Aquatic Macrophytes Occurrence in Mediterranean Farm Ponds: Preliminary Investigations in North-Western Sicily (Italy)
Abstract
:1. Introduction
2. Study Area Settings
3. Materials and Methods
4. Results
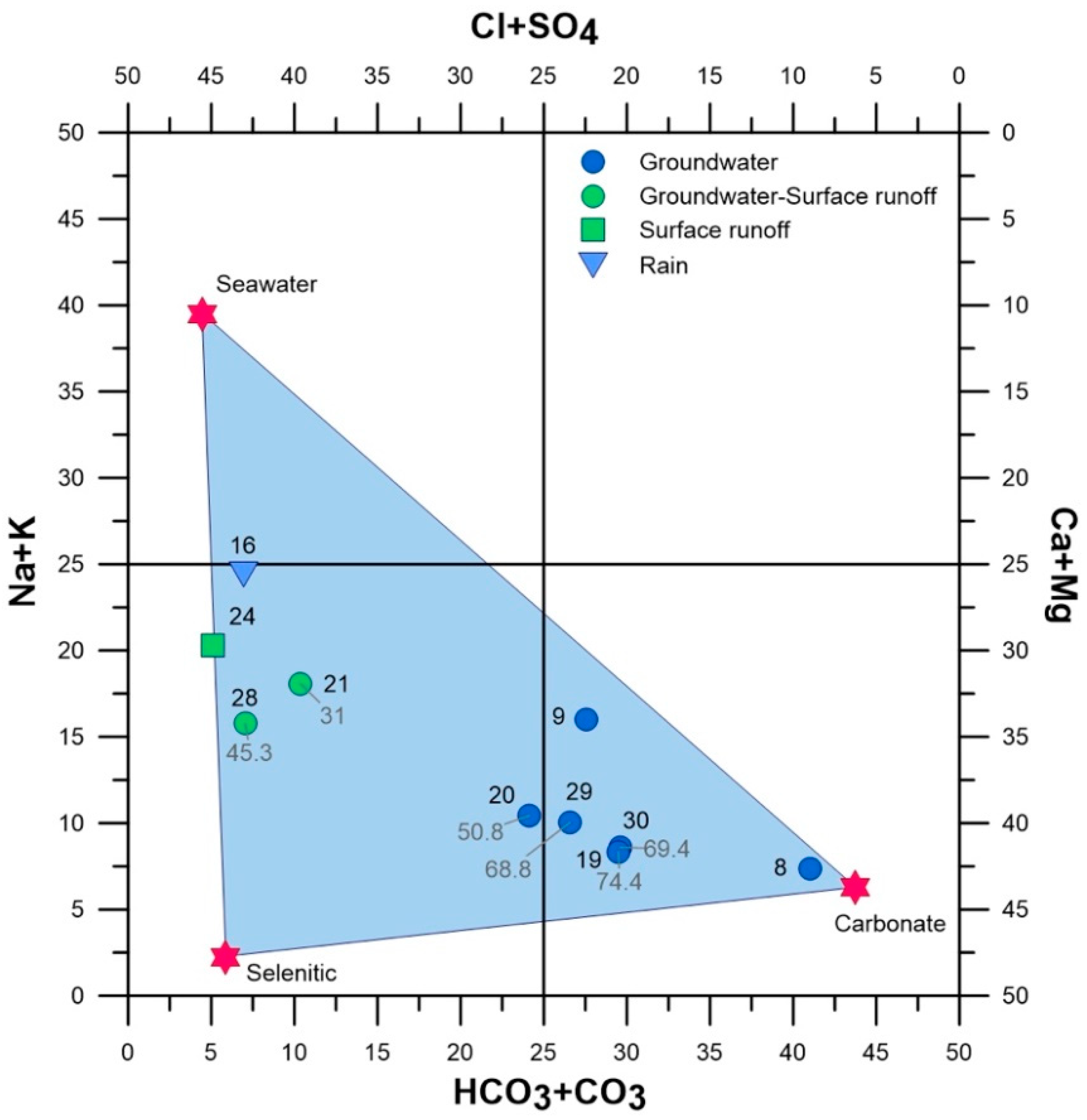
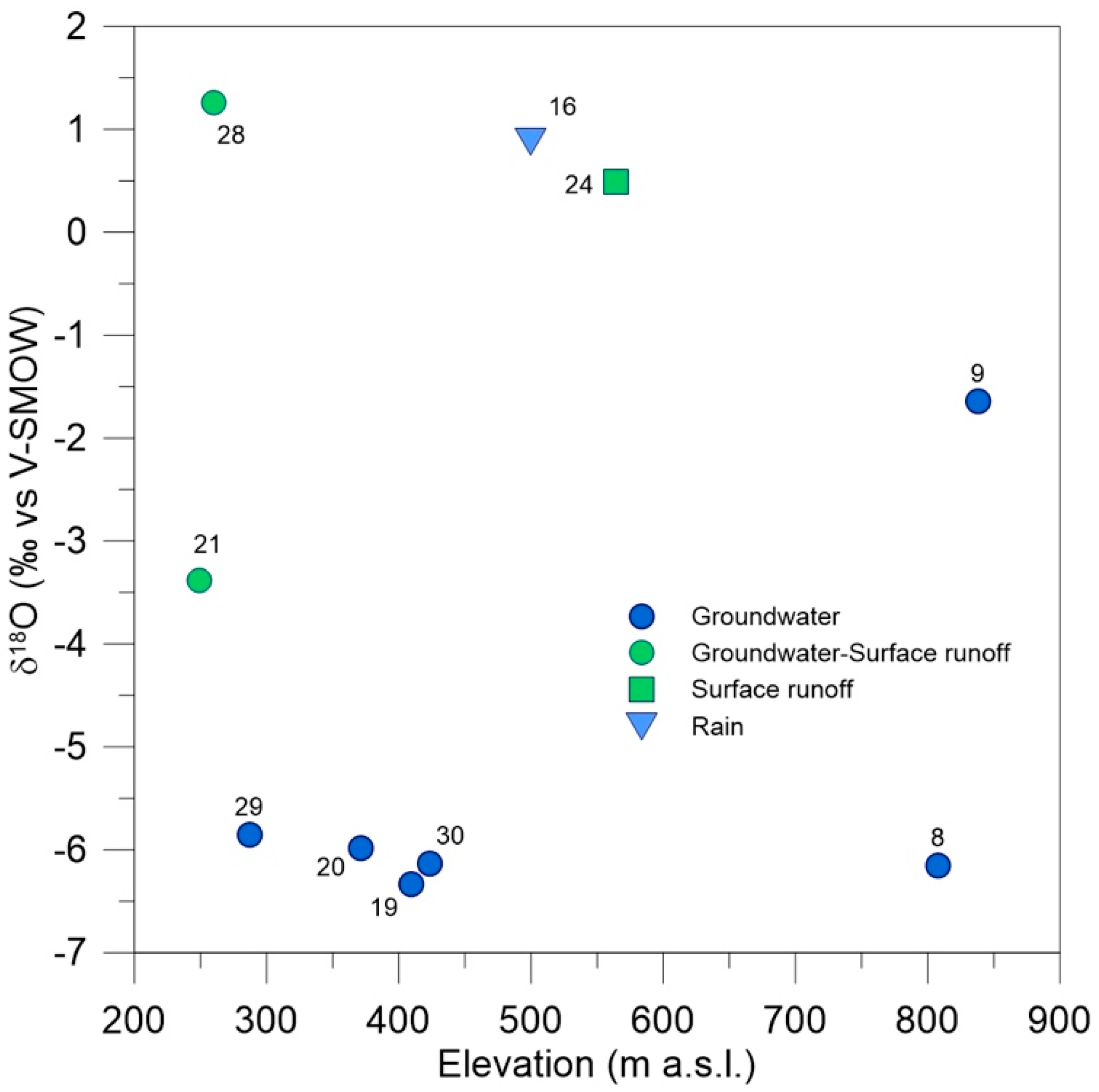
4.1. Physical and Chemical Characterization of the Ponds
4.2. Flora of the Ponds
5. Discussion
5.1. Role of Man-Made Farm Ponds for the Biodiversity of the Agricultural Landscape
5.2. Use of Aquatic Macrophytes for Monitoring the Quality of Inland Waters in the Mediterranean Area
- -
- Ponds entirely or partially fed by groundwater, rich in nitrates (29, 30, 19, 20) or with values close to the eutrophication threshold (28 and 21); the sole exception is site 8, located in the poorly anthropized area of Monte San Calogero, where nitrates are absent.
- -
- Ponds fed by surface waters, characterized by low nitrate concentrations (24, 16 and 9).
