Successful Cryopreservation of Dormant Buds of Blackcurrant (Ribes nigrum L.) by Using Greenhouse-Grown Plants and In Vitro Recovery
Abstract
:1. Introduction
2. Results
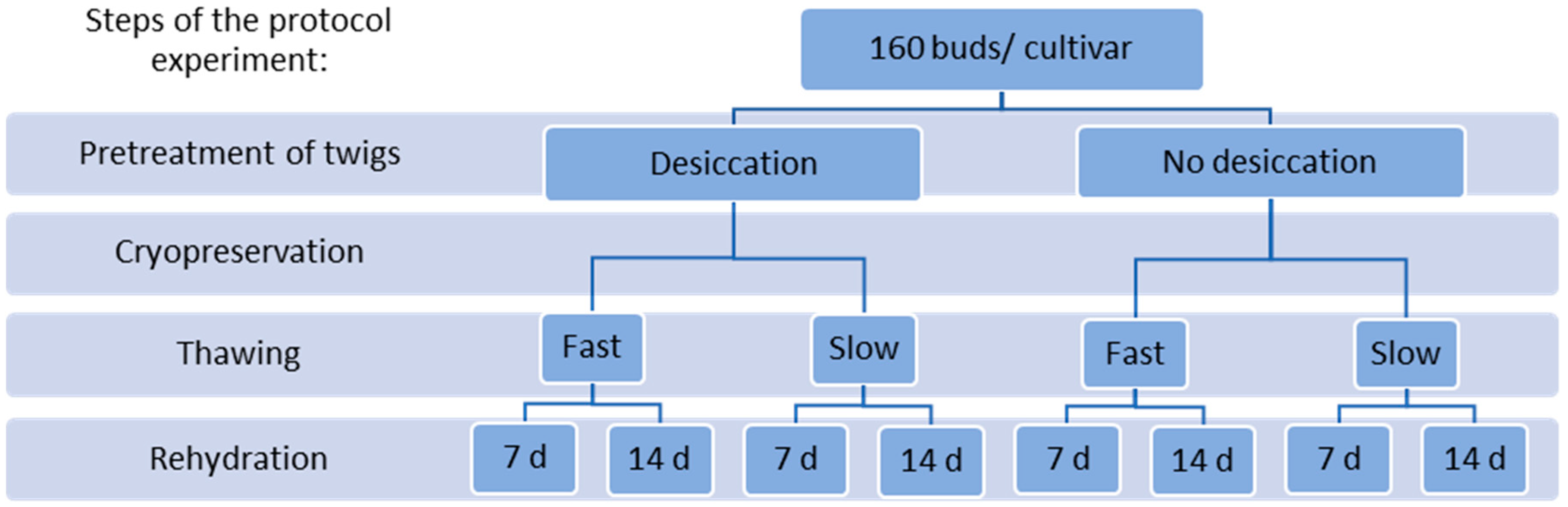
2.1. Protocol Experiments
2.1.1. Preliminary Quality Evaluation
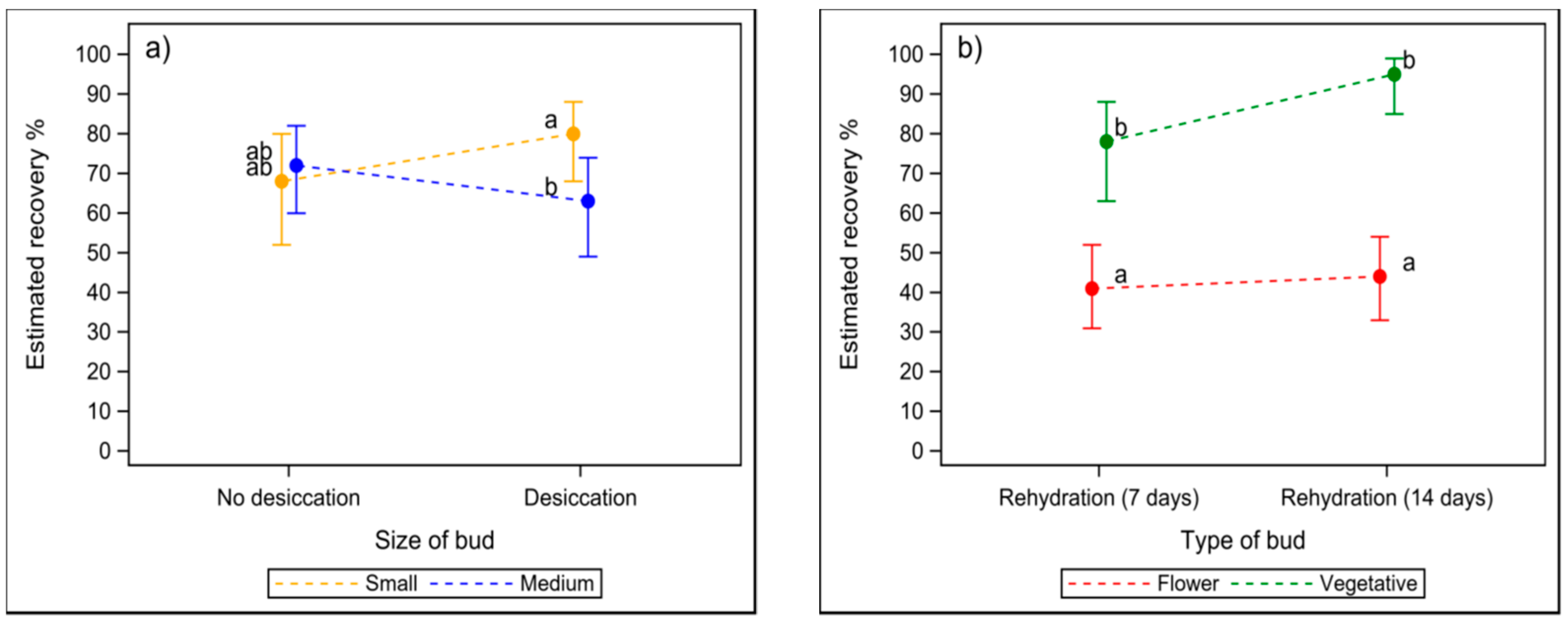
2.1.2. The Effect of Desiccation on the Recovery of Thawed Buds In Vitro
2.1.3. The Effect of Thawing Speed and Rehydration on the Recovery of Buds In Vitro
2.1.4. The Necessity of Rehydration
2.2. Viability Testing When Cryobanking a Collection of Cultivars
3. Discussion
4. Materials and Methods
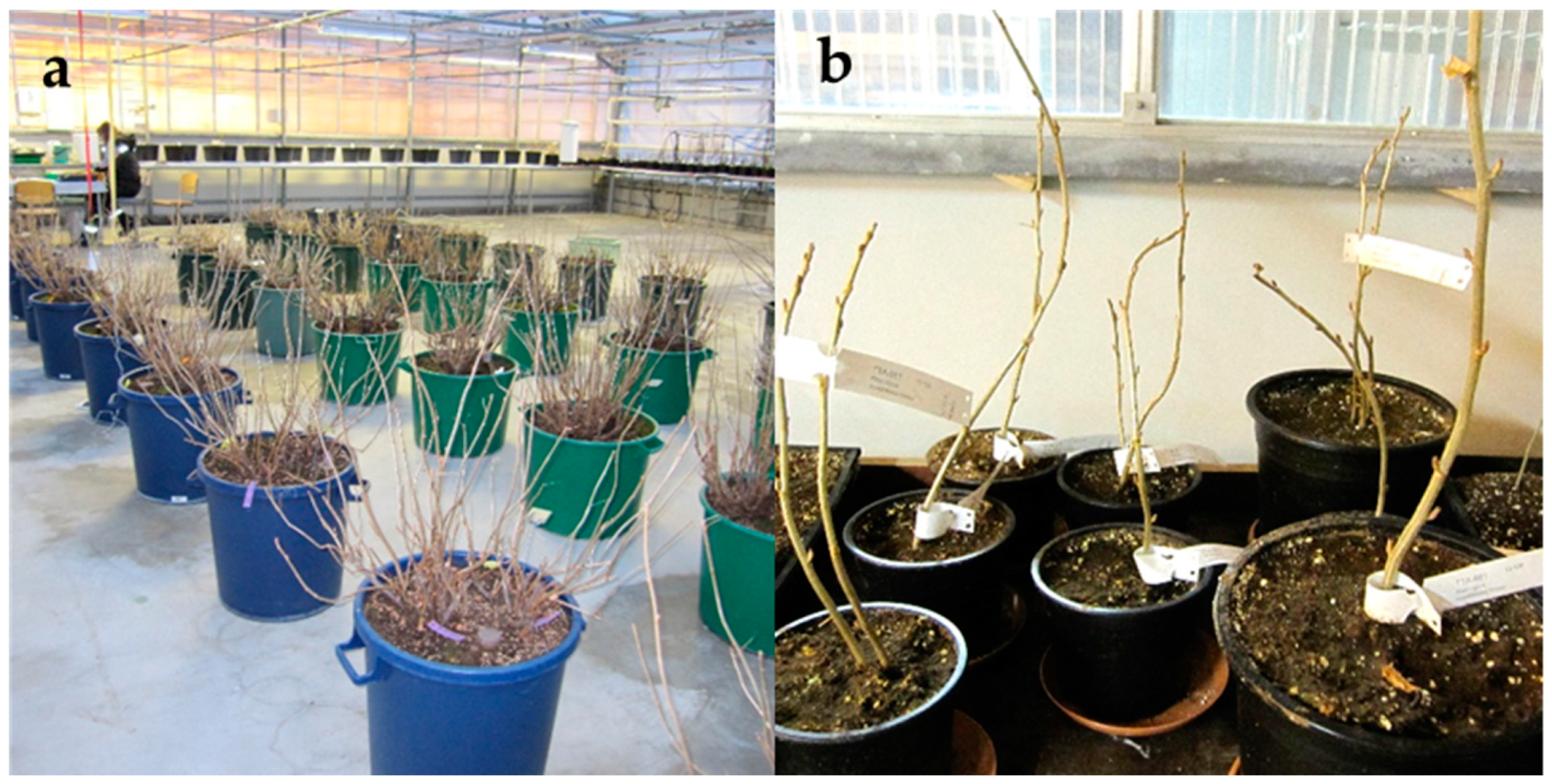
4.1. Plant Material
4.2. Collection and Handling of Bud Sections
4.2.1. Protocol Experiment
4.2.2. Cryobanking of Dormant Buds
4.3. Cooling and Cryopreservation of Bud Sections
4.4. Thawing and Rehydration of Bud Sections
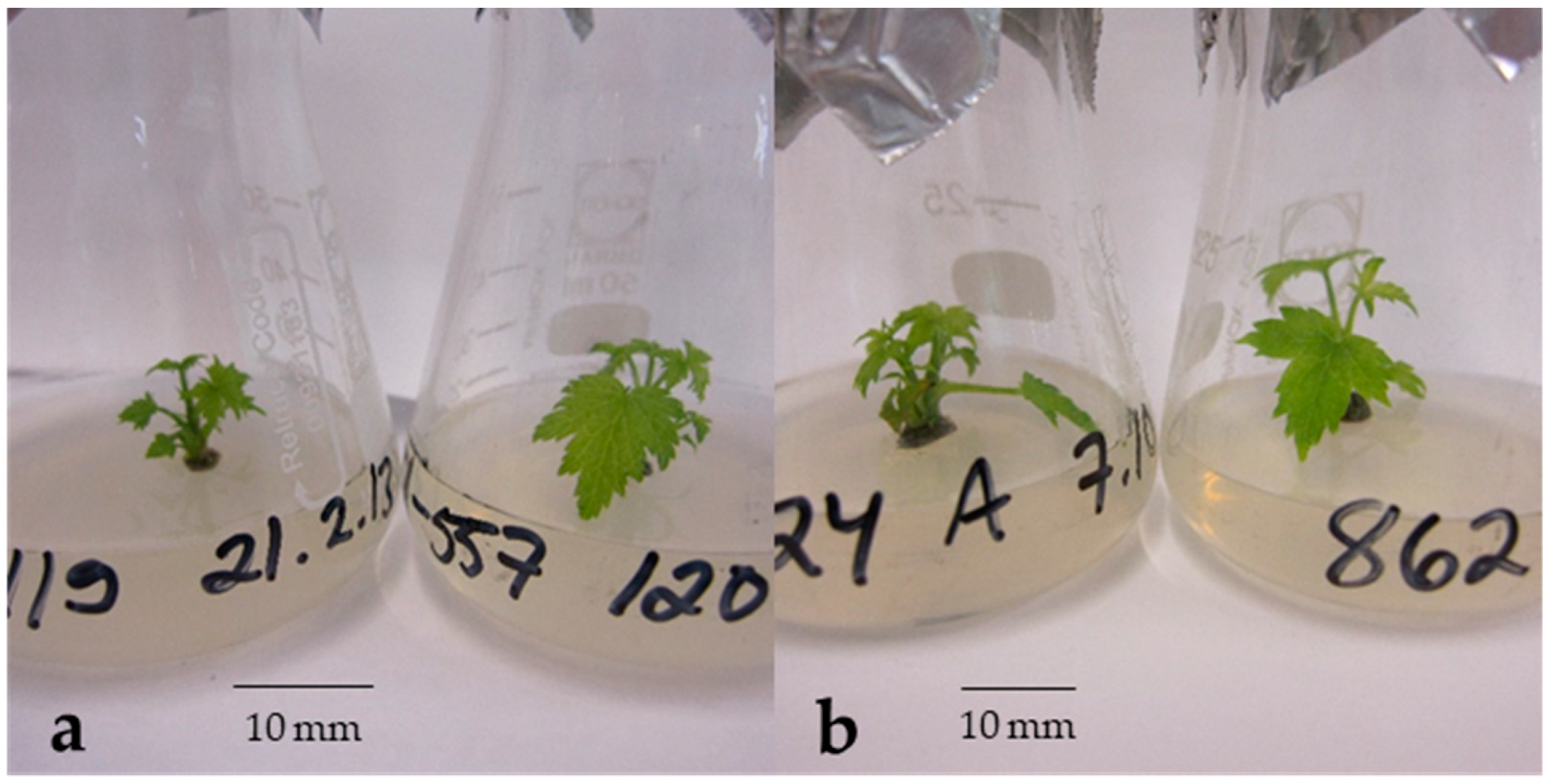
4.5. Recovery of Buds In Vitro
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2013. [Google Scholar]
- Panis, B.; Nagel, M.; Van den Houwe, I. Challenges and prospects for the conservation of crop Genetic resources in field genebanks, in in vitro collections and/or in liquid nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.E. Cryopreservation of Phytodiversity: A Critical Appraisal of Theory & Practice. Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar]
