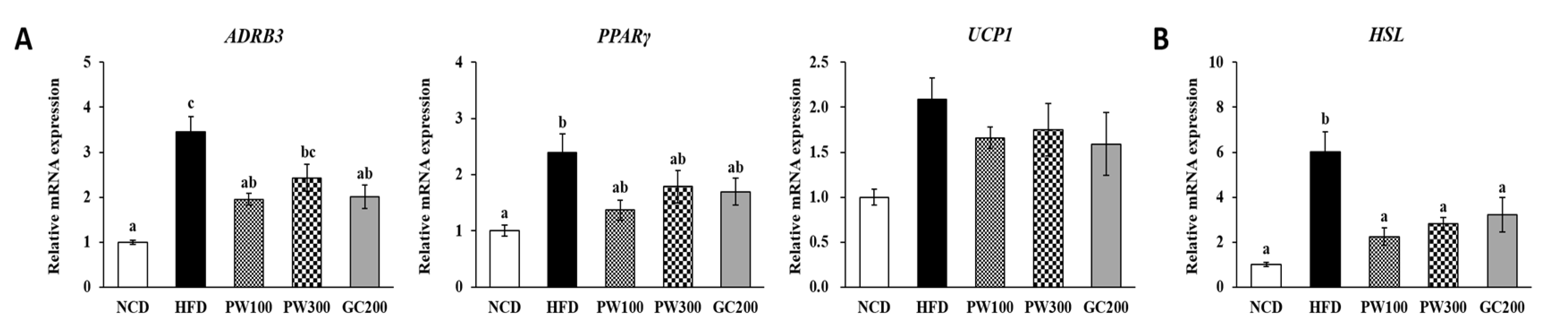
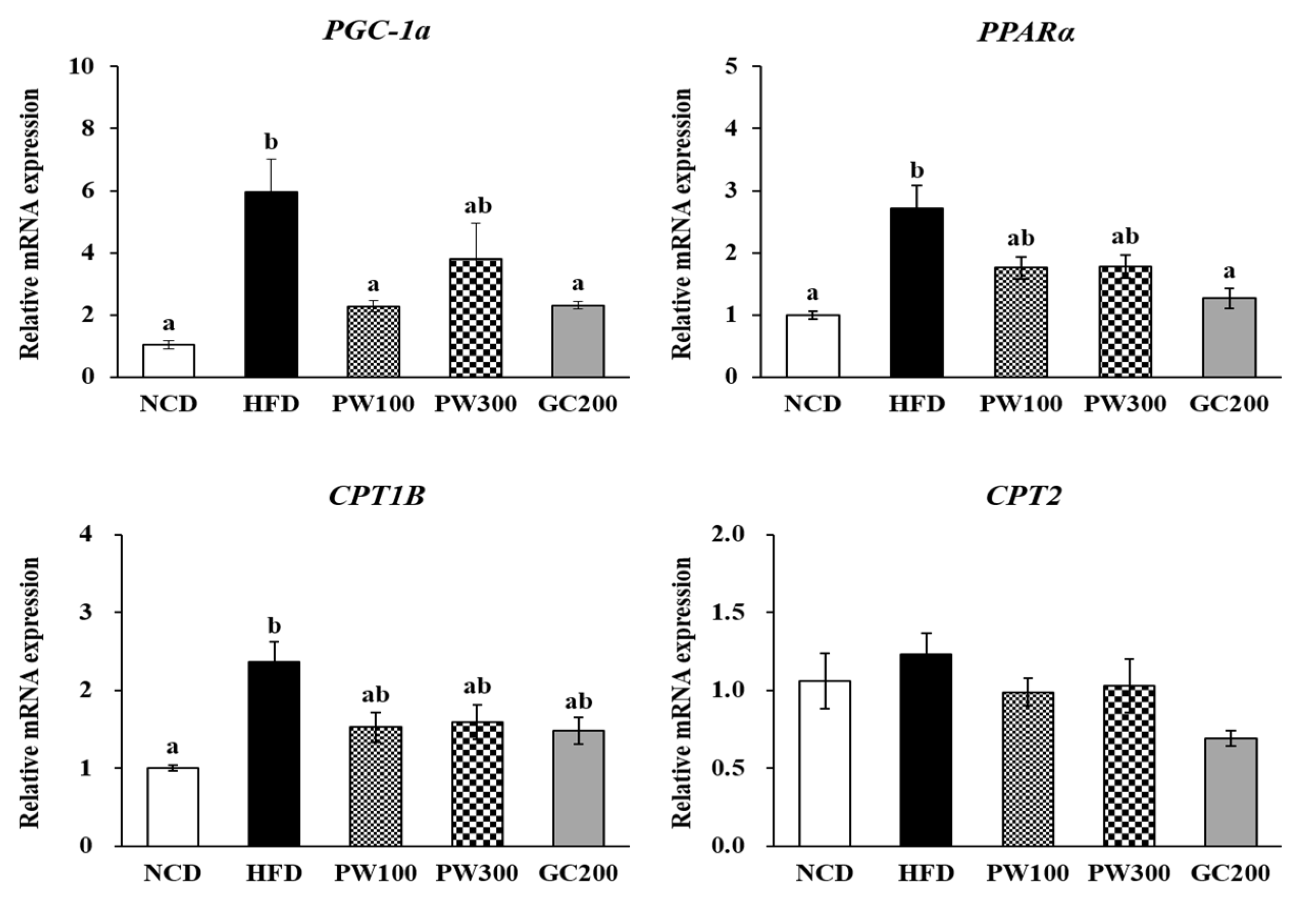
2.4. Effects of PW on Lipid Metabolism Gene Expression in Interscapular BAT
2.5. Effects of PW on Serum Biochemical Parameters and Lipid Profiles
2.6. Effects of PW on Hyperinsulinemia and Insulin Resistance
3. Discussion
4. Materials and Methods
4.1. Preparation of the PW Extract
4.2. Animal Experiments
4.3. Intraperitoneal Insulin Tolerance Test and Oral Glucose Tolerance Test
4.4. Serum Markers Levels and Lipid Contents
4.5. Histological Analysis
4.6. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulya, A.; Kirwan, J.P. Brown and beige adipose tissue: Therapy for obesity and its comorbidities? Endocrinol. Metab. Clin. North. Am. 2016, 45, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, A.; Oh, K.-J.; Lee, S.C.; Kim, W.K.; Bae, K.-H. The role of adipose tissue mitochondria: Regulation of mitochodrial function for the treatment of metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4924. [Google Scholar] [CrossRef] [PubMed]
- Sugita, J.; Yoneshiro, T.; Hatano, T.; Aita, S.; Ikemoto, T.; Uchiwa, H.; Iwanaga, T.; Kameya, T.; Kawai, Y.; Saito, M. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br. J. Nutr. 2013, 110, 733–738. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Ohtomo, T.; Ino, K.; Miyashita, R.; Chigira, M.; Nakamura, M.; Someya, K.; Inaba, N.; Fujita, M.; Takagi, M.; Yamada, J. Chronic high-fat feeding impairs adaptive induction of mitochondrial fatty acid combustion-associated proteins in brown adipose tissue of mice. Biochem. Biophys. Rep. 2017, 10, 32–38. [Google Scholar] [CrossRef]
- Bounda, G.-A.; Feng, Y.U. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacogn. Res. 2015, 7, 225–236. [Google Scholar] [CrossRef]
- Lin, E.-Y.; Chagnaadorj, A.; Huang, S.-J.; Wang, C.-C.; Chiang, Y.-H.; Cheng, C.-W. Hepatoprotective activity of the ethanolic extract of Polygonum multiflorum Thunb. against oxidative stress-induced liver injury. Evid. Based Complement. Alternat. Med. 2018, 2018, 4130307. [Google Scholar] [CrossRef]
- Ham, J.R.; Lee, H.-I.; Choi, R.-Y.; Ryu, H.-S.; Yee, S.-T.; Kang, K.-Y.; Lee, M.-K. Heshouwu (Polygonum multiflorum Thunb.) extract attenuates bone loss in diabetic mice. Prev. Nutr. Food Sci. 2019, 24, 121–127. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Wang, M.-F.; Chen, Y.-C.; Yang, D.-Y.; Lee, M.-S.; Cheng, F.-C. Long-term administration of Polygonum multiflorum Thunb: Reduces cerebral ischemia-induced infarct volume in gerbils. Am. J. Chin. Med. 2003, 31, 71–77. [Google Scholar] [CrossRef]
