Abstract
The development of biotechnologies based on beneficial microorganisms for improving soil fertility and crop yields could help to address many current agriculture challenges, such as food security, climate change, pest control, soil depletion while decreasing the use of chemical fertilizers and pesticides. Plant growth-promoting (PGP) microbes can be used as probiotics in order to increase plant tolerance/resistance to abiotic/biotic stresses and in this context strains belonging to the Pseudomonas chlororaphis group have shown to have potential as PGP candidates. In this study a new P. chlororaphis isolate is reported and tested for (i) in vitro PGP features, (ii) whole-genome sequence analysis, and (iii) its effects on the rhizosphere microbiota composition, plant growth, and different plant genes expression levels in greenhouse experiments. Results showed that P. chlororaphis ST9 is an efficient rice root colonizer which integrates into the plant resident-microbiota and affects the expression of several plant genes. The potential use of this P. chlororaphis strain as a plant probiotic is discussed.
1. Introduction
The agriculture of the 21st century has several challenges to face. Among them are the increase in population and the growing demand for food in the context of climate change, soil depletion, competition between different land uses, and the need to reduce chemical fertilizers and pesticides. The use of beneficial microorganisms in crop production is an appealing solution as they can have beneficial effects on soil and plant by improving soil fertility, plant yields and reducing the use of agrochemicals [1]. The development of next-generation sequencing methodologies has led to numerous studies on plant microbiomes documenting that plants are colonized and live in association with a large number of microorganisms. Many of these, indicated as PGP (plant growth-promoting) microbes, play important roles in plant health and resistance to biotic and abiotic stresses [2,3,4,5]. PGP microbes allow the reduction of fertilizers requirements, improving crop nutrient use efficiency (nitrogen, phosphate, etc.), and protecting the host plant by pathogens invasion through niche exclusion mechanisms and antibacterial/antifungal compound production [6,7]. PGP microorganisms can also elicit transcriptional changes in hormone-, defense- and cell wall- related genes [8], increase root length [9], and activate auxin-response genes that reinforce plant growth [10]. In the last decades, the interest in developing PGP probiotic microorganisms has increased intending to reduce chemical fertilization [11] and pesticide use, both in conventional and organic farming, to offer healthier food and improving the sustainability of crop production [12]. One class of microorganisms that has been studied for many years for their application is known as plant growth-promoting rhizobacteria (PGPR) [13]. Among the group of PGPR, strains belonging to the Pseudomonas chlororaphis species have been found in association with a wide range of plants, both mono- and dicotyledonous, and both wild and cultivated [14]. P. chlororaphis is currently classified into four subspecies, namely chlororaphis, aureofaciens, aurantiaca, and piscium [15,16]. Several strains of P. chlororaphis have shown potential for application as plant probiotics [17,18,19] due to their rhizosphere colonization abilities and plant-associated beneficial phenotypes such as chemotaxis and motility [20], biofilm formation [21], P solubilization [22], ACC deaminase [23], IAA production [24,25] and biocontrol. P. chlororaphis strains produce different antifungal compounds such as Prn (pyrrolnitrin), PCN (phenazine-1-carboxamide), PCA (phenazine-1-carboxylic acid), 2-OH-PHZ (2-hydroxyphenazine), HPR (2-hexyl-5-propyl-alkylresorcinol) and HCN (hydrogen cyanide). These molecules inhibit the growth of various phytopathogens belonging to the Fusarium group [26,27] and different species of Colletotrichum, Phytophthora, Pythium, Sclerotinia, Magnaporthe oryzae [28] and Rhizoctonia [29], protecting plants such as maize [30], tomato [31]. Besides, good formulation protocols have been developed and products containing P. chlororaphis strains have been produced and commercialized [32]. In this work, we report the isolation and characterization of P. chlororaphis strain ST9. This strain was able to colonize and persist in rice roots for the entire rice vegetative cycle and studies are presented of its effect on the root microbiota, on plant growth and on the expression of several plant genes. The potential use of this P. chlororaphis strain as a plant probiotic for rice cultivation is discussed.
2. Results
2.1. Identification and Characterization of P. chlororaphis ST9
A culture collection of bacterial isolates was generated from two soil samples as described in the Materials and Methods section. It was of interest to then focus our studies on a new isolate of P. chlororaphis called strain ST9, since this species is used in microbial inoculants for agriculture and is known to have several plant beneficial properties. The genome sequence of P. chlororaphis ST9 was determined and its chromosome was 6.7 Mb long with a GC content of 63%; according to the RASTtk annotation scheme it contains 6185 predicted protein-coding sequences (CDSs) and 83 RNAs. Phylogenetic analyses based on MSLA using 6 loci (16S rRNA, recA, gyrB, rpoD, carA and atpD; [33]) and 16 sequences of P. chlororaphis isolates revealed that strain ST9 belongs to the Pseudomonas chlororaphis subsp. aurantiaca group (Supplementary Figure S1). Similar to other P. chlororaphis strains, its genome possesses several loci encoding for anti-bacterial/anti-fungal compounds including operons for the biosynthesis of phenazine, two R-tailocins bacteriocines and pyrrolnitrin. Other loci potentially involved in microbial pathogen antagonism, cell-cell signal interferences, niche colonization, plant protection and plant-beneficial traits were identified and reported in Table 1. In summary, P. chlororaphis ST9 possesses many features that can potentially make this strain a plant probiotic and biocontrol agent.

Table 1.
P. chlororaphis genome mining for PGP and biocontrol related genetic loci. ST9 genes ID were assigned by the RAST Annotation Server [34] during automatic annotation and can be used for retrieve gene sequences from the RAST server (https://rast.nmpdr.org/?page=JobDetails&job=828746, accessed on 21 February 2020); “guest” as login and password).
In order to assess the possible PGP potential of P. chlororaphis ST9, several in vitro and in vivo phenotype tests, summarized in Table 2, were performed. Among the performed tests, the ability of ST9 to inhibit the growth of a wide variety of plant pathogens, including Dickeya zeae, Pseudomonas fuscovaginae, Magnaporthe oryzae, Aspergillus nidulans and Fusarium graminearum was observed in vitro (Supplementary Figure S2). An in planta biocontrol assay was also performed: ST9 treated plants displayed a slight but significant decrease of the severity of the disease as reported in Figure 1.

Table 2.
Phenotypic characterization of PGP features of P. chlororaphis ST9.
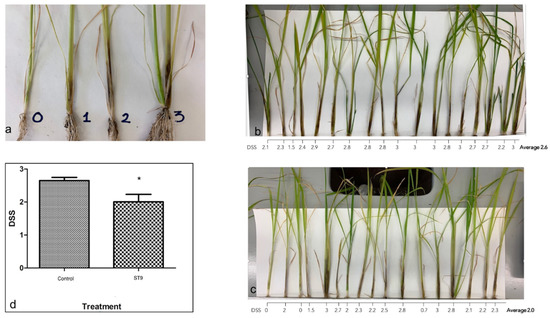
Figure 1.
Biocontrol activity of P. chlororaphis ST9 against Dickeya zeae. (a) Disease Severity Score Scale; (b,c) control and ST9 treated plants respectively, 7 days after D. zeae infection: the average of the DSS (Disease Severity Score) assigned to each plant by 7 different persons is reported; (d) graph reporting the average of the DSS assigned to the control and ST9 treated plants: the difference proved to be significant (Mann–Whitney t-test: * p-value < 0.05).