- Forsline, P.L. Procedures for collection, conservation, evaluation and documentation of Malus germplasm. Acta Hort. 2000, 522, 223–234. [Google Scholar] [CrossRef]
- Popova, E.; Shukla, M.; Kim, H.H.; Saxena, P.K. Plant Cryopreservation for Biotechnology and Breeding. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Cham, Switzerland, 2015; pp. 66–93. [Google Scholar] [CrossRef]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Kingsley, W.; Dixon, W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.-C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A.; Engelman, F. Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis. Biotechhnol. Adv. 2013, 31, 175–185. [Google Scholar] [CrossRef]
- Towill, L.E.; Ellis, D.D. Cryopreservation of Dormant Buds. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 421–441. [Google Scholar]
- Tanner, J.D.; Chen, K.Y.-C.; Bonnart, R.M.; Minas, I.S.; Volk, G.M. Considerations for large-scale implementation of dormant budwood cryopreservation. Plant Cell Tissue Organ Cult. 2020. [Google Scholar] [CrossRef]
- Sakai, A. Survival of the twig of woody plants at −196 °C. Nature 1960, 185, 392–394. [Google Scholar] [CrossRef]
- Tyler, N.J.; Stushnoff, C. The effects of prefreezing and controlled dehydration on cryopreservation of dormant vegetative apple buds. Can. J. Plant Sci. 1988, 68, 1163–1167. [Google Scholar] [CrossRef]