- Chen, H.-S.; Liu, Y.; Lin, L.-Q.; Zhao, J.-L.; Zhang, C.-P.; Jin, J.-C.; Wang, L.; Bai, M.-H.; Wang, Y.-C.; Liu, M.; et al. Anti-proliferative effect of an extract of the root of Polygonum multiflorum Thunb. on MCF-7 human breast cancer cells and the possible mechanisms. Mol. Med. Rep. 2011, 4, 1313–1319. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Cheng, F.-C.; Wang, M.-F. Beneficial effects of different Polygonum multiflorum Thunb. extracts on memory and hippocampus morphology. J. Nutr. Sci. Vitaminol. 2002, 48, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Xian, Z.; Liu, Y.; Xu, W.; Duan, F.; Guo, Z.; Xiao, H. The Anti-hyperlipidemia effects of raw Polygonum multiflorum extract in vivo. Biol. Pharm. Bull. 2017, 40, 1839–1845. [Google Scholar] [CrossRef][Green Version]
- Lin, P.; He, Y.R.; Lu, J.M.; Li, N.; Wang, W.G.; Gu, W.; Yu, J.; Zhao, R.H. In vivo lipid regulation mechanism of Polygoni multiflori radix in high-fat diet fed rats. Evid. Based Complement. Alternat. Med. 2014, 2014, 642058. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-Y.; Almofti, M.R.; Lu, L.; Kang, H.; Zhang, J.; Li, T.-J.; Rui, Y.-C.; Sun, L.-N.; Chen, W.-S. Reduction of atherosclerosis in cholesterol-fed rabbits and decrease of expressions of intracellular adhesion molecule-1 and vascular endothelial growth factor in foam cells by a water-soluble fraction of Polygonum multiflorum. J. Pharmacol. Sci. 2005, 99, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.-Y.; Lee, H.-I.; Ham, J.R.; Yee, S.-T.; Kang, K.-Y.; Lee, M.-K. Heshouwu (Polygonum multiflorum Thunb.) ethanol extract suppresses pre-adipocytes differentiation in 3T3-L1 cells and adiposity in obese mice. Biomed. Pharmacother. 2018, 106, 355–362. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, M.; Liu, J.; Li, H. Influence factors on the hepatotoxicity of Polygoni Multiflori Radix. Evid. Based Complement. Altern. Med. 2019, 2019, 5482896. [Google Scholar] [CrossRef]