Other PGP-related in vitro phenotypes such as lipolytic and proteolytic activities and motility were observed. In summary, this isolate possesses several phenotypic abilities which can be of importance in plant colonization and PGP.
2.2. P. chlororaphis ST9 Root Colonization Ability, Persistence and Effect on the Rhizosphere Microbiota
The ability of P. chlororaphis ST9 to colonize and persist in the rhizosphere of rice plants was evaluated. Rice seeds were inoculated with P. chlororaphis ST9, grown in the greenhouse and roots were then collected at T1 (10 dpi), T2 (28 dpi) and T4 (90 dpi) together with untreated controls for ST9 strain CFU counts. Results are summarized in Figure 2, which shows that the level of colonization after 10 days was significantly high (107 cfu/g of root, -wet weight) then it decreased and stabilized around a value of 104 cfu/g at 90 dpi. Colonies grown on TSA rifampicin plates were randomly chosen for identification through 16S rRNA gene amplification and sequencing to confirm their identity as P. chlororaphis ST9. Rifampicin-resistant colonies present in the untreated plants resulted to be a mixture of different bacteria: some of them were part of an analogue experiment carried out in concomitance (data not shown). These results evidenced that under the conditions tested, P. chlororaphis ST9 was an efficient root colonizer and able to persist in the rhizosphere.
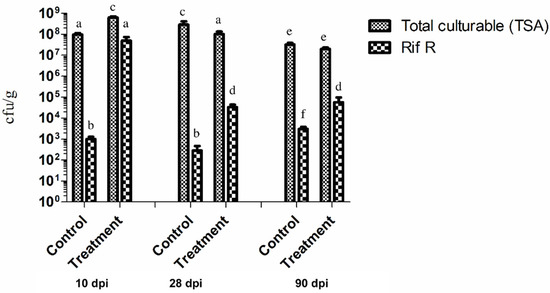
Figure 2.
Rice root colonization by P. chlororaphis ST9 was evaluated at three time points: 10, 28 and 90 dpi (days post inoculation). The average of the cfu/g of fresh weight root of 10 plants for each treatment and time point is presented. Each sample was plated on TSA to count all cultivable bacteria, and on TSArif to count just the rifampicin resistant CFUs, mainly P. chlororaphis ST9. Statistical analysis: a–f letters indicate statistical differences: same letter means no significant difference (t-test, non-parametric, Mann–Whitney, p-value < 0.05).
The possible effects of strain ST9 colonization on the rhizosphere microbial diversity were also investigated. 16S-rRNA community profiling was used to compare the microbial root community at 28 and 90 dpi in ST9 treated and untreated plants. The richness and diversity values of the bacterial communities after normalization are shown in Figure 3. The Pseudomonas genus was significantly more abundant (p-value < 0.001) in the P. chlororaphis ST9 inoculated plants as presented in Figure 3A. The presence of P. chlororaphis ST9 was confirmed by finding its 16S V3–V4 region sequence among the reads obtained by the NGS experiment. Significant differences (p-value = 0.000086) in Shannon alpha diversity (Figure 3B) were observed between inoculated and un-inoculated samples either at 28 or 90 dpi. Beta diversity analysis based on Bray Curtis distance (Figure 3C) was performed to compare the microbial community compositions of the two different tested conditions. Results highlighted differences of the microbial populations in samples that received different treatments. When P. chlororaphis was inoculated, several genera resulted differently distributed as evidenced by the heatmap (Figure 4 and in Supplementary Figures S3 and S4). Among the genera more abundant in untreated samples were Duganella, Clostridium, Aeromonas, Enterobacter and Vogesella; the majority of them are known to be animal/human pathogens, while only a few species, such as Clostridium puniceum [43] and Enterobacter cloacae [44], are correlated with plant diseases. On the other hand, genera such as Janthinobacterium, known for its antifungal features [45], Flavobacterium, often reported as plant growth-promoting rhizobacterium [46], Bacillus, Paenibacillus, Bradyrhizobium and others known for their PGP potential [47,48,49,50,51,52] were significantly more abundant in the P. chlororaphis ST9 treated communities.
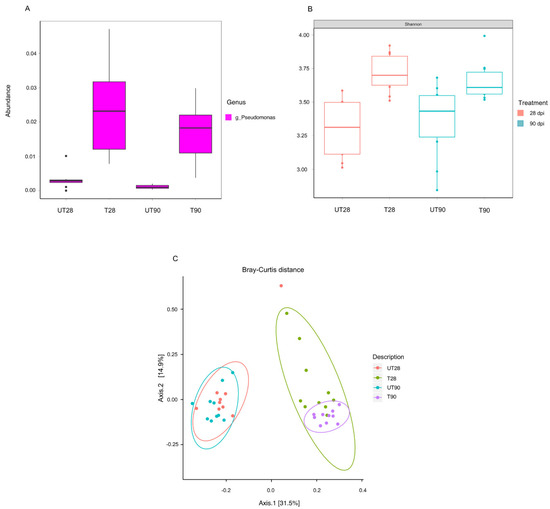
Figure 3.
Microbiota analysis. (A) Pseudomonas genus abundance according to the plant treatment and time point; the Welch two sample t-test was used to compare untreated versus treated samples at 28 dpi (p-value = 0.0006793) and at 90 dpi (p-value = 0.0003417). (B) Alpha diversity (Shannon index) at community level in accordance with the treatment and the time point; the Wilcoxon rank sum test was used, p-value for untreated versus treated samples both at 28 and 90 dpi results < 0.01. (C) Principal component analysis of the samples in accordance to the treatment and the time point, similarity between the different communities was evaluated using the Bray–Curtis test; the bacterial communities of the untreated plants cluster together and differently from the bacterial communities colonizing the ST9 treated samples (p-value < 0.001).UT28: untreated samples at 28 dpi; T28: ST9 treated samples at 28 dpi; UT90: untreated samples at 90 dpi; T90: ST9 treated samples at 90 dpi.
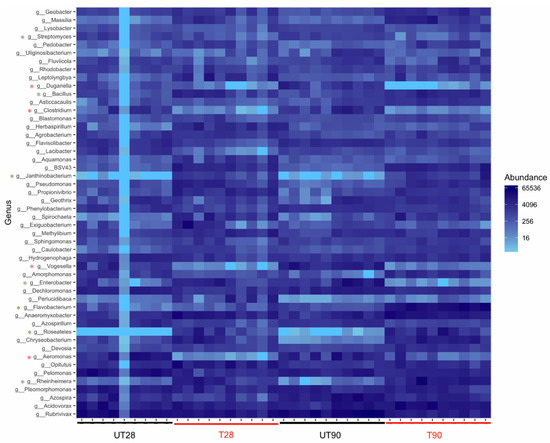
Figure 4.
Heatmap showing the relative abundance (% of sequencing reads) of the 50 predominant genera. Rows are bacterial genera. Columns are samples. Colors indicate taxa with a higher (blue) or lower (light blue) relative abundance in each sample. Genera showing a different distribution and abundance level between the samples are highlighted with *: green if enriched in the treated samples, red if enriched in the untreated ones. UT28: untreated samples at 28 dpi; T28: ST9 treated samples at 28 dpi; UT90: untreated samples at 90 dpi; T90: ST9 treated samples at 90 d.