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galewski, T.; Balkiz, O.; Beltrame, C.; Chazée, L.; Elloumi, M.-J.; Grillas, P.; Jalbert, J.; Khairallah, M.; Korichi, N.; Logotheti, A.; et al. Biodiversity–Status and Trends of Species in Mediterranean Wetlands; Mediterranean Wetlands Observatory: Tour du Valat, France, 2012. [Google Scholar]
- Leberger, R.; Geijzendorffer, I.R.; Gaget, E.; Gwelmami, A.; Galewski, T.; Pereira, H.M.; Guerra, C.A. Mediterranean wetland conservation in the context of climate and land cover change. Reg. Environ. Chang. 2020, 20, 67. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Ruggiero, A.; Céréghino, R.; Figuerola, J.; Marty, P.; Angélibert, S. Farm ponds make a contribution to the biodiversity of aquatic insects in a French agricultural landscape. Comptes Rendus Biol. 2008, 331, 298–308. [Google Scholar] [CrossRef]
- Casas, J.; Toja, J.; Bonachela, S.; Fuentes, F.; Gallego, I.; Juan, M.; León, D.; Peñalver, P.; Pérez, C.; Sánchez, P. Artificial ponds in a Mediterranean region (Andalusia, southern Spain): Agricultural and environmental issues. Water Environ. J. 2011, 25, 308–317. [Google Scholar] [CrossRef]
- Fuentes-Rodríguez, F.; Juan, M.; Gallego, I.; Lusi, M.; Fenoy, E.; León, D.; Peñalver, P.; Toja, J.; Casas, J.J. Diversity in Mediterranean farm ponds: Trade-offs and synergies between irrigation modernisation and biodiversity conservation. Freshw. Biol. 2013, 58, 63–78. [Google Scholar] [CrossRef]
- Briggs, A.; Pryke, J.S.; Samways, M.J.; Conlong, D.E. Macrophytes promote aquatic insect conservation in artificial ponds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 1–12. [Google Scholar] [CrossRef]
- Della Bella, V.; Bazzanti, M.; Dowgiallo, M.G.; Iberite, M. Macrophyte diversity and physico-chemical characteristics of Tyrrhenian coast ponds in central Italy: Implications for conservation. Hydrobiologia 2008, 597, 85–95. [Google Scholar] [CrossRef]
- Bubíková, K.; Hrivnák, R. Artificial ponds in Central Europe do not fall behind the natural ponds in terms of macrophyte diversity. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 8. [Google Scholar] [CrossRef] [Green Version]
- Stefanidis, K.; Sarika, M.; Papastergiadou, E. Exploring environmental predictors of aquatic macrophytes in water-dependent Natura 2000 sites of high conservation value: Results from a long-term study of macrophytes in Greek lakes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1133–1148. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiaoussi, V.; Tsiripidis, I. HeLM: A macrophyte-based method for monitoring and assessment of Greek lakes. Environ. Monit. Assess. 2018, 190, 326. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.C.; Trajanovska, S.; Biberdžić, V.; Marković, A.; Talevska, M.; Imeri, A.; Veljanoska-Sarafiloska, E.; Đurašković, P.; Jovanović, K.; Cara, M. The Balkan macrophyte index (BMI) for assessment of eutrophication in lakes. Acta Zool. Bulg. 2020, 72, 439–454. [Google Scholar]
- Rodrigo, M.A.; Puche, E.; Rojo, C. On the tolerance of charophytes to high-nitrate concentrations. Chem. Ecol. 2018, 34, 22–42. [Google Scholar] [CrossRef]
- Gallego, I.; Pérez-Martínez, C.; Sánchez-Castillo, P.M.; Fuentes-Rodríguez, F.; Juan, M.; Casa, J.J. Physical, chemical, and management-related drivers of submerged macrophyte occurrence in Mediterranean farm ponds. Hydrobiologia 2015, 762, 209–222. [Google Scholar] [CrossRef]
- European Commission. E.C. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Comm. 2000, 327, 1–71. [Google Scholar]
- Climatologia Della Sicilia 16; Regione Siciliana, Assessorato Agricoltura e Foreste, Gruppo IV, Unità di Agrometeorologia: Palermo, Italy, 1998; p. 69. Available online: http://www.sias.regione.sicilia.it/pdf/Climatologia_sicilia.pdf (accessed on 12 April 2021). (In Italian)
- Andreas, W.H.; Hairigh, A. 18O-Equilibration on Water, Fruit Juice and Wine Using Thermo Scientific GasBench II; Thermo Fisher Scientific: Brema, Germany, 2008. [Google Scholar]