- Li, T.; Gong, H.; Yuan, Q.; Du, M.; Ren, F.; Mao, X. Supplementation of polar lipids-enriched milk fat globule membrane in high-fat diet-fed rats during pregnancy and lactation promotes brown/beige adipocyte development and prevents obesity in male offspring. FASEB J. 2020, 34, 4619–4634. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lee, G.-S.; Cheon, S.-Y.; Cha, Y.-Y.; An, H.-J. The anti-obesity effects of Tongbi-san in a high-fat diet-induced obese mouse model. BMC Complement. Altern. Med. 2019, 19, 1. [Google Scholar] [CrossRef]
- Yuan, H.; Chung, S.; Ma, Q.; Ye, L.I.; Piao, G. Combination of deep sea water and Sesamum indicum leaf extract prevents high-fat diet-induced obesity through AMPK activation in visceral adipose tissue. Exp. Ther. Med. 2016, 11, 338–344. [Google Scholar] [CrossRef]
- Lu, Y.A.; Lee, H.G.; Li, X.; Hyun, J.-M.; Kim, H.-S.; Kim, T.H.; Kim, H.-M.; Lee, J.J.; Kang, M.-C.; Jeon, Y.-J. Anti-obesity effects of red seaweed, Plocamium telfairiae, in C57BL/6 mice fed a high-fat diet. Food Funct. 2020, 11, 2299–2308. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schön, M.R.; Abumrad, N.A.; Blüher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nat. Cell Biol. 2012, 484, 333–338. [Google Scholar] [CrossRef]
- Eissing, L.; Scherer, T.; Tödter, K.; Knippschild, U.; Greve, J.W.; Buurman, W.A.; Pinnschmidt, H.O.; Rensen, S.S.; Wolf, A.M.; Bartelt, A.; et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 2013, 4, 1528. [Google Scholar] [CrossRef]
- Ortega-Prieto, P.; Postic, C. Carbohydrate sensing through the transcription factor ChREBP. Front. Genet. 2019, 10, 472. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Lin, P.; Li, Y.; Sun, R.; Gu, W.; Yu, J.; Zhao, R. In vitro effects of active components of Polygonum Multiflorum Radix on enzymes involved in the lipid metabolism. J. Ethnopharmacol. 2014, 153, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Lee, J.; Teraminami, A.; Kim, Y.; Hirai, S.; Uemura, T.; Inoue, H.; Takahashi, N.; Kawada, T. Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J. Lipid Res. 2011, 52, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.; Staels, B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J. Clin. Invest. 2006, 116, 571–580. [Google Scholar] [CrossRef]
- Tsuchida, A.; Yamauchi, T.; Takekawa, S.; Hada, Y.; Ito, Y.; Maki, T.; Kadowaki, T. Peroxisome proliferator–activated receptor (PPAR) α activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: Comparison of activation of PPARα, PPARγ, and their combination. Diabetes 2005, 54, 3358–3370. [Google Scholar] [CrossRef] [PubMed]
