Chitosan-Induced Activation of the Antioxidant Defense System Counteracts the Adverse Effects of Salinity in Durum Wheat
Abstract
:1. Introduction
2. Results
2.1. Effect of CTS on the Growth Performance of NaCl-Treated Seedlings
2.2. Effect of CTS on ROS Production and MDA Content in the Shoot of NaCl-Treated Seedlings
2.3. Effect of CTS on Total Phenolic Content, Total Flavonoid Content and Total Antioxidant Activity in the Shoot of NaCl-Treated Seedlings
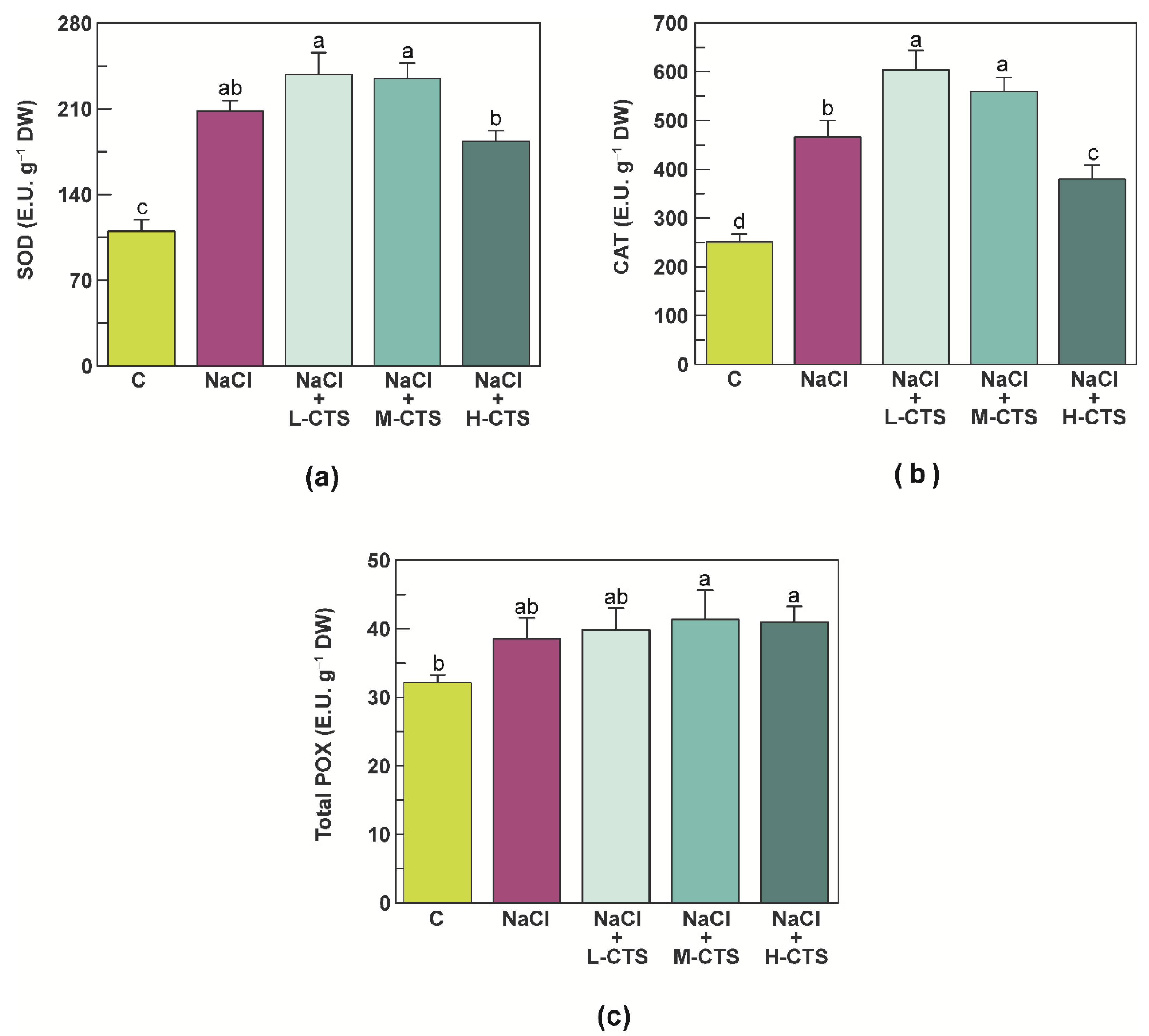
2.4. Effect of CTS on the Activities of the Antioxidant Enzymes in the Shoot of NaCl-Treated Seedlings
2.5. Effect of CTS on the Activities of NADPH-Producing Enzymes in the Shoot of NaCl-Treated Seedlings
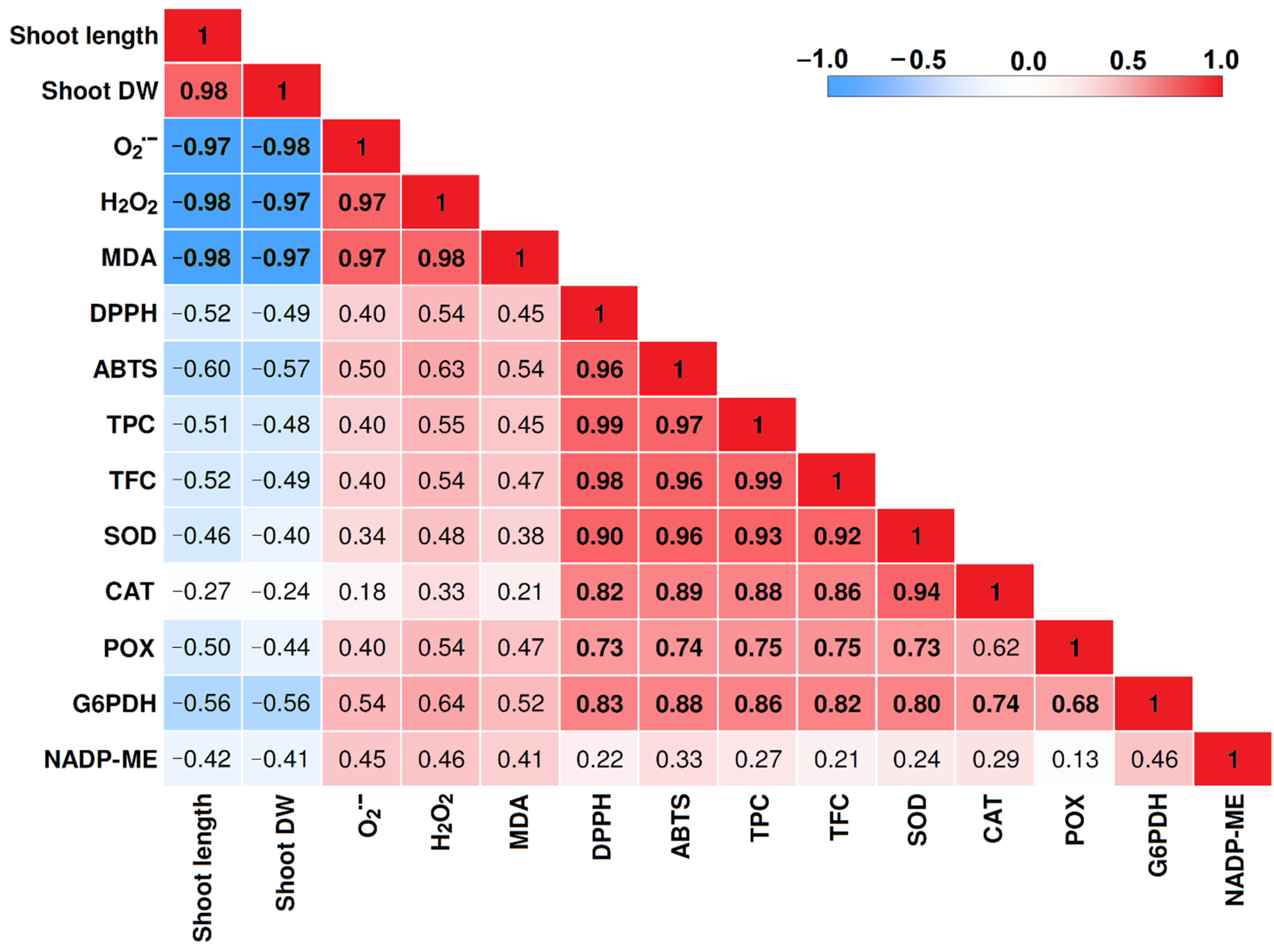
2.6. Correlation Matrix among Traits
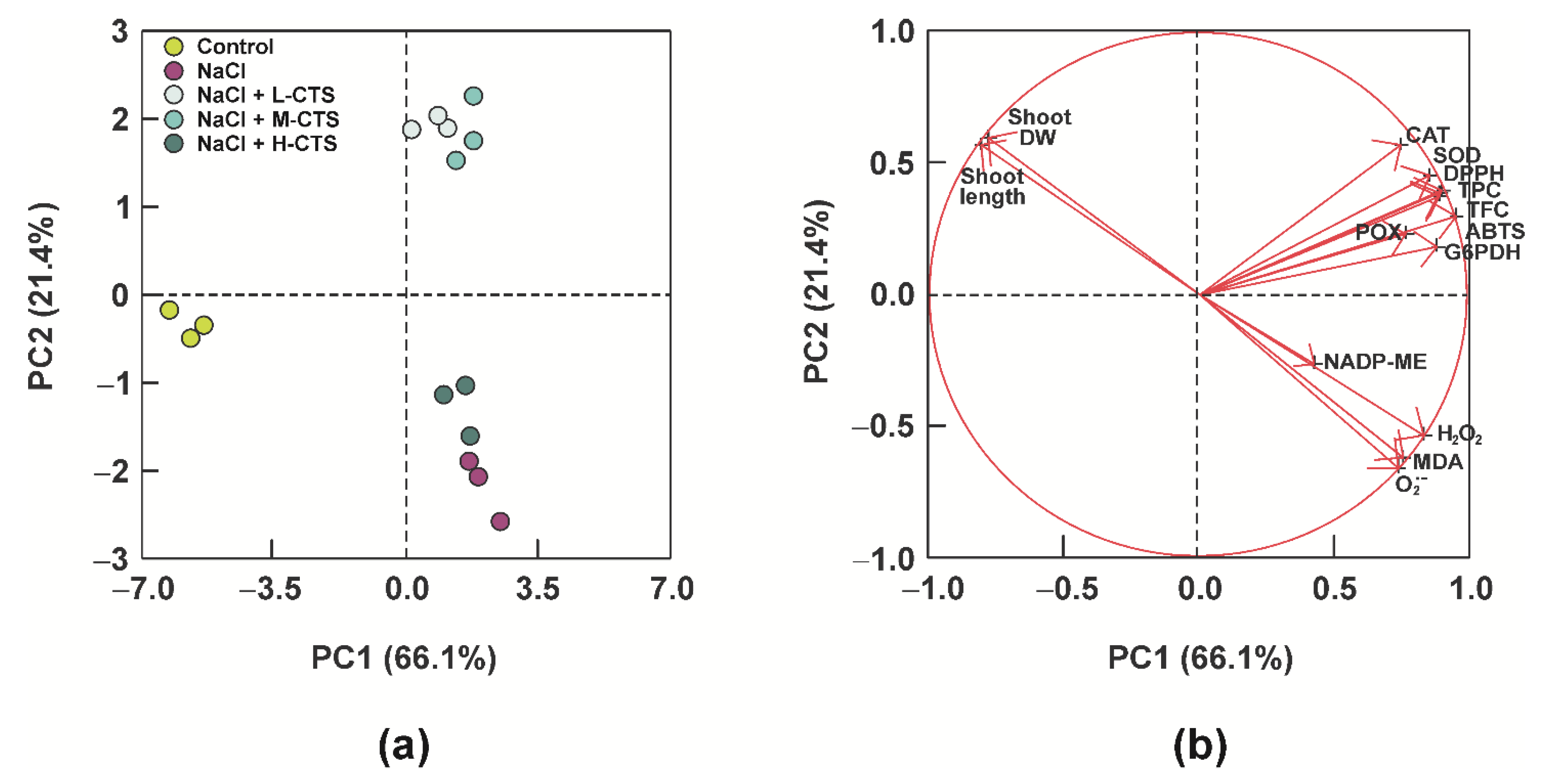
2.7. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of CTS Solutions
4.2. Experimental Design and Treatments
4.3. Determination of Growth Parameters
4.4. Preparation of Shoot Extracts
4.5. Determination of ROS Production
4.6. Determination of MDA Content
4.7. Determination of TPC and TFC
4.8. Determination of TAA
4.9. Determination of the Enzymatic Activities
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Royo, C.; Nazco, R.; Villegas, D. The climate of the zone of origin of Mediterranean durum wheat (Triticum durum Desf.) landraces affects their agronomic performance. Genet. Resour. Crop Evol. 2014, 61, 1345–1358. [Google Scholar] [CrossRef] [Green Version]
- Rana, G.; Katerji, N. Measurement and estimation of actual evapotranspiration in the field under Mediterranean climate: A review. Eur. J. Agron. 2000, 13, 125–153. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Fagnano, M.; Barbieri, G. Saline agriculture in Mediterranean environments. Ital. J. Agron. 2011, 6, e7. [Google Scholar] [CrossRef]
- Bar, S.; Kumari, B.; Gupta, S.K. Salinization of coastal groundwater resource in the perspective of climate change. In Fate and Transport of Subsurface Pollutants; Pankaj Kumar Gupta, P.K., Bharagava, R.N., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 315–326. [Google Scholar]