2.3. Plant Gene Expression and Phenotypic Analysis of P. chlororaphis Inoculated Plants
In order to determine the effects of P. chlororaphis ST9 on plant growth, several phenotypic parameters were assayed. Statistical analyses were carried out on chlorophyll, flavonoid content and nitrogen balance index (NBI) as physiological parameters. In addition, plant height and dry shoot biomass at 90 dpi (after the flowering stage) were also established. Results did not show any statistical differences between control and ST9 inoculated plants [p(F) < 0.05] (Figure 5), although a tendency on higher NBI and lower flavonoid contents was measured in inoculated plants compared with the control. It was concluded that under the conditions tested, no significant plant beneficial effect was observed upon seed inoculation of P. chlororaphis ST9.
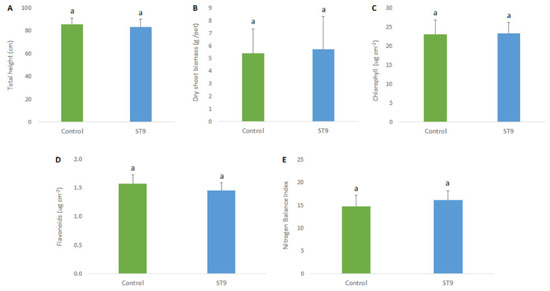
Figure 5.
Total height (A), dry shoot biomass (B), chlorophyll (C) and flavonoid content (D), and NBI (E) in control un-inoculated and in ST9 inoculated plants. a indicate statistical differences. No statistical significance was observed (t-test, JMP7 (JMP®, Version 7. SAS Institute Inc., Cary, NC, USA, 1989-202).
In order to investigate the effect on PGPR beneficial plant-bacteria interactions, RT-qPCR was performed on 14 genes selected for their role in ethylene and auxin pathways (Table 3).

Table 3.
List of the genes considered for plant gene expression.
In Supplementary Table S2 the fold change was shown for genes that were significantly and not significantly differentially expressed, while in Figure 6 the heat map representation of the transcript levels coupled to a hierarchical clustering in ST9 inoculated plants is presented. At T2, significant differences in the expression of some loci were evidenced when compared to the other two time points. The most up- and down-regulated genes at T2 were OsETR3 and Osmetallothionein, respectively. At T1, except for Osmetallothionein, there were no significantly differentially regulated genes. At T3 the most up-regulated gene was OsIAA14, while OsERF3 was the most down-regulated. The only genes that were never significantly differentially expressed were OsIAA11 and OsIAA4. The PCA on ΔCT data of control (un-inoculated) and ST9 inoculated samples for each replicate and time point showed, without outlier observations, a clear distinction between control and inoculated samples at T2, where we have found the highest number of differently regulated genes (Figure 7). On the other hand, there was no separation between data at T1 and at T3, where we have found a reduced number of regulated genes. In summary, several loci displayed different levels of gene expression upon inoculation with strain ST9; one gene was regulated at T1, while 12 and five genes were regulated at T2 and T3, respectively.
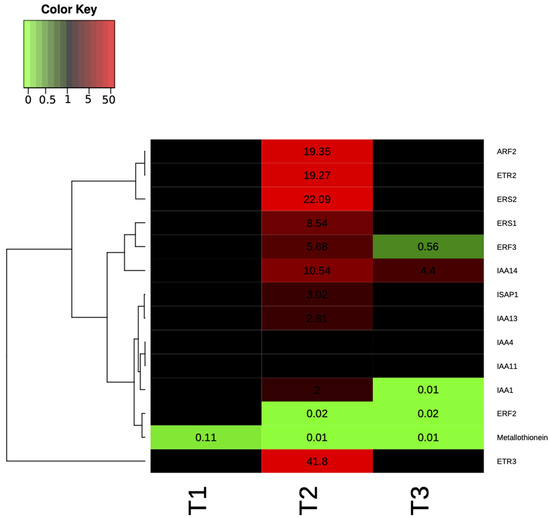
Figure 6.
Heat map representation of the transcript levels (as result of the fold change calculated following 2−ΔΔCT) coupled to a hierarchical clustering in ST9 inoculated plants. Each column represents a time point, while each row represents a gene. Expression levels are coloured green for low intensities and red for high intensities (see scale at the top left corner). The black cells represent genes not significantly different from those of the untreated samples.
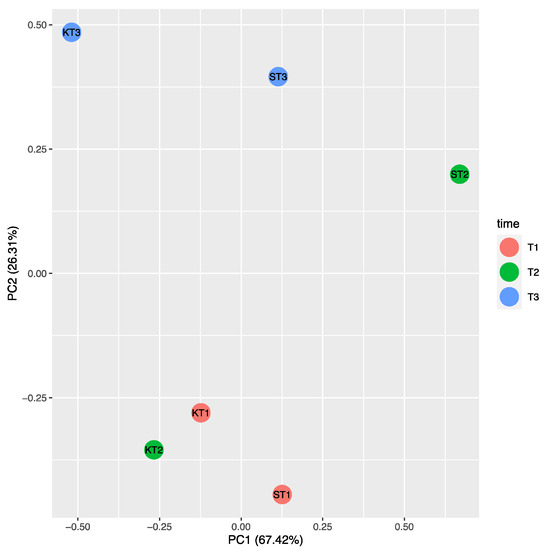
Figure 7.
PCA carried out on ΔCT of untreated control (K) and ST9 inoculated plants (S) at each time point (T1 = 10 dpi; T2 = 28 dpi; T3 = 40 dpi). The cumulative variability percentage is given by the sum of 67.42% for PC1 and 26.31% for PC2, resulting in 93.73%. KT2 and ST2 are clearly separated in the space, unlike the other time point.
3. Discussion
This study presents the identification and characterization of a new P. chlororaphis strain. The work performed included root colonization and persistence, the effect on the rhizosphere microbiota, effect on targeted plant gene expression and a greenhouse plant growth promotion test. Results showed that P. chlororaphis ST9 is an efficient rice root colonizer: when inoculated it is integrated into the plant resident-microbiota affecting the expression of several plant genes.
After inoculation, P. chlororaphis was able to persist in the root compartment for 90 days without decreasing its abundance below a concentration of 104 cfu/g of root. An initial good root colonization rate then slowly decreased in the later stages of plant growth has also been observed for other PGPR strains. For example, in Chaudhary et al. (2013) [57] Azotobacter strain ST24 colonized well the wheat rhizosphere decreasing during growth and reaching a density of 104 cfu/mL at 90 days after sowing. Similarly, Solanki and Garg (2014) [58] reported that Azotobacter on Brassica campestris persisted at 30 and 60 day post-inoculation being approximately 2 × 104 cfu per plant at 60 dpi. Mosquito et al. (2020) [59] demonstrated that a Kosakonia sp. strain inoculated in rice plants was persistent at 30 dpi, while it was undetectable at 60 and 90 dpi. The effect of strain ST9 inoculation on the total bacterial population evidenced an increase in biodiversity with enrichment of certain bacterial genera (e.g., Janthinobacterium, Flavobacterium, Bacillus, Paenibacillus, Bradyrhizobium) which are known to establish a beneficial association with plants [47,48,49,50,51,52].