- Hiuge, A.; Tenenbaum, A.; Maeda, N.; Benderly, M.; Kumada, M.; Fisman, E.Z.; Tanne, D.; Matas, Z.; Hibuse, T.; Fujita, K.; et al. Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin level. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Loviscach, M.; Rehman, N.; Carter, L.; Mudaliar, S.; Mohadeen, P.; Ciaraldi, T.P.; Veerkamp, J.H.; Henry, R.R. Distribution of peroxisome proliferator-activated receptors (PPARs) in human skeletal muscle and adipose tissue: Relation to insulin action. Diabetologia 2000, 43, 304–311. [Google Scholar] [CrossRef]
- Guzmán, M.; Verme, J.L.; Fu, J.; Oveisi, F.; Blázquez, C.; Piomelli, D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α). J. Biol. Chem. 2004, 279, 27849–27854. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.-I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.-J.; Joshi, Y.; Patil, Y.; Noland, R.C.; Chang, J.S. NT-PGC-1α activation attenuates high-fat diet–induced obesity by enhancing brown fat thermogenesis and adipose tissue oxidative metabolism. Diabetes 2014, 63, 3615–3625. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, G.; Harper, M.-E. Invited review: Uncoupling proteins and thermoregulation. J. Appl. Physiol. 2002, 92, 2187–2198. [Google Scholar] [CrossRef]
- Hibi, M.; Oishi, S.; Matsushita, M.; Yoneshiro, T.; Yamaguchi, T.; Usui, C.; Yasunaga, K.; Katsuragi, Y.; Kubota, K.; Tanaka, S.; et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int. J. Obes. 2016, 40, 1655–1661. [Google Scholar] [CrossRef]
- Chernogubova, E.; Hutchinson, D.S.; Nedergaard, J.; Bengtsson, T. α1- and β1-Adrenoceptor signaling fully compensates for β3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology 2005, 146, 2271–2284. [Google Scholar] [CrossRef]
- Lee, H.J. Exercise and activation of brown adipose tissue. Asian J. Kinesiol. 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Hoeke, G.; Kooijman, S.; Boon, M.R.; Rensen, P.C.; Berbée, J.F. Role of brown fat in lipoprotein metabolism and atherosclerosis. Circ. Res. 2016, 118, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Dominguez, M.; Mir, J.F.; Fucho, R.; Weber, M.; Serra, D.; Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2016, 5, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, W.G.; Shen, Z.F.; Yuan, T.; Liu, S.N.; Liu, Q.; Fu, Y.; Sun, W. Comparative proteome analysis of brown adipose tissue in obese C57BL/6J mice using iTRAQ-coupled 2D LC-MS/MS. PLoS ONE 2015, 10, e0119350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, T.; Li, J.; Zhao, W.