- Tyler, N.; Stushnoff, C. Dehydration of dormant apple buds at different stages of cold acclimation to induce cryopreservability in different cultivars. Can. J. Plant Sci. 1988, 68, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Yakuwa, H.; Oka, S. Plant regeneration through meristem culture from vegetative buds of mulberry (Morus bombycis Koidz.) stored in liquid nitrogen. Ann. Bot. 1988, 62, 79–82. [Google Scholar] [CrossRef]
- Jenderek, M.M.; Tanner, J.D.; Chao, C.T.; Blackburn, H. How applicable are dormant buds in cryopreservation of horticultural woody plant crops? The Malus case. In Proceedings of the III International Symposium on Plant Cryopreservation; International Society for Horticultural Science: Leuven, Belgium, 2019; pp. 317–322. [Google Scholar]
- Harvengt, L.; Meier-Dinkel, A.; Dumas, E.; Collin, E. Establishment of a a cryopreserved gene bank of European elms. Can. J. For. Res. 2004, 34, 43–55. [Google Scholar] [CrossRef]
- Matsumoto, T.; Niino, T.; Shirata, K.; Kurahashi, T.; Matsumoto, S.; Maki, S.; Itamura, H. Long term conservation of Diospyros germplasm using dormant buds by a prefreezing method. Plant Biotechnol. 2004, 21, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Atmakuri, A.R.; Chaudhury, R.; Malik, S.K.; Kumar, S.; Ramachandran, R.; Qadri, S.M.H. Mulberry biodiversity conservation through cryopreservation In vitro Cell. Dev. Biol. Plant 2009, 45, 639–649. [Google Scholar] [CrossRef]