Regardless of the efficient rhizosphere colonization ability, no plant beneficial effect on the rice inoculated with strain ST9 was observed under the condition tested here. In order to fully test potential PGP properties of strain ST9, more experimentation and conditions need to be tested, including challenging rice with biotic and abiotic stresses. The differences between the physiological parameters of the inoculated and uninoculated plants are not statistically significant; however, their tendency could be an indication of a decreased stress condition in ST9 inoculated plants. A similar trend was also observed by Andreozzi et al. (2019) [60] where NBI was significantly lower in the uninoculated control with respect to the Herbaspirillum huttiense RCA24 + Enterobacter cloacae RCA25 inoculated Baldo rice plants.
Gene expression studies were performed with 14 rice loci related to ethylene and auxin pathways together with genes coding for a metallothionein-like protein and a multiple stress-responsive zinc-finger protein; a role for these genes during rice-PGPR interaction has been demonstrated [53,54,56,61]. Genes OsERS1, OsERS2, OsETR2, OsETR3, homologs to Arabidopsis thaliana ethylene receptors, were transcribed at a higher level in the P. chlororaphis ST9 inoculated plants. Similarly, Vargas et al. (2012) [53] also observed an increase in expression of these loci when inoculated with Azospirillum brasilense and Burkholderia kururiensis. In their study, rice varieties having more BNF (biological nitrogen fixation) capacities showed higher bacterial colonization as well as up-regulation of ethylene receptors genes. In our study, these loci were significantly up-regulated at 28 dpi indicating that the Baldo rice variety displays good nitrogen-fixing capacity as previously reported [60]. The OsERF2 and OsERF3 genes, encoding for transcriptional factors related to ethylene, were also differentially expressed in ST9 inoculated plants indicating that this strain could be involved in the regulation of ethylene hormone levels. Indole-3-acetic acid (IAA) pathway modulation is important during an effective plant colonization, both by pathogenic and by nonpathogenic organisms [56]. In P. chlororaphis inoculated plants it was established that loci OsIAA1, OsIAA13 and OslAA14 were up-regulated at T2, OsIAA1 was down-regulated at T3, while OsIAA11 and OsIAA4 were not differently transcribed in the three considered time points. Some of these trends are in accordance and others in contrast with previous observations [56,61]; this could be due to the different timing and experimental conditions applied. Expression of genes associated with defense can be also affected by bacterial presence in the rhizosphere [56]. OsISAP1, encoding for a multiple stress-responsive zinc-finger protein, was up-regulated at T2, suggesting an activation of the plant defense mechanisms. The gene coding for a metallothionein, which is a metal-binding protein involved in metal homeostasis, was always down-regulated at each time point in agreement with previous data [56]. The plant defense system could be primed by P. chlororaphis ST9 maintaining a low level of stress; future studies on the response to biotic stress of strain ST9 inoculated plant are needed in order to determine whether the changes in the expression levels of these loci provides immunity against microbial pathogens.
4. Material and Methods
4.1. Strain Isolation, Growth and Identification
It was of interest to identify new bacterial isolates with PGP potential. Strains were recovered from one gram of uncultivated bulk soil from two distinct but very close sites, located in Padriciano, Trieste, Italy (45°39′32″ N, 13°50′28″ E). They were resuspended in 5 mL of PBS (phosphate buffer solution) and serial dilutions were performed and plated on 1/6 Tryptic Soy medium (BD, Sparks, MD, USA), solidified with 1.5% agar. Plates were incubated at 28 °C for 72 h and colonies were counted: the amount of colony-forming units of bacteria in both sites was of 1.3 × 105 cfu/gr. Approximately 150 isolates showing distinct colony morphology (color and texture), were isolated through further streaking and also characterized for some biochemical features (KOH, catalase and oxidase activities). Bacteria showing different morphological and biochemical profiles were stored individually at −80 °C constituting a bacterial collection of 63 isolates. In vitro testing was then performed for P solubilization, IAA production and antagonistic activity against the plant pathogen Dickeya zeae. Sixteen isolates having at least 2 independent in vitro PGP-related phenotypes were identified by 16S rRNA sequencing (data not shown): briefly, primers fD1 and rR2 were used for the amplification of the 16S rRNA gene and primers 518F and 800R (Supplementary Table S1) were used for the sequencing control (Eurofins, Ebersberg, Germany). P. chlororaphis ST9 was one of these isolates and it was chosen for the rice inoculation experiments based on the activities observed in the primary screening and for the literature on its PGP and biocontrol (BC) potential.
4.2. In Vitro Phenotypic Characterization
The presence of lipolytic and proteolytic activities was determined by streaking the bacterial isolates on 1/6 TSA (Tryptic Soy Agar) medium amended with 1% Glyceryl tributyrin [62] and 2% of powder milk [63], respectively. Exopolysaccharide (EPS) production was tested by streaking the bacterial isolates on yeast extract mannitol medium [64] and indole acetic acid (IAA) production was verified as described by [65] using the Salkowski reagent. The 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity was detected using M9 minimal medium with ACC as a unique N source [66], while the ability to solubilize P was verified using the NPRBB growth medium [67]. N-acyl homoserine lactone (AHL) production was assessed by T-streak technique, using the biosensor Chromobacterium violaceum CV026 after incubation for 1–2 days [68]. Motility was checked on M8 medium plates with 0.3% (swimming) or 0.5% (swarming) agar [69]. Anti-bacterial activity was tested by streaking plant pathogens Dickeya zeae and Pseudomonas fuscovaginae adjacent to a 24 h old streak of P. chlororaphis ST9 on LB medium; plates were incubated for 48 h at 28 °C and then evaluated. Antifungal activity was tested by streaking strain ST9 on TSA medium plates and placing, at 2 cm distance, a fragment of PDA agar contaminated with either Magnaporthe oryzae, or Fusarium graminearum or Aspergillus nidulans. Plates were checked after 7 days of incubation at 28 °C. Plant growth promotion traits were tested on 300 surface-sterilized rice seeds. In detail, 16 h pre-germinated seeds were submerged for 2 h in a ST9 bacterial suspension (OD600 0.5) or PBS, as control; after inoculum application seeds were rinsed with sterile water and allowed to germinate in the dark at 30 °C. One hundred seeds were evaluated for emergence after 3 days, 100 seeds were grown for 10 days and then used to measure coleoptile length and the growth of the last 100 seeds was interrupted after 12 days for dry mass weight.
4.3. Generation of a Rifampicin-Resistant Spontaneous Mutants
The P. chlororaphis ST9 strain was grown in 1/6 TS medium for 16 h at 30 °C with 100 rpm shaking. A 1/100 dilution of the overnight culture was put in 1/6 TS medium with 15 µg/mL rifampicin and the culture was grown again in the same growth conditions. The procedure was repeated, increasing the concentration of Rifampicin (25, 50 and 100 µg/mL), and the final culture was plated on 1/6 TS. One colony was chosen and streaked on TSArif100; its identity, P. chlororaphis ST9, was confirmed again by 16S rRNA sequencing.