- Edelstein, M.; Plaut, Z.; Ben-Hur, M. Water salinity and sodicity effects on soil structure and hydraulic properties. Adv. Hort. Sci. 2010, 24, 154–160. [Google Scholar]
- Francois, L.E.; Maas, E.V. Crop response and management on salt-affected soils. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 149–181. [Google Scholar]
- Pasternak, D.; De Malach, Y. Crop irrigation with saline water. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 599–622. [Google Scholar]
- Borrelli, G.M.; Ficco, D.B.M.; Giuzio, L.; Pompa, M.; Cattivelli, L.; Flagella, Z. Durum wheat salt tolerance in relation to physiological, yield and quality characters. Cereal Res. Commun. 2011, 39, 525–534. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Fragasso, M.; Nigro, F.; Platani, C.; Papa, R.; Beleggia, R.; Trono, D. Analysis of metabolic and mineral changes in response to salt stress in durum wheat (Triticum turgidum ssp. durum) genotypes, which differ in salinity tolerance. Plant Physiol. Biochem. 2018, 133, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, B. Measurements of proline and malondialdehyde contents and antioxidant enzyme activities in leaves of drought stressed cotton. Bio-Protocol 2016, 6, e1913. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Descourvieres, P.; Kunert, K.J. Protection against oxygen radicals: An important defence mechanism studied in transgenic plants. Plant Cell Environ. 1994, 17, 507–523. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waskiewicz, A.; Besztedra, M.; Goliński, P. Nonenzymatic antioxidants in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Acdemic Press: San Diego, CA, USA, 2014; pp. 201–234. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidant defence mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013, 64, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Aguayo-Trinidada, S.; Ogawa, T.; Yoshimura, K.; Shigeoka, S. Activation of NADPH-recycling systems in leaves and roots of Arabidopsis thaliana under arsenic-induced stress conditions is accelerated by knock-out of Nudix hydrolase 19 (AtNUDX19) gene. J. Plant Physiol. 