- Ryynänen, L.; Jokipii, S.; Häggman, H. Controlled rate cooling of silver birch and aspen dormant buds. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 432–435. [Google Scholar]
- Towill, L.; Volk, G.; Waddel, J.; Bonnart, R.; Widrlechner, M. Cryopreservation of Willow (Salix) Dormant Buds in Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 436–437. [Google Scholar]
- Jenderek, M.M.; Ambruzs, B.D.; Holman, G.E.; Carstens, J.D.; Ellis, D.D. Salix dormant bud cryotolerance varies by taxon, harvest year, and stemsegment length. Crop Sci. 2020, 60, 1965–1973. [Google Scholar] [CrossRef]
- Tanner, J.D.; Minas, I.S.; Chen, K.Y.; Jenderek, M.M.; Wallner, S.J. Antimicrobial forcing solution improves recovery of cryopreserved temperate fruit tree dormant buds. Cryobiology 2020, 92, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Yamamoto, S.; Fukui, K.; Rafique, T.; Engelman, F.; Niino, T. Cryopreservation of persimmon shoot tips from dormant buds using the D cryo-plate technique. Hortic. J. 2015, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Forsline, P.L.; Towill, L.E.; Waddel, J.W.; Stushnoff, C.; Lamboy, W.F.; McFerson, J.R. Recovery and longevity of cryopreserved dormant apple buds. J. Am. Soc. Hort. Sci. 1998, 123, 365–370. [Google Scholar] [CrossRef]