4.4. Bacterial Genomic DNA Extraction and Sequencing
Genomic DNA extraction for genome sequencing was performed with the pronase Sarkosyl lysis method [70]. Two micrograms of DNA, quantified through Nanodrop (Thermo Scientific, Waltham, MA, USA) and checked by gel electrophoresis, were used for genome sequencing by the Exeter Sequencing Service (University of Exeter, Exeter, UK). The sequence was carried out using the Illumina technique with the Hiseq 2500 platform with the 125 base pair-paired end system. Reads were assembled using SPAdes 3.9.03 [71]. The assembled P. chlororaphis ST9 genome was uploaded in the RAST Annotation Server [34] and was automatically annotated using the RASTtk annotation scheme [72]. The genome sequence is presented as a unique contig and it is available on the RAST server https://rast.nmpdr.org/?page=JobDetails&job=828746 (accessed on 21 February 2020); “guest” as login and password).
4.5. P. chlororaphis ST9 Taxonomy
Multi-locus Sequence Analysis (MLSA) was performed using 5 housekeeping genes: recA (recombinase A), gyrB (DNA gyrase subunit B), rpoD (RNA polymerase sigma factor), carA (Carbamoyl-phosphate synthase small chain) and atpD (ATP synthase subunit beta), plus the 16S rRNA locus. Sequences of these loci were obtained from the ST9 genome sequence. For the phylogenetic analysis, we concatenated the gene sequences in the following order: 16S-recA-gyrB-rpoD-carA-atpD resulting in a single sequence. For the same genes, orthologue sequences from 16 P. chlororaphis species were obtained from the NCBI database and chained. The used sequences are reported in Supplementary Materials 1. As an outgroup, we used the orthologue concatenate from Pseudomonas putida KT2440 and P. fluorescens Pf-01. The phylogenetic analysis was performed using the NGPhylogeny.fr public platform [73].
4.6. Plant Inoculation
Seeds of O. sativa L. cv. Baldo were surface-sterilized with 50% sodium hypochlorite solution (commercial bleach) for 60 min, rinsed with sterile water and incubated in a wet dark environment for seven days at 30 °C for germination. Two growth conditions (treatments) were considered: with and without the ST9 inoculation. The roots of 70 seedlings were soaked for 60 min in an ST9 RifR bacterial solution at 0.5 of OD600 (treated or inoculated plants), while the control was performed soaking the roots of 70 seedlings in a sterile PBS solution (untreated or un-inoculated plants). The inoculated and the control untreated seedlings were transferred to plastic tubes containing 0.4% water-agar and Hoagland solution for 72 h and then transplanted into pots in the greenhouse.
4.7. Greenhouse Experiments
For each of the two treatments, fourteen plastic pots (23 cm × 21 cm) were used, filled with non-sterile paddy field soil (47.8% sand, 9.4% clay, 42.8% silt, pH 6.4, organic matter 1.45%) taken from the experimental rice field of CREA-CI in Vercelli (VC, Italy). Pots were placed in the greenhouse under uncontrolled temperature, light and humidity parameters following the natural season trend from June to September 2019. In each pot, five seedlings, undergoing the same treatment, were sown. Plants were watered every day with tap water and kept in a greenhouse for 90 days, following the natural photoperiod. Four time points were considered: T1, 10 days post-inoculation (dpi); T2, 28 dpi; T3, 40 dpi and T4, 90 dpi. At T1, T2 and T3 ten plants per treatment were sampled, washed and stored at −80 °C for gene expression analysis. At T1, T2 and T4 ten plants per treatment were sampled and utilized for bacterial counting and microbiota analysis. A timeline of the experiment is presented in Supplementary Figure S5. The remaining 20 plants were used for physiological and morphological evaluations. In particular, nitrogen balance index (NBI), an indicator of the plant nitrogen status at the beginning of the flowering stage [74] was calculated as the ratio between chlorophyll (CHL) and flavonoid (FLA) concentration recorded by the DUALEX 4 Scientific (Dx4) chlorophyll meter (Force-A, Paris, France) [75]. Measurements were carried out on both adaxial and abaxial faces of the panicle leaf for each plant. The total height was also measured for each plant as well as the dry weight of shoots (obtained after 48 h at 65 °C).
4.8. Colonization Counts
The roots of 10 rice plants for each treatment at each time point were cleaned from the soil and washed thoroughly under tap water before processing them. For T1, complete roots were macerated, while for T2 and T4, 600 mg of root samples were macerated and resuspended in 2 mL of PBS. Serial dilutions, up to 10−5, were made and 100 μL of each dilution was plated in triplicate on TSA (Tryptic soy agar) and TSA with rifampicin (50 µg/L). Plates were incubated at 28 °C for 48 h and the emerged colonies were counted considering the dilution factor and the starting material weight in order to determine the number of total cultivable CFUs (colony forming units) and of rifampicin-resistant CFUs present in 1 g of roots.
4.9. In Vivo Biocontrol Activity of P. chlororaphis ST9 against Dickeya Zeae
Baldo rice seeds were surfaced sterilized and germinated for one week. Thirty seedling roots were submerged in PBS (control plants) while the other 30 were submerged in an ST9 bacterial suspension (OD600 = 0.5) for 60 min (ST9 treated plants). Seedlings were transplanted in soil (five seedlings per pot) and one week after transplantation 1 mL of an ST9 bacterial suspension (OD600 = 0.3) was inoculated directly into the soil, near the stem emergence site, of the already ST9 treated plants as a second inoculum. Twenty days after transplantation, 1 μL of D. zeae bacterial suspension OD600 = 0.3 was injected, at the base of the stem, in the control and ST9 treated plants. 10 plants of each treatment were used as control and injected with PBS. Seven days after infection lesions were scored. Lesions assume diverse phenotypes: very dark for few centimeters, light brown for longer fragment or discontinuous blackening. A Disease Severity Score Scale was set and a Disease Severity Score (DSS) was scored for each plant by 7 different persons.
4.10. Bacterial Genomic DNA Extraction, 16S rRNA Gene Amplicon Library Preparation and Sequencing
Five hundred mg of root samples from 10 ST9-treated and untreated plants at T2 and T4 time points were used to extract DNA and perform microbiome analysis. DNA was extracted using the PowerSoil Kit (Qiagen, Hilden, Germany) following supplier instructions. For the 16S amplicon libraries preparation, 12.5 ng of DNA, quantified using Nanodrop, was used for each sample to prepare the 16S rRNA amplicon libraries. Library preparation was done using the Illumina methodology following the 16S Metagenomic Sequencing Library Preparation protocol (https://emea.support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf, accessed on 17 July 2021). Sequencing was performed at CBM scrl (Trieste, Italy) with MiSeq sequencing platform.
4.11. Microbiome Sequence Analysis
FASTQ files were demultiplexed using the QIIME 1.9.1 split_libraries_fastq.py script and then analyzed using DADA2 v1.4.0 [76] adapting the methods from the DADA2 Pipeline Tutorial (1.4) and including dereplication, singletons and chimera removing (sample inference was performed using the inferred error model, while chimeric sequences were removed using the removeBimeraDenovo function). The Greengenes (GG) database [77], giving a final Operational Taxonomic Unit (OTU) table, was employed to assign bacterial taxonomy using the assign Taxonomy function with a 97% sequence similarity. The resulting OTUs were clustered at genus taxonomic level obtaining the final profile and abundance of bacterial taxa in the different samples. Statistical analysis was performed using the vegan package version 2.5–4 [78] and phyloseq package [79] in R version 3.5.2 [80]. Relative abundances of OTUs between samples were calculated. To test the differential representation of microbial taxa in diverse samples the Deseq2 package [81] was used.