-G.; Sun, W.; Liu, S.-N.; Liu, Q.; Fu, Y.; Shen, Z.-F. Effects of metformin on metabolism of white and brown adipose tissue in obese C57BL/6J mice. Diabetol. Metab. Syndr. 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, J.; Shin, S.S.; Yoon, M. Ascorbic acid inhibits visceral obesity and nonalcoholic fatty liver disease by activating peroxisome proliferator-activated receptor α in high-fat-diet-fed C57BL/6J mice. Int. J. Obes. 2019, 43, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Li, Y.; Gao, H.; Zhang, H.; Han, J.; Zhang, D.; Li, Y.; Zhou, J.; Lu, C.; Su, X. Modulation of the gut microbiota by the mixture of fish oil and krill oil in high-fat diet-induced obesity mice. PLoS ONE 2017, 12, e0186216. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, H.-S.; Kim, Y.-E.; Kim, B.-M.; Kim, I.-H.; Lee, C.-H. Effect of Polygonum multiflorum Thunberg extract on lipid metabolism in rats fed high-cholesterol diet. J. Korean Soc. Food Sci. Nutr. 2012, 41, 957–962. [Google Scholar] [CrossRef]
- Zheng, S.; Hoos, L.; Cook, J.; Tetzloff, G.; Davis, H., Jr.; van Heek, M.; Hwa, J.J. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur. J. Pharmacol. 2008, 584, 118–124. [Google Scholar] [CrossRef]
- Li, D.-K.; Chen, J.; Ge, Z.-Z.; Sun, Z.-X. Hepatotoxicity in rats induced by aqueous extract of Polygoni Multiflori Radix, root of Polygonum multiflorum related to the activity inhibition of CYP1A2 or CYP2E1. Evid. Based Complement. Altern. Med. 2017, 2017, 9456785. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Son, H.; Hwang, C.; Cho, K.; Park, S.; Kim, H.; Kim, H.J. The Root of Polygonum multiflorum Thunb. alleviates non-alcoholic steatosis and insulin resistance in high fat diet-fed mice. Nutrients 2020, 12, 2353. [Google Scholar] [CrossRef]
- Kim, E.-S.; Kwon, H.-J. Development of Korean herbal medicine cosmetics containing Polygonum multiflorum extracts. Med. Leg. Update 2019, 19, 388. [Google Scholar] [CrossRef]
- Ezez, D.; Tefera, M. Effects of solvents on total phenolic content and antioxidant activity of ginger extracts. J. Chem. 2021, 2021, 6635199. [Google Scholar] [CrossRef]