- Volk, G.M.; Waddell, J.; Bonnart, R.; Towill, L.; Ellis, D.; Luffman, M. High viability of dormant Malus buds after 10 years of storage in liquid nitrogen vapour. CryoLetters 2008, 29, 89–94. [Google Scholar]
- Benson, E.E. Cryopreservation Theory. In Plant Cryopreservation A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 15–32. [Google Scholar]
- Volk, G.M.; Bonnart, J.; Waddel, J.; Widrlechner, M.P. Cryopreservation of dormant buds from diverse Fraxinus species. CryoLetters 2009, 30, 262–267. [Google Scholar]
- Jenderek, M.M.; Tanner, J.; Ambruzs, B.D.; West, M.; Postman, J.D.; Hummer, K.E. Twig pre-harvest temperature significantly influences effective cryopreservation of Vaccinium dormant buds. Cryobiology 2017, 74, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Towill, L.E.; Widrlechner, M. Cryopreservation of Salix species using sections from winter vegetative scions. CryoLetters 2004, 27, 71–80. [Google Scholar]
- Towill, L.E.; Bonnart, R. Cryopreservation of apple using non-desiccated sections from winter-collected scions. CryoLetters 2005, 26, 323–332. [Google Scholar] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT Database; FAO: Rome, Italy, 2021; Available online: http://www.fao.org/faostat/en/#home (accessed on 15 June 2021).