4.12. RNA Extraction and cDNA Conversion
The RNA from three biological replicates for each thesis at each time point was extracted using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. After the RNA extraction, any amount of DNA was removed using DNase (RQ1 RNase-Free DNase, Promega, Madison, WI, USA) and measured using Qubit (Invitrogen, ThermoFisher Scientific, Waltham, MA, USA). The absence of genomic DNA was verified through PCR on RNA with the primers for the reference gene [55]. Total RNA was used for each sample to synthesize the cDNA, according to the SuperScript II Reverse Transcriptase (Invitrogen, ThermoFisher Scientific, Waltham, MA, USA) procedure using random primers.
4.13. Primer Selection
The genes analyzed in this study (Table 3) were selected based on their regulation and role during the interaction between PGPR and rice. In particular, in addition to gene coding for a metallothionein—like protein and a multiple stress-responsive zinc-finger protein [56], genes involved in ethylene and auxin pathways were considered [53,54,56]. Before RT-qPCR, all primers (Supplementary Table S1) were tested in silico on PRIMERBLAST and in PCR reactions on genomic DNA extracted from Baldo rice. The DNA extraction was performed using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
4.14. Gene Expression Analysis
Quantitative RT-PCR was carried out with 7500 Fast Real Time Systems (Applied Biosystem, ThermoFisher Scientific, Waltham, MA, USA). Each PCR reaction was conducted on a total volume of 10 μL, containing 1 μL cDNA, 5 μL SYBR Green Reaction Mix and 0.3 µL of each primer (10 μM) using a 96-well plate. The used primers are listed in ST1. The following PCR program, which includes the calculation of a melting curve, was used: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. All the reactions were performed for three biological and technical replicates. The baseline range and CT (cycle threshold) values were automatically calculated using the 7500 Fast Real Time Systems software. In order to compare data from different PCR runs or cDNA samples, the CT values of all the genes were normalized to the CT value of OsACT1, the reference gene. The candidate gene expression was normalized to that of the reference gene by subtracting the Ct value of the reference gene from the Ct value of the candidate gene efficiency correction, from the equation 2−ΔΔCT [82], where ΔΔCT represents the ΔCT sample−ΔCT control.
4.15. Data Analysis
The statistical analysis of the phenotyping data was done using JMP7 (SAS Institute Inc., Cary, NC, USA, 1989-2019). Concerning gene expression, statistical analyses were carried out using REST 2009, version 2.0.13 (Qiagen, Hilden, Germany) [83], considering 0.05 as the p-value. Only significant expression values were considered and visualized as heat maps by a custom R (version 3.6.3) script (command “heatmap.2”). In order to reduce the data set dimension, a PCA (Principal Component Analysis) was carried out on ΔCT data for each biological replicate through R (version 3.6.3) (CRAN package “ggfortify”). The input files for R analysis were tabular filed in.csv including ID# genes and ID# samples.
5. Conclusions
Inoculated PGPR must interact or compete with other microorganisms in the rhizosphere microbiome and this can cause the short persistence of the inoculated bacteria possibly affecting its probiotic effects [2]. The ability of P. chlororaphis ST9 to successfully colonize and persist in the rhizosphere is an important trait for its possible use as bioinoculant for agriculture. Furthermore, the plethora of secondary metabolites and antimicrobial activities encoded in its genome and a biocontrol test against D. zeae infection make P. chlororaphis ST9 a potential candidate for biotic stress tolerance tests upon its inoculation. Plant gene expression analysis demonstrated that the presence of P. chlororaphis ST9 positively affects the expression of some plant hormonal pathways such as IAA and ethylene; further investigation should be carried out on rice defense genes in order to better clarify the type of interaction. P. chlororaphis strains are considered safe for the environment and human health (EPA, 2009) and their use in agriculture has been permitted through the application of live microorganism formulations [84,85] and via the production and purification of metabolites [35,86]. Future experiments with P. chlororaphis ST9 will reveal its full potential as a bioinoculant for PGP and its possible role in tolerance to abiotic and biotic stresses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10071466/s1, Figure S1: Phylogenetic tree reconstructed by the MSLA method, Figure S2: In vitro antimicrobial activity of P. chlororaphis ST9Phylogenetic tree reconstructed by the MSLA method, Figure S3: Differential representation of OTUs between ST9 inoculated and control samples at 28 dpi, Figure S4: Differential representation of OTUs between ST9 inoculated and control samples at 90 dpi, Figure S5: Time line of the sampling and use of the samples, Table S1: List of the primers used in this study, Table S2: Fold change at each time point, Supplementary Material 1: Sequences used for the taxonomic analysis of P. chlororaphys ST9.
Author Contributions
V.V., S.M., I.B. and E.Z. contributed in the conceptualization of the work; I.B. and E.Z. performed experiments; C.B. and A.V. analyzed the data and prepared figures; I.B., E.Z., V.V. and S.M. were involved in the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Risobiosystems project (DM n. 94667, MiPAAF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The genome sequence is presented as a unique contig and it is available on the RAST server (https://rast.nmpdr.org/?page=JobDetails&job=828746 (accessed 21 February 2020); “guest” as login and password).