2016, 192, 81–89. [Google Scholar] [CrossRef]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Gómez-Rodríguez, M.V.; Chaki, M.; Pedrajas, J.R.; Fernández-Ocana, A.; del Río, L.A.; Barroso, J.B. The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ. 2006, 29, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Ursini, M.V. A new lease of life for an old enzyme. Bioassay 1996, 18, 631–637. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitin- and chitosan-based derivatives in plant protection against biotic and abiotic stresses and in recovery of contaminated soil and water. Polysaccharides 2020, 1, 21–30. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Falcón-Rodríguez, A.B.; Costales, D.; Cabrera, J.C.; Martínez-Téllez, M.A. Chitosan physico–chemical properties modulate defense responses and resistance in tobacco plants against the oomycete Phytophthora nicotianae. Pestic. Biochem. Physiol. 2011, 100, 221–228. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Flagella, Z.; Cantore, V.; Giuliani, M.M.; Tarantino, E.; De Caro, A. Crop salt tolerance: Physiological, yield and quality aspects. Rec. Res. Devel. Plant Biol. 2002, 2, 155–186. [Google Scholar]
- Mehmood, S.; Ahmed, W.; Ikram, M.; Imtiaz, M.; Mahmood, S.; Tu, S.; Chen, D. Chitosan modified biochar increases soybean (Glycine max L.) resistance to salt-stress by augmenting root morphology, antioxidant defense mechanisms and the expression of stress-responsive genes. Plants 2020, 9, 1173. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Brestic, M.; Skalicky, M. Chitosan-selenium nanoparticle (Cs-Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef]
- Turk, H. Chitosan-induced enhanced expression and activation of alternative oxidase confer tolerance to salt stress in maize seedlings. Plant Physiol. Biochem. 2019, 141, 415–422. [Google Scholar] [CrossRef]
- Zou, P.; Li, K.; Liu, S.; Xing, R.; Qin, Y.; Yu, H.; Zhou, M.; Li, P. Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohydr. Polym. 2015, 126, 62–69. [Google Scholar] [CrossRef]
- Moolphuerk, N.; Pattanagul, W. Pretreatment with different molecular weight chitosans encourages drought tolerance in rice (Oryza sativa L.) seedling. Not. Bot. Horti. Agrobo. 2020, 48, 2072–2084. [Google Scholar] [CrossRef]