- Tuovinen, T.; Parikka, P.; Lemmetty, A. Plant protection in currant production in Finland. Acta Hortic. 2008, 777, 333–338. [Google Scholar] [CrossRef]
- Jones, A.T. Important virus diseases of Ribes, their diagnosis, detection and control. Acta Hortic. 2002, 585, 279–285. [Google Scholar] [CrossRef]
- Jones, A.T. Black currant reversion disease—The probable causal agent, eriophyid mite vectors, epidemiology and prospects for control. Virus Res. 2000, 71, 71–84. [Google Scholar] [CrossRef]
- Lemmetty-Kaukoranta, A. Isolation and Identification of the Causal Agent of Black Currant Reversion Disease. Ph.D. Thesis, University of Turku, Turku, Finland, 30 March 2001. ISBN 951-29-1883-8. [Google Scholar]
- Rajamäki, M.-L.; Lemmetty, A.; Laamanen, J.; Roininen, E.; Vishwakarma, A.; Streng, J.; Latvala, S.; Valkonen, J.P.T. Small-RNA analysis of pre-basic mother plants and conserved accessions of plant genetic resources for the presence of viruses. PLoS ONE 2019, 14, e0220621. [Google Scholar] [CrossRef] [Green Version]
- Antonius, K.; Karhu, S.; Kaldmäe, H.; Lacis, G.; Rugenius, R.; Baniulis, D.; Sasnauskas, A.; Schulte, E.; Kuras, A.; Korbin, M.; et al. Development of the Northern European Ribes core collection based on a microsatellite (SSR) marker diversity analysis. Plant Genet. Resour. Charact. Util. 2012, 10, 70–73. [Google Scholar] [CrossRef]
- Karhu, S.; Antonius, K.; Rantala, S.; Pluta, S.; Ryliskis, D.; Schulte, E.; Toldam-Andersen, T.B.; Kaldmäe, H.; Rumpunen, K.; Sasnauskas, A.; et al. A multinational approach for conserving the European genetic resources of currants and gooseberry. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Berries: From Genomics to Sustainable Production, Quality and Health; Mezzetti, B., Brás de Oliveira, P., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2012; Volume 926, pp. 27–32. [Google Scholar]
- Benson, E.E.; Reed, B.M.; Brennan, R.M.; Clacher, K.A.; Ross, D.A. Use of thermal analysis in the evaluation of cryopreservation protocols for Ribes nigrum L. germplasm. CryoLetters 1996, 17, 347–362. [Google Scholar]
- Reed, B.M.; Dumet, D.; Denoma, J.M.; Benson, E.E. Validation of cryopreservation protocols for plant germplasm conservation: A pilot study using Ribes L. Biodivers. Conserv. 2001, 10, 939–949. [Google Scholar] [CrossRef]
- Reed, B.M.; Schumacher, L.; Dumet, D.; Benson, E.E. Evaluation of a modified encapsulation-dehydration procedure incorporating sucrose pretreatments for the cryopreservation of Ribes germplasm. In Vitro Cell. Dev. Biol. Plant 2005, 41, 431–436. [Google Scholar] [CrossRef]
- Rantala, S.; Kaseva, J.; Nukari, A.; Laamanen, J.; Karhu, S.; Veteläinen, M.; Häggman, H. Droplet vitrification technique for cryopreservation of a large diversity of blackcurrant (Ribes nigrum L.) cultivars. Plant Cell Tissue Organ Cult. PCTOC 2021, 144, 79–90. [Google Scholar] [CrossRef]
- Green, J.; Grout, B. Direct cryopreservation of winter buds of nine cultivars of blackcurrant (Ribes nigrum L.). CryoLetters 2010, 31, 341–346. [Google Scholar]
- Rantala, S.; Kaseva, J.; Karhu, S.; Veteläinen, M.; Uosukainen, M.; Häggman, H. Cryopreservation of Ribes nigrum (L.) dormant buds: Recovery via in vitro culture to the field. Plant Cell Tissue Organ Cult. 2019, 138, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Verzhuk, V.; Pavlov, A.; Novikova, L.; Filipenko, G. Viability of Red currant (Ribes rubrum L.) and Black currant (Ribes nigrum L.) Cuttings in Field Conditions after Cryopreservation in Vapors of Liquid Nitrogen. Agriculture 2020, 10, 476. [Google Scholar] [CrossRef]