- Noda, T.; Yamada, T.; Ohkubo, T.; Omura, T.; Ono, T.; Adachi, T.; Awaya, T.; Tasaki, Y.; Shimizu, K.; Matsubara, K. Hot-water-extracts of Polygonum multiflorum do not induce any toxicity but elicit limited beneficial effects on the liver in mice. J. Health Sci. 2009, 55, 720–725. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.; Rushing, P.A.; D’Alessio, D.; Tso, P. A Controlled high-fat diet induces an obese syndrome in rats. J. Nutr. 2003, 133, 1081–1087. [Google Scholar] [CrossRef]
- Woods, S.C.; D’Alessio, D.A.; Tso, P.; Rushing, P.A.; Clegg, D.J.; Benoit, S.C.; Gotoh, K.; Liu, M.; Seeley, R.J. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol. Behav. 2004, 83, 573–578. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Lo, J.C.; Ljubicic, S.; Leibiger, B.; Kern, M.; Leibiger, I.B.; Moede, T.; Kelly, M.E.; Bhowmick, D.C.; Murano, I.; Cohen, P.; et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell 2014, 158, 41–53. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kang, K.Y.; Kim, J.J.; Lee, S.J.; Son, Y.J.; Paik, S.H.; Yee, S.T. Effects of hot water extracts from Polygonum multiflorum on ovariectomy induced osteopenia in mice. Evid. Based Complement. Alternat. Med. 2016, 2016, 8970585. [Google Scholar] [CrossRef]
- Maia-Landim, A.; Lancho, C.; Poblador, M.S.; Lancho, J.L.; Ramírez, J.M. Garcinia cambogia and Glucomannan reduce weight, change body composition and ameliorate lipid and glucose blood profiles in overweight/obese patients. J. Herb. Med. 2021, 26, 100424. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| NCD | HFD | PW100 | PW300 | GC200 | |
|---|---|---|---|---|---|
| Body weight (g) | |||||
| 0 weeks | 20.26 ± 0.23 | 20.55 ± 0.35 | 20.18 ± 0.24 | 20.34 ± 0.31 | 20.39 ± 0.31 |
| 1 weeks | 21.61 ± 0.36 | 22.50 ± 0.36 | 21.19 ± 0.54 | 20.96 ± 0.42 | 22.35 ± 0.36 |
| 2 weeks | 22.46 ± 0.41 | 23.64 ± 0.35 | 22.30 ± 0.45 | 22.28 ± 0.61 | 24.03 ± 0.27 |
| 3 weeks | 23.20 ± 0.45 | 24.67 ± 0.32 | 23.83 ± 0.54 | 23.52 ± 0.54 | 24.65 ± 0.36 |
| 4 weeks | 24.30 ± 0.56 | 26.18 ± 0.32 | 24.75 ± 0.50 | 24.64 ± 0.60 | 26.28 ± 0.48 |
| 5 weeks | 25.13 ± 0.70 | 27.06 ± 0.42 | 25.67 ± 0.48 | 24.90 ± 0.65 | 26.69 ± 0.48 |
| 6 weeks | 26.53 ± 0.75 ab | 28.84 ± 0.44 b | 26.97 ± 0.53 ab | 26.33 ± 0.56 a | 28.14 ± 0.58 ab |
| 7 weeks | 27.50 ± 0.65 a | 30.35 ± 0.41 c | 28.20 ± 0.55 ab | 27.92 ± 0.50 a | 30.06 ± 0.38 bc |
| 8 weeks | 27.70 ± 0.91 a | 31.35 ± 0.49 c | 28.98 ± 0.61 abc | 28.10 ± 0.55 ab | 30.23 ± 0.42 bc |