- Zong, H.; Liu, S.; Xing, R.; Chen, X.; Li, P. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef]
- Khalil, M.S.; Badawy, M.E.I. Nematicidal activity of a biopolymer chitosan at different molecular weights against root-knot nematode, Meloidogyne incognita. Plant Protect. Sci. 2012, 48, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Arif, Y.; Siddiqui, H.; Sami, F.; Zaidi, R.; Azam, A.; Alam, P.; Hayat, S. Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol. Environ. Saf. 2021, 213, 112020. [Google Scholar] [CrossRef]
- Sami, F.; Siddiqui, H.; Alam, P.; Hayat, S. Glucose-induced response on photosynthetic efficiency, ROS homeostasis, and antioxidative defense system in maintaining carbohydrate and ion metabolism in Indian mustard (Brassica juncea L.) under salt-mediated oxidative stress. Protoplasma 2021, 258, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Chen, L.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism. PeerJ 2020, 8, e10486. [Google Scholar] [CrossRef] [PubMed]
- Fardus, J.; Hossain, M.S.; Fujita, M. Modulation of the antioxidant defense system by exogenous L-glutamic acid application enhances salt tolerance in lentil (Lens culinaris Medik.). Biomolecules 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Ahanger, M.A.; Alam, P.; Alyemeni, M.N.; Wijaya, L.; Ali, S.; Ashraf, M. Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physiobiochemical attributes and key antioxidant enzymes. J. Plant Growth Regul. 2019, 38, 70–82. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Ouhibi, C.; Attia, H.; Rebah, F.; Msilini, N.; Chebbi, M.; Aarrouf, J.; Urban, L.; Lachaal, M. Salt stress mitigation by seed priming with UV-C in lettuce plants: Growth, antioxidant activity and phenolic compounds. Plant Physiol. Biochem. 2014, 83, 126–133. [Google Scholar] [CrossRef]
- Lucini, L.; Borgognone, D.; Rouphael, Y.; Cardarelli, M.; Bernardi, J.; Colla, G. Mild potassium chloride stress alters the mineral composition, hormone network, and phenolic profile in artichoke leaves. Front. Plant Sci. 2016, 7, 948. [Google Scholar] [CrossRef] [Green Version]