- Stushnoff, C. Cryophysiology of woody plant dormant buds. In Proceedings of the II International Symposium on Plant Cryopreservation; Reed, B.M., Ed.; International Society for Horticultural Science: Leuven, Belgium, 2014; Volume 1039, pp. 63–72. [Google Scholar]
- Vogiatzi, C.; Grout, B.W.W.; Wetten, A.; Toldam-Andersen, B.T. Cryopreservation of winter-dormant apple buds: II tissue water status after desiccation at −4 °C and before further cooling. CryoLetters 2011, 32, 367–376. [Google Scholar] [PubMed]
- Vogiatzi, C.; Grout, B.W.W.; Wetten, A.; Orididge, M.; Clausen, S.K. Cryopreservation of winter-dormant apple buds IV: Critical temperature variation that can compromise survival. CryoLetters 2018, 39, 245–250. [Google Scholar] [PubMed]
- Niedz, R.P.; Bausher, M.G. Control of in vitro contamination of explants from greenhouse- and field-grown trees. In Vitro Cell. Dev. Biol. Plant 2002, 38, 468–471. [Google Scholar] [CrossRef]
- Dziedzic, E.; Jagla, J. Micropropagation of Rubus and Ribes spp. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ed.; Springer Science + Business Media: New York, NY, USA, 2013; Methods in molecular Biology; Volume 11013, pp. 149–160. [Google Scholar]
- Aronen, T.; Ryynänen, L. Cryopreservation of dormant in vivo-buds of hybrid aspen: Timing as critical factor. CryoLetters 2014, 35, 385–394. [Google Scholar] [CrossRef]
- Nasr, T.A.A.; Warein, P.F. Studies on flower initiation in black currant 1. Some internal factors affecting flowering. J. Hortic. Sci. 1961, 36, 1–10. [Google Scholar] [CrossRef]
- Lambardi, M.; Benelli, C.; De Carlo, A.; Ozudogru, E.A.; Previati, A.; Ellis, D. Cryopreservation of ancient apple cultivars of Vento: A comparison between PVS2-vitrification and dormant-bud techniques. In Proceedings of the First International Symposium on Cryopreservation in Horticultural Species; Panis, P., Lynch, P., Eds.; ISHS: Leuven, Belgium, 2011; pp. 191–198. [Google Scholar]
- Reed, B.M.; Kovalchuk, I.; Kushnarenko, S.; Meier-Dinkel, A.; Schoenweiss, K.; Pluta, S.; Straczynska, K.; Benson, E.E. Evaluation of critical points in technology transfer of cryopreservation protocols to international plant conservation laboratories. CryoLetters 2004, 25, 341–352. [Google Scholar]
- Bettoni, C.J.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. PCTOC 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Towill, L.E. Cryopreservation of apple (Malus domestica) dormant Buds. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 427–429. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc. Int. Plant Propag. Soc. 1980, 30, 421–427. [Google Scholar]
- Uosukainen, M. Rooting and weaning of apple rootstock YP. Agronomie 1992, 12, 803–806. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 231–232. ISBN 0-471-36093-7. [Google Scholar]
- Kenward, M.G.; Roger, J.H. An improved approximation to the precision of fixed effects from restricted maximum likelihood. Comput. Stat. Data Anal. 2009, 53, 2583–2595. [Google Scholar] [CrossRef]
- Westfall, P.; Tobias, R.D.; Wolfinger, R.D. Multiple Comparisons and Multiple Tests Using SAS; SAS Publishing: Cary, NC, USA, 2011. [Google Scholar]


| Mikael | Marski | Vilma | |||||
|---|---|---|---|---|---|---|---|
| Treatment Combination | Number of Floral Buds | Recovery of Thawed Buds (%) | Number of Floral Buds | Recovery of Thawed Buds (%) | Number of Floral Buds | Recovery of Thawed Buds (%) | |
| Desiccated bud sections | |||||||
| Fast thawing + rehydration 7 d | 18/20 | 30 | 18/20 | 40 | 14/19 * | 47 | |