| 9 weeks | 28.65 ± 0.97 a | 32.41 ± 0.51 b | 30.14 ± 0.76 ab | 28.75 ± 0.65 a | 30.95 ± 0.50 ab |
| 10 weeks | 30.22 ± 0.91 a | 34.45 ± 0.54 b | 31.68 ± 0.78 ab | 30.62 ± 0.69 a | 32.10 ± 0.59 ab |
| 11 weeks | 31.34 ± 0.88 a | 35.71 ± 0.50 b | 33.39 ± 0.76 ab | 32.04 ± 0.85 a | 33.09 ± 0.63 ab |
| 12 weeks | 31.85 ± 0.89 a | 36.54 ± 0.51 b | 34.42 ± 0.83 ab | 33.16 ± 0.77 a | 33.73 ± 0.57 ab |
| Body weight gain (g) | 11.61 ± 0.75 a | 16.00 ± 0.61 b | 14.23 ± 0.63 ab | 12.82 ± 0.71 a | 13.33 ± 0.40 a |
| NCD | HFD | PW100 | PW300 | GC200 | |
|---|---|---|---|---|---|
| Food intake (g/day) | 3.24 ± 0.05 b | 2.83 ± 0.03 a | 2.73 ± 0.02 a | 2.72 ± 0.03 a | 2.75 ± 0.03 a |
| FER | 0.0423 ± 0.0022 a | 0.0671 ± 0.0021 c | 0.0619 ± 0.0025 bc | 0.0558 ± 0.0025 b | 0.0576 ± 0.0013 b |
| Serum marker levels | |||||
| AST (U/L) | 54.62 ± 2.92 | 62.60 ± 3.06 | 62.00 ± 3.86 | 52.40 ± 2.07 | 54.87 ± 2.96 |
| ALT (U/L) | 23.12 ± 2.01 a | 34.40 ± 2.70 b | 32.10 ± 3.55 ab | 24.50 ± 2.12 ab | 28.00 ± 2.34 ab |
| Glucose (mmol/L) | 11.76 ± 1.37 | 12.95 ± 0.73 | 10.64 ± 0.97 | 11.07 ± 1.01 | 13.54 ± 0.68 |
| Serum lipid contents | |||||
| Triglyceride (mmol/L) | 0.80 ± 0.05 | 0.93 ± 0.05 | 0.94 ± 0.03 | 0.93 ± 0.05 | 0.83 ± 0.02 |
| Free fatty acid (mmol/L) | 0.84 ± 0.05 b | 0.79 ± 0.07 b | 0.76 ± 0.03 b | 0.65 ± 0.05 ab | 0.43 ± 0.05 a |
| Total cholesterol (mmol/L) | 3.96 ± 0.17 | 4.60 ± 0.16 | 4.27 ± 0.16 | 4.13 ± 0.09 | 4.40 ± 0.14 |
| HDL-cholesterol (mmol/L) | 2.56 ± 0.05 a | 3.01 ± 0.10 b | 2.97 ± 0.09 ab | 2.90 ± 0.05 ab | 3.21 ± 0.19 b |
| HTR (%) | 65.38 ± 2.52 | 66.92 ± 2.02 | 69.79 ± 1.52 | 69.30 ± 1.11 | 74.62 ± 4.61 |
| Gene | Full Name | Sequences of Forward and Reverse Primer (5′-3′) |
|---|---|---|
| PGC-1α | Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha | GTCATGTGACTGGGGACTGTAG/TCCACTCTGACACACAGCAC |
| PPARα | Peroxisome proliferator-activated receptor alpha | GCTGGAGGGTTCGTGGAGTC/CGGTGAGATACGCCCAAATGC |
| UCP1 | Uncoupling protein1 (mitochondrial, proton carrier) | CCTGCCTCTCTCGGAAACAA/TCTGGGCTTGCATTCTGACC |
| CPT1B | Carnitine palmitoyltransferase 1b | TGGCTACGGGGTCTCTTACA/AAGTTCGGCGATGTCCAACA |
| CPT2 | Carnitine palmitoyltransferase 2 | GCCTGCTGTTGCGTGACTG/TGGTGGGTACGATGCTGTGC |
| HSL | Lipase, hormone sensitive | GTGAATGAGATGGCGAGGGTC/TGAGGAGTCGCGTTAGAGTC |