- Hichem, H.; Mounir, D.; Naceur, E.A. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crops and Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Picchi, V.; Gobbi, S.; Fattizzo, M.; Zefelippo, M.; Faoro, F. Chitosan nanoparticles loaded with N-acetyl cysteine to mitigate ozone and other possible oxidative stresses in durum wheat. Plants 2021, 10, 691. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Di Loreto, A.; Bosi, S.; Montero, L.; Bregola, V.; Marotti, I.; Sferrazza, R.E.; Dinelli, G.; Herrero, M.; Cifuentes, A. Determination of phenolic compounds in ancient and modern durum wheat genotypes. Electrophoresis 2018, 39, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic compounds and bioaccessibility thereof in functional pasta. Antioxidants 2020, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem. Toxicol. 1999, 37, 949–962. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Martínez Gonzáles, G.; Reyes, G.; Falcón, R.; Núñez, V. Effect of seed treatment with chitosan on the growth of rice (Oryza sativa L.) seedlings cv. INCA LP-5 in saline medium. Cultivos Tropicales 2015, 36, 143–150. [Google Scholar]
- Jabeen, N.; Ahmad, R. The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. J. Sci. Food Agric. 2013, 93, 1699–1705. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Yu, C.; Wang, Y.; Li, X.; Li, N.; Bu, N. Alleviation of exogenous oligochitosan on wheat seedlings growth under salt stress. Protoplasma 2012, 249, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.M.S.; Ramadan, A.A.; El-Bassiouny, H.M.S.; Bakry, B.A. Regulation of antioxidant system in wheat cultivars by using chitosan or salicylic acid to improve growth and yield under salinity stress. Asian J. Plant Sci. 2020, 19, 114–126. [Google Scholar]
- Leterrier, M.; Barroso, J.B.; Valderrama, R.; Palma, J.M.; Corpas, F.J. NADP-dependent isocitrate dehydrogenase (NADP-ICDH) from Arabidopsis roots contributes I n the mechanism of defence against the nitro-oxidative stress induced by salinity. Sci. World J. 2012, 2012, 694740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, G.S.M.; Ali, A.S.; Eldebawy, E.M.M.; Saber, N.E.-S. Role of cellular NADP+/NADPH ratio in the acclimative mechanism of two common bean cultivars toward salt stress. J. Appl. Bot. Food Qual. 2017, 90, 43–51. [Google Scholar]