| Fast thawing + rehydration 14 d | 18/20 | 30 | 12/20 | 80 | 16/20 | 60 | |
| Slow thawing + rehydration 7 d | 16/20 | 50 | 16/20 | 50 | 11/20 | 60 | |
| Slow thawing + rehydration 14 d | 18/20 | 35 | 17/20 | 65 | 12/20 | 55 | |
| Non-desiccated bud sections | |||||||
| Fast thawing + rehydration 7 d | 18/20 | 50 | 16/20 | 70 | 18/20 | 25 | |
| Fast thawing + rehydration 14 d | 17/20 | 50 | 17/20 | 80 | 9/20 | 70 | |
| Slow thawing + rehydration 7 d | 18/20 | 40 | 10/20 | 70 | 13/20 | 55 | |
| Slow thawing + rehydration 14 d | 18/20 | 20 | 17/20 | 60 | 10/13 ** | 46 | |
| Cultivar | Actual Recovery of Thawed Buds% | Estimated Recovery of Thawed Buds % (CI) | Actual Number of Regenerated Shoots Per Recovered Bud Mean (CI) | Estimated Number of Regenerated Shoots Per Recovered Bud Mean (CI) |
|---|---|---|---|---|
| Karila | 95 | 90 (52–99) | 8.4 (5.9–10.8) | 5.5 (3.9–7.6) |
| Vilma * | 95 | 90 (52–99) | 3.6 (2.9–4.2) | 2.7 (1.9–3.7) |
| Ri 289 * | 90 | 85 (54–96) | 5.9 (4.6–7.2) | 4.8 (3.5–6.5) |
| Suvi-7 | 95 | 81 (48–95) | 7.7 (5.5–9.9) | 5.2 (3.8–7.2) |
| Hedda | 75 | 78 (51–92) | 5.1 ((3.9–6.3) | 3.9 (2.8–5.5) |
| Venny * | 65 | 74 (49–90) | 2.6 (1.6–3.6) | 2.2 (1.6–3.1) |
| Marski | 85 | 72 (40–90) | 4.2 (3.0–5.4) | 3.0 (2.1–4.1) |
| Öjebyn | 75 | 70 (42–88) | 3.7 (2.2–5.2) | 2.4 (1.7–3.3) |
| Mortti | 80 | 68 (38–89) | 5.1 (4.2–6.0) | 3.9 (2.6–5.6) |
| Vertti * | 55 | 68 (44–85) | 3.4 (1.6–5.1) | 3.0 (2.0–4.4) |
| Mikael | 70 | 66 (39–85) | 3.7 (3.0–4.5) | 2.9 (2.0–4.1) |
| Ola | 70 | 65 (38–86) | 2.1 (1.6–2.6) | 1.6 (1.1–2.2) |
| Pyhtilän Musta | 85 | 63 (26–89) | 1.7 (1.2–2.1) | 1.1 (0.7–1.6) |
| Osmola | 75 | 63 (35–84) | 5.9 (3.7–8.1) | 3.5 (2.5–5.0) |
| Nikkala | 80 | 60 (29–85) | 3.9 (2.3–5.5) | 2.4 (1.7–3.5) |
| Åström | 75 | 59 (32–82) | 3.3 (2.0–4.5) | 2.1 (1.5–2.9) |
| Kangosfors | 80 | 59 (29–83) | 4.6 (3.1–6.1) | 2.9 (2.0–4.1) |
| Osmolan musta | 75 | 56 (30–78) | 5.0 (2.7–2.6) | 3.0 (2.1–4.2) |
| Kuoksan Musta | 80 | 55 (23–84) | 2.0 (1.4–2.6) | 1.3 (0.9–2.0) |
| Gerby | 70 | 55 (29–78) | 3.0 (2.1–3.9) | 2.0 (1.4–2.8) |
| Matkakoski | 65 | 54 (29–77) | 1.5 (1.1–1.9) | 1.2 (0.8–1.7) |
| Lepaan Musta | 63 | 42 (16–73) | 3.5 (2.5–4.5) | 2.2 (1.6–3.7) |
| Jänkisjärvi | 15 | 9 (3–29) | 1.3 (0–2.8) | 1.1 (0.6–2.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rantala, S.; Kaseva, J.; Nukari, A.; Laamanen, J.; Veteläinen, M.; Häggman, H.; Karhu, S. Successful Cryopreservation of Dormant Buds of Blackcurrant (Ribes nigrum L.) by Using Greenhouse-Grown Plants and In Vitro Recovery. Plants 2021, 10, 1414. https://doi.org/10.3390/plants10071414
Rantala S, Kaseva J, Nukari A, Laamanen J, Veteläinen M, Häggman H, Karhu S. Successful Cryopreservation of Dormant Buds of Blackcurrant (Ribes nigrum L.) by Using Greenhouse-Grown Plants and In Vitro Recovery. Plants. 2021; 10(7):1414. https://doi.org/10.3390/plants10071414
Chicago/Turabian StyleRantala, Saija, Janne Kaseva, Anna Nukari, Jaana Laamanen, Merja Veteläinen, Hely Häggman, and Saila Karhu. 2021. "Successful Cryopreservation of Dormant Buds of Blackcurrant (Ribes nigrum L.) by Using Greenhouse-Grown Plants and In Vitro Recovery" Plants 10, no. 7: 1414. https://doi.org/10.3390/plants10071414
APA StyleRantala, S., Kaseva, J., Nukari, A., Laamanen, J., Veteläinen, M., Häggman, H., & Karhu, S. (2021). Successful Cryopreservation of Dormant Buds of Blackcurrant (Ribes nigrum L.) by Using Greenhouse-Grown Plants and In Vitro Recovery. Plants, 10(7), 1414. https://doi.org/10.3390/plants10071414