| PPARγ | Peroxisome proliferator-activated receptor gamma | TCGCTGATGCACTGCCTATG/GAGAGGTCCACAGAGCTGAT |
| C/EBPα | CCAAT/enhancer binding protein (C/EBP), alpha | GCGCAAGAGCCGAGATAAA/GGTGAGGACACAGACTCAAATC |
| SREBP1c | Sterol regulatory element binding transcription factor 1 | AACCTCATCCGCCACCTG/TGGTAGACAACAGCCGCATC |
| ADRB3 | Adrenergic receptor, beta 3 | CAGGCTCTGTGTCTCTGGTTAG/GTGAGGAGACAGGGATGAAACC |
| ChREBP | Carbohydrate-responsive element-binding protein | GAAGGAATGGGTCCAGACATAC/TCACACTGGTCACTCCTACA |
| CD36 | CD36 antigen | GCTGTCAGGCGTCAGGATAA/TGGCTTCAGGGAGACTGTTG |
| FABP4 | Fatty acid binding protein 4, adipocyte | TTTGGTCACCATCCGGTCAG/CCCGCCATCTAGGGTTATGA |
| FAS | Fatty acid synthase | TTGGAGCTAAGGCATGGTGG/GCAGTTGTCCTCTGGATGCT |
| SCD1 | Stearoyl-coenzyme A desaturase 1 | TTCTTCATCGACTGCATGGC/ACTCAGAAGCCCAAAGCTCAG |
| DGAT2 | Diacylglycerol O-acyltransferase 2 | CTGGCTGATAGCTGTGCTCTACTTC/TGCGATCTCCTGCCACCTTTC |
| NF-κB | Nuclear factor of kappa light polypeptide gene enhancer in B cells | GAAGTGAGAGAGTGAGCGAGAGAG/CGGGTGGCGAAACCTCCTC |
| TNF-α | Tumor necrosis factor | AAAGACACCATGAGCACAGAAAGC/GCCACAAGCAGGAATGAGAAGAG |
| IL-6 | Interleukin 6 | AGTCCTTCCTACCCCAATTTCC/TGGTCTTGGTCCTTAGCCAC |
| MCP1 | Chemokine (C–C motif) ligand 2 | GAAGGAATGGGTCCAGACATAC/TCACACTGGTCACTCCTACA |
| RPLP0 | Ribosomal protein, large, P0 | GCAGGTGTTTGACAACGGCAG/GATGATGGAGTGTGGCACCGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, R.-Y.; Lee, M.-K. Polygonum multiflorum Thunb. Hot Water Extract Reverses High-Fat Diet-Induced Lipid Metabolism of White and Brown Adipose Tissues in Obese Mice. Plants 2021, 10, 1509. https://doi.org/10.3390/plants10081509
Choi R-Y, Lee M-K. Polygonum multiflorum Thunb. Hot Water Extract Reverses High-Fat Diet-Induced Lipid Metabolism of White and Brown Adipose Tissues in Obese Mice. Plants. 2021; 10(8):1509. https://doi.org/10.3390/plants10081509
Chicago/Turabian StyleChoi, Ra-Yeong, and Mi-Kyung Lee. 2021. "Polygonum multiflorum Thunb. Hot Water Extract Reverses High-Fat Diet-Induced Lipid Metabolism of White and Brown Adipose Tissues in Obese Mice" Plants 10, no. 8: 1509. https://doi.org/10.3390/plants10081509
APA StyleChoi, R.-Y., & Lee, M.-K. (2021). Polygonum multiflorum Thunb. Hot Water Extract Reverses High-Fat Diet-Induced Lipid Metabolism of White and Brown Adipose Tissues in Obese Mice. Plants, 10(8), 1509. https://doi.org/10.3390/plants10081509