- Liu, S.; Cheng, Y.; Zhang, X.; Guan, Q.; Nishiuchi, S.; Hase, K.; Takano, T. Expression of an NADP-malic enzyme gene in rice (Oryza sativa. L) is induced by environmental stresses; over-expression of the gene in Arabidopsis confers salt and osmotic stress tolerance. Plant Mol. Biol. 2007, 64, 49–58. [Google Scholar] [CrossRef]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef] [Green Version]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet. 2019, 51, 885–895. [Google Scholar] [CrossRef] [Green Version]
- De Simone, V.; Soccio, M.; Borrelli, G.M.; Pastore, D.; Trono, D. Stay-green trait-antioxidant status interrelationship in durum wheat (Triticum durum) flag leaf during post-flowering. J. Plant Res. 2014, 127, 159–171. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Menga, V.; Trono, D. The molecular and functional characterization of the durum wheat lipoxygenase TdLOX2 suggests its role in hyperosmotic stress response. Plants 2020, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Antioxidant potential of Artemisia argentea L’Hér alcoholic extract and its relation with the phenolic composition. Food Res. Int. 2011, 44, 1620–1631. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6065. [Google Scholar] [CrossRef]
- Del Río, L.A.; Ortega, M.G.; López, A.L.; Gorgé, J.L. A more sensitive modification of the catalase assay with the Clark oxygen electrode. Application to the kinetic study of the pea leaf enzyme. Anal. Biochem. 1977, 80, 409–415. [Google Scholar] [CrossRef]
- Amako, K.; Chen, G.-X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar]
- Muto, S.; Uritani, I. Glucose-6-phosphate dehydrogenase from sweet potato. Plant Cell Physiol. 1970, 11, 767–776. [Google Scholar] [CrossRef]
- Day, D.A.; Mannix, M. Malate oxidation by soybean nodule mitochondria and the possible consequences for nitrogen fixation. Plant Physiol. Biochem. 1988, 26, 567–573. [Google Scholar]