Acknowledgments
Authors thank F. Sillo for his help in heatmap generation and in PCA analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol. 2014, 201, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Glick, B.R.; Pasternak, J. Plant-microbial interaction under gnotobiotic conditions: A scanning electron microscope study. Curr. Microbiol. 1991, 23, 111–114. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Mehnaz, S. An overview of globally available bioformulations. Bioformulations Sustain. Agric. 2016, 267–281. [Google Scholar] [CrossRef]
- Hazra, K.; Swain, D.; Bohra, A.; Singh, S.; Kumar, N.; Nath, C. Organic rice: Potential production strategies, challenges and prospects. Org. Agric. 2018, 8, 39–56. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 140682. [Google Scholar] [CrossRef] [PubMed]
- Biessy, A.; Novinscak, A.; Blom, J.; Léger, G.; Thomashow, L.S.; Cazorla, F.M.; Josic, D.; Filion, M. Diversity of phytobeneficial traits revealed by whole-genome analysis of worldwide-isolated phenazine-producing Pseudomonas Spp. Environ. Microbiol. 2019, 21, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Burr, S.E.; Gobeli, S.; Kuhnert, P.; Goldschmidt-Clermont, E.; Frey, J. Pseudomonas chlororaphis subsp. piscium subsp. nov., isolated from freshwater fish. Int. J. Syst. Evol. Microbiol. 2010, 60, 2753–2757. [Google Scholar] [CrossRef][Green Version]
- Peix, A.; Valverde, A.; Rivas, R.; Igual, J.M.; Ramirez-Bahena, M.-H.; Mateos, P.F.; Santa-Regina, I.; Rodriguez-Barrueco, C.; Martínez-Molina, E.; Velázquez, E. Reclassification of Pseudomonas aurantiaca as a synonym of Pseudomonas chlororaphis and proposal of three subspecies, P. chlororaphis subsp. chlororaphis subsp. nov., P. chlororaphis subsp. aureofaciens subsp. nov., comb. nov. and P. chlororaphis subsp. aurantiaca subsp. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1286–1290. [Google Scholar]
- Anderson, A.J.; Kim, Y.C. Insights into plant-beneficial traits of probiotic Pseudomonas chlororaphis isolates. J. Med. Microbiol. 2020, 69, 361–371. [Google Scholar] [CrossRef]
- Arrebola, E.; Tienda, S.; Vida, C.; De Vicente, A.; Cazorla, F.M. Fitness features involved in the biocontrol interaction of Pseudomonas chlororaphis with host plants: The case study of PcPCL1606. Front. Microbiol. 2019, 10, 719. [Google Scholar] [CrossRef]
- Zhao, L.F.; Xu, Y.J.; Ma, Z.Q.; Deng, Z.S.; Shan, C.J.; Wei, G.H. Colonization and plant growth promoting characterization of endophytic Pseudomonas chlororaphis strain Zong1 isolated from Sophora alopecuroides root nodules. Braz. J. Microbiol. 2013, 44, 629–637. [Google Scholar] [CrossRef]
- Arrebola, E.; Cazorla, F.M. Aer receptors influence the Pseudomonas chlororaphis PCL1606 lifestyle. Front. Microbiol. 2020, 11, 1560. [Google Scholar] [CrossRef]
- Calderón, C.E.; Pérez-García, A.; de Vicente, A.; Cazorla, F.M. The dar genes of Pseudomonas chlororaphis PCL1606 are crucial for biocontrol activity via production of the antifungal compound 2-hexyl, 5-propyl resorcinol. Mol. Plant Microbe Interact. 2013, 26, 554–565. [Google Scholar] [CrossRef]
- Ahemad, M. Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: A review. 3 Biotech 2015, 5, 111–121. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.R.; Yang, K.Y.; Cho, B.H.; Han, T.H.; Kim, I.S.; Lee, M.C.; Anderson, A.J.; Kim, Y.C. Production of indole-3-acetic acid in the plant-beneficial strain Pseudomonas chlororaphis O6 is negatively regulated by the global sensor kinase GacS. Curr. Microbiol. 2006, 52, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Kim, J.S.; Park, J.Y.; Han, S.H.; Dimkpa, C.; Anderson, A.J.; Kim, Y.C. The RpoS sigma factor negatively regulates production of IAA and siderophore in a biocontrol rhizobacterium, Pseudomonas chlororaphis O6. Plant Pathol. J. 2013, 29, 323. [Google Scholar] [CrossRef]
- Chin-A-Woeng, T.F.; Bloemberg, G.V.; van der Bij, A.J.; van der Drift, K.M.; Schripsema, J.; Kroon, B.; Scheffer, R.J.; Keel, C.; Bakker, P.A.; Tichy, H.-V. Biocontrol by phenazine-1-carboxamide-producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporum f. sp. radicis-lycopersici. Mol. Plant Microbe Interact. 1998, 11, 1069–1077. [Google Scholar] [CrossRef]
- Hu, W.; Gao, Q.; Hamada, M.S.; Dawood, D.H.; Zheng, J.; Chen, Y.; Ma, Z. Potential of Pseudomonas chlororaphis subsp. aurantiaca strain Pcho10 as a biocontrol agent against Fusarium graminearum. Phytopathology 2014, 104, 1289–1297. [Google Scholar] [CrossRef]
- Spence, C.A.; Raman, V.; Donofrio, N.M.; Bais, H.P. Global gene expression in rice blast pathogen Magnaporthe oryzae treated with a natural rice soil isolate. Planta 2014, 239, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, Y.; Jiang, H.; Peng, H.; Huang, X.; Zhang, X.; Thomashow, L.S.; Xu, Y. Characterization of a phenazine-producing strain Pseudomonas chlororaphis GP72 with broad-spectrum antifungal activity from green pepper rhizosphere. Curr. Microbiol. 2007, 54, 302–306. [Google Scholar] [CrossRef]
- Tagele, S.B.; Lee, H.G.; Kim, S.W.; Lee, Y.S. Phenazine and 1-undecene producing Pseudomonas chlororaphis subsp. aurantiaca strain KNU17Pc1 for growth promotion and disease suppression in Korean maize cultivars. J. Microbiol. Biotechnol. 2019, 29, 66–78. [Google Scholar] [CrossRef]
- Zhang, L.; Khabbaz, S.; Wang, A.; Li, H.; Abbasi, P. Detection and characterization of broad-spectrum antipathogen activity of novel rhizobacterial isolates and suppression of Fusarium crown and root rot disease of tomato. J. Appl. Microbiol. 2015, 118, 685–703. [Google Scholar] [CrossRef]
- Nam, H.S.; Anderson, A.J.; Kim, Y.C. Biocontrol efficacy of formulated Pseudomonas chlororaphis O6 against plant diseases and root-knot nematodes. Plant Pathol. J. 2018, 34, 241. [Google Scholar] [CrossRef] [PubMed]
- Hilario, E.; Buckley, T.R.; Young, J.M. Improved resolution on the phylogenetic relationships among Pseudomonas by the combined analysis of atp D, car A, rec A and 16S rDNA. Antonie Van Leeuwenhoek 2004, 86, 51–64. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Liu, K.; Hu, H.; Wang, W.; Zhang, X. Genetic engineering of Pseudomonas chlororaphis GP72 for the enhanced production of 2-hydroxyphenazine. Microb. Cell Factories 2016, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dorosky, R.J.; Yu, J.M.; Pierson, L.S.; Pierson, E.A. Pseudomonas chlororaphis produces two distinct R-tailocins that contribute to bacterial competition in biofilms and on roots. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Nandi, M.; Selin, C.; Brassinga, A.K.C.; Belmonte, M.F.; Fernando, W.D.; Loewen, P.C.; De Kievit, T.R. Pyrrolnitrin and hydrogen cyanide production by Pseudomonas chlororaphis strain PA23 exhibits nematicidal and repellent activity against Caenorhabditis elegans. PLoS ONE 2015, 10, e0123184. [Google Scholar] [CrossRef]
- Zhu, X.; Van Pee, K.-H.; Naismith, J.H. The ternary complex of PrnB (the second enzyme in the pyrrolnitrin biosynthesis pathway), tryptophan, and cyanide yields new mechanistic insights into the indolamine dioxygenase superfamily. J. Biol. Chem. 2010, 285, 21126–21133. [Google Scholar] [CrossRef]
- Cazorla, F.M.; Duckett, S.B.; Bergström, E.T.; Noreen, S.; Odijk, R.; Lugtenberg, B.J.; Thomas-Oates, J.E.; Bloemberg, G.V. Biocontrol of avocado dematophora root rot by antagonistic Pseudomonas fluorescens PCL1606 correlates with the production of 2-hexyl 5-propyl resorcinol. Mol. Plant Microbe Interact. 2006, 19, 418–428. [Google Scholar] [CrossRef]