| Treatment | Shoot | Root | ||
|---|---|---|---|---|
| Length (cm) | Dried Biomass (mg) | Length (cm) | Dried Biomass (mg) | |
| Control | 30.37 ± 1.08 a | 57.26 ± 1.26 a | 18.68 ± 1.61 a | 26.89 ± 0.96 a |
| NaCl | 19.52 ± 1.16 d | 35.57 ± 1.09 e | 7.38 ± 1.52 d | 10.89 ± 1.28 d |
| NaCl + L-CTS | 25.27 ± 0.85 b | 46.89 ± 1.34 c | 13.31 ± 1.38 b | 20.39 ± 1.34 b |
| NaCl + M-CTS | 26.59 ± 1.46 b | 51.23 ± 1.46 b | 14.22 ± 1.42 b | 17.88 ± 1.03 c |
| NaCl + H-CTS | 21.44 ± 1.67 c | 39.49 ± 1.26 d | 10.49 ± 1.14 c | 16.84 ± 1.13 c |
| Treatment | TPC (mg FE g−1 DW) | TFC (mg CE g−1 DW) | TAA (μmol TE g−1 DW) | |
|---|---|---|---|---|
| DPPH | ABTS | |||
| Control | 9.28 ± 0.45 d | 3.87 ± 0.25 c | 132.3 ± 1.0 c | 100.3 ± 2.6 c |
| NaCl | 15.52 ± 0.37 c | 5.85 ± 0.29 b | 163.8 ± 3.5 b | 132.4 ± 0.1 b |
| NaCl + L-CTS | 19.08 ± 0.24 a | 6.99 ± 0.32 a | 181.2 ± 0.8 a | 140.1 ± 0.8 a |
| NaCl + M-CTS | 17.73 ± 0.49 b | 6.55 ± 0.33 ab | 174.9 ± 0.8 a | 134.9 ± 1.2 ab |
| NaCl + H-CTS | 16.88 ± 0.20 b | 6.33 ± 0.12 ab | 172.9 ± 1.3 ab | 129.2 ± 1.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quitadamo, F.; De Simone, V.; Beleggia, R.; Trono, D. Chitosan-Induced Activation of the Antioxidant Defense System Counteracts the Adverse Effects of Salinity in Durum Wheat. Plants 2021, 10, 1365. https://doi.org/10.3390/plants10071365
Quitadamo F, De Simone V, Beleggia R, Trono D. Chitosan-Induced Activation of the Antioxidant Defense System Counteracts the Adverse Effects of Salinity in Durum Wheat. Plants. 2021; 10(7):1365. https://doi.org/10.3390/plants10071365
Chicago/Turabian StyleQuitadamo, Filippo, Vanessa De Simone, Romina Beleggia, and Daniela Trono. 2021. "Chitosan-Induced Activation of the Antioxidant Defense System Counteracts the Adverse Effects of Salinity in Durum Wheat" Plants 10, no. 7: 1365. https://doi.org/10.3390/plants10071365
APA StyleQuitadamo, F., De Simone, V., Beleggia, R., & Trono, D. (2021). Chitosan-Induced Activation of the Antioxidant Defense System Counteracts the Adverse Effects of Salinity in Durum Wheat. Plants, 10(7), 1365. https://doi.org/10.3390/plants10071365