- Shah, N.; Gislason, A.S.; Becker, M.; Belmonte, M.F.; Fernando, W.D.; de Kievit, T.R. Investigation of the quorum-sensing regulon of the biocontrol bacterium Pseudomonas chlororaphis strain PA23. PLoS ONE 2020, 15, e0226232. [Google Scholar]
- Cho, S.-T.; Chang, H.-H.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B.; Kuo, C.-H. Genome analysis of Pseudomonas fluorescens PCL1751: A rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS ONE 2015, 10, e0140231. [Google Scholar] [CrossRef] [PubMed]
- Flury, P.; Aellen, N.; Ruffner, B.; Péchy-Tarr, M.; Fataar, S.; Metla, Z.; Dominguez-Ferreras, A.; Bloemberg, G.; Frey, J.; Goesmann, A. Insect pathogenicity in plant-beneficial pseudomonads: Phylogenetic distribution and comparative genomics. ISME J. 2016, 10, 2527–2542. [Google Scholar] [CrossRef] [PubMed]
- Lund, B.; Brocklehurst, T.; Wyatt, G. Characterization of Strains of Clostridium puniceum sp. no v., a Pink-pigmented, Pectolytic Bacterium. Microbiology 1981, 122, 17–26. [Google Scholar] [CrossRef][Green Version]
- García-González, T.; Sáenz-Hidalgo, H.K.; Silva-Rojas, H.V.; Morales-Nieto, C.; Vancheva, T.; Koebnik, R.; Ávila-Quezada, G.D. Enterobacter cloacae, an emerging plant-pathogenic bacterium affecting chili pepper seedlings. Plant Pathol. J. 2018, 34, 1. [Google Scholar] [CrossRef] [PubMed]
- Haack, F.S.; Poehlein, A.; Kröger, C.; Voigt, C.A.; Piepenbring, M.; Bode, H.B.; Daniel, R.; Schäfer, W.; Streit, W.R. Molecular keys to the Janthinobacterium and Duganella spp. interaction with the plant pathogen Fusarium graminearum. Front. Microbiol. 2016, 7, 1668. [Google Scholar] [CrossRef] [PubMed]
- Kolton, M.; Erlacher, A.; Berg, G.; Cytryn, E. The Flavobacterium genus in the plant holobiont: Ecological, physiological, and applicative insights. In Microbial Models: From Environmental to Industrial Sustainability; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–207. [Google Scholar]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.M.; Soares, H.M.; Soares, E.V. Promising bacterial genera for agricultural practices: An insight on plant growth-promoting properties and microbial safety aspects. Sci. Total Environ. 2019, 682, 779–799. [Google Scholar] [CrossRef] [PubMed]
- Greetatorn, T.; Hashimoto, S.; Sarapat, S.; Tittabutr, P.; Boonkerd, N.; Uchiumi, T.; Teaumroong, N. Empowering rice seedling growth by endophytic Bradyrhizobium sp. SUTN 9-2. Lett. Appl. Microbiol. 2019, 68, 258–266. [Google Scholar] [CrossRef]
- Radhapriya, P.; Ramachandran, A.; Palani, P. Indigenous plant growth-promoting bacteria enhance plant growth, biomass, and nutrient uptake in degraded forest plants. 3 Biotech 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Sansinenea, E. Bacillus spp.: As plant growth-promoting bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 225–237. [Google Scholar] [CrossRef]
- Souza, R.d.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Vargas, L.; de Carvalho, T.L.G.; Ferreira, P.C.G.; Baldani, V.L.D.; Baldani, J.I.; Hemerly, A.S. Early responses of rice (Oryza sativa L.) seedlings to inoculation with beneficial diazotrophic bacteria are dependent on plant and bacterial genotypes. Plant Soil 2012, 356, 127–137. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Xiong, L. Comprehensive expression profiling analysis of OsIAA gene family in developmental processes and in response to phytohormone and stress treatments. Planta 2009, 229, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, J.; Kim, S. First report of leaf blight caused by Pantoea agglomerans on rice in Korea. Plant Dis. 2010, 94, 1372. [Google Scholar] [CrossRef] [PubMed]
- Brusamarello-Santos, L.; Pacheco, F.; Aljanabi, S.; Monteiro, R.; Cruz, L.; Baura, V.; Pedrosa, F.; Souza, E.; Wassem, R. Differential gene expression of rice roots inoculated with the diazotroph Herbaspirillum seropedicae. Plant Soil 2012, 356, 113–125. [Google Scholar] [CrossRef]
- Chaudhary, D.; Narula, N.; Sindhu, S.; Behl, R. Plant growth stimulation of wheat (Triticum aestivum L.) by inoculation of salinity tolerant Azotobacter strains. Physiol. Mol. Biol. Plants 2013, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.; Garg, F.C. The use of lacZ marker in enumeration of Azotobacter chroococcum in carrier based inoculants. Braz. J. Microbiol. 2014, 45, 595–601. [Google Scholar] [CrossRef]
- Mosquito, S.; Bertani, I.; Licastro, D.; Compant, S.; Myers, M.P.; Hinarejos, E.; Levy, A.; Venturi, V. In planta colonization and role of T6SS in two rice Kosakonia endophytes. Mol. Plant Microbe Interact. 2020, 33, 349–363. [Google Scholar] [CrossRef]
- Andreozzi, A.; Prieto, P.; Mercado-Blanco, J.; Monaco, S.; Zampieri, E.; Romano, S.; Valè, G.; Defez, R.; Bianco, C. Efficient colonization of the endophytes Herbaspirillum huttiense RCA24 and Enterobacter cloacae RCA25 influences the physiological parameters of Oryza sativa L. cv. Baldo rice. Environ. Microbiol. 2019, 21, 3489–3504. [Google Scholar] [CrossRef]
- Ambreetha, S.; Chinnadurai, C.; Marimuthu, P.; Balachandar, D. Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere 2018, 5, 57–66. [Google Scholar] [CrossRef]
- Smeltzer, M.S.; Hart, M.E.; Iandolo, J.J. Quantitative spectrophotometric assay for staphylococcal lipase. Appl. Environ. Microbiol. 1992, 58, 2815–2819. [Google Scholar] [CrossRef]
- Huber, B.; Riedel, K.; Hentzer, M.; Heydorn, A.; Gotschlich, A.; Givskov, M.; Molin, S.; Eberl, L. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 2001, 147, 2517–2528. [Google Scholar] [CrossRef]
- Zlosnik, J.E.; Hird, T.J.; Fraenkel, M.C.; Moreira, L.M.; Henry, D.A.; Speert, D.P. Differential mucoid exopolysaccharide production by members of the Burkholderia cepacia complex. J. Clin. Microbiol. 2008, 46, 1470–1473. [Google Scholar] [CrossRef] [PubMed]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Steindler, L.; Venturi, V. Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol. Lett. 2007, 266, 1–9. [Google Scholar] [CrossRef]
- Köhler, T.; Curty, L.K.; Barja, F.; Van Delden, C.; Pechère, J.-C. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 2000, 182, 5990–5996. [Google Scholar] [CrossRef]
- Better, M.; Lewis, B.; Corbin, D.; Ditta, G.; Helinski, D.R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell 1983, 35, 479–485. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny. fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. Community Ecol. Package Version 2013, 2, 1–295. [Google Scholar]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language And Environment For Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Anderson, J.A.; Staley, J.; Challender, M.; Heuton, J. Safety of Pseudomonas chlororaphis as a gene source for genetically modified crops. Transgenic Res. 2018, 27, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Rosas, S.B. Pseudomonas chlororaphis subsp. aurantiaca SR1: Isolated from rhizosphere and its return as inoculant. A review. Int. Biol. Rev. 2017, 1. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, P.; Bilal, M.; Wang, W.; Hu, H.; Zhang, X. Enhanced biosynthesis of phenazine-1-carboxamide by engineered Pseudomonas chlororaphis HT66. Microb. Cell Factories 2018, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).