- Raunkiaer, C.C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: Oxford, UK, 1934. [Google Scholar]
- Mouronval, J.B.; Baudouin, S.; Borel, N.; Soulié-Märsche, I.; Klesczewski, M.; Grillas, P. Guide des Characées de France Méditerranéenne; Office National de la Chasse et Faune Sauvage: Paris, France, 2015. [Google Scholar]
- Pignatti, S.; La Rosa, M.; Guarino, R. Flora d’Italia, 2nd ed.; Edagricole—New Business Media: Milano-Bologna, Italy, 2017; Volume 2. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Langelier, W.F.; Ludwig, F. Graphical methods for indicating the mineral character of natural waters. J. Am. Water Works Assoc. 1942, 34, 335–352. [Google Scholar] [CrossRef]
- Paladino, O.; Massabò, M.; Gandoglia, E. Assessment of Nitrate Hazards in Umbria Region (Italy) Using Field Datasets: Good Agriculture Practices and Farms Sustainability. Sustainability 2020, 12, 9497. [Google Scholar] [CrossRef]
- Liotta, M.; Grassa, F.; D’Alessandro, W.; Favara, R.; Gagliano Candela, E.; Pisciotta, A.; Scaletta, C. Isotopic composition of precipitation and groundwater in Sicily, Italy. Appl. Geochem. 2013, 34, 199–206. [Google Scholar] [CrossRef]
- Raimondo, F.M.; Bazan, G.; Troia, A. Taxa a rischio nella flora vascolare della Sicilia. Biogeographia 2011, 30, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, T.; Rákosy, L. Changes of traditional agrarian landscapes and their conservation implications: A case study of butterflies in Romania. Divers. Distrib. 2007, 13, 855–862. [Google Scholar] [CrossRef]
- Hartel, T.; Schweiger, O.; Ollerer, K.; Cogalniceanu, D.; Arntzen, J.W. Amphibian distribution in a traditionally managed rural landscape of Eastern Europe: Probing the effect of landscape composition. Biol. Conserv. 2010, 143, 1118–1124. [Google Scholar] [CrossRef]
- Williams, P.; Whitfield, M.; Biggs, J.; Bray, S.; Fox, G.; Nicolet, P.; Sear, D. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol. Conserv. 2004, 115, 329–341. [Google Scholar] [CrossRef]
- Davies, B.; Biggs, J.; Williams, P.; Whitfield, M.; Nicolet, P.; Sear, D.; Bray, S.; Maund, S. Comparative biodiversity of aquatic habitats in the European agricultural landscape. Agric. Ecosyst. Environ. 2008, 125, 1–8. [Google Scholar] [CrossRef]
- Sebastián-González, E.; Sánchez-Zapata, J.A.; Botella, F. Agricultural ponds as alternative habitat for waterbirds: Spatial and temporal patterns of abundance and management strategies. Eur. J. Wildl. Res. 2010, 56, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Casas, J.J.; Toja, J.; Peñalver, P.; Juan, M.; León, D.; Fuentes-Rodríguez, F.; Gallego, I.; Fenoy, E.; Pérez-Martínez, C.; Sánchez, P.; et al. Farm Ponds as Potential Complementary Habitats to Natural Wetlands in a Mediterranean Region. Wetlands 2012, 32, 161–174. [Google Scholar] [CrossRef]
- Bolpagni, R.; Laini, A.; Stanzani, C.; Chiarucci, A. Aquatic Plant Diversity in Italy: Distribution, Drivers and Strategic Conservation Actions. Front. Plant Sci. 2018, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Marcenò, C.; Minissale, P.; Sciandrello, S.; Cuena-Lombraña, A.; Fois, M.; Bacchetta, G. The MedIsWet project in Sicily and Sardinia. An opportunity for improving wetland knowledge and conservation. Preliminary results. In Abstract Book of the 28th Meeting Proceedings of the European Vegetation Survey, Vegetation Diversity and Global Change, Madrid, Spain, 2–6 September 2019; Gavilán, R.G., Gutiérrez-Girón, A., Eds.; University of Cagliari: Cagliari, Italy, 2019; p. 68. [Google Scholar]
- Zelnik, I.; Potisek, M.; Gaberščik, A. Environmental conditions and macrophytes of Karst Ponds. Pol. J. Environ. Stud. 2012, 21, 1911–1920. [Google Scholar]
- Guarino, R.; Marcenò, C.; Ilardi, V.; Mannino, A.M.; Troia, A. One Chara does not make Charetea in the Mediterranean aquatic vegetation. Webbia 2019, 74, 139–147. [Google Scholar] [CrossRef]
- Brullo, S.; Minissale, P.; Spampinato, G. Studio fitosociologico della vegetazione lacustre dei Monti Nebrodi (Sicilia settentrionale). Fitosociologia 1994, 27, 5–50. [Google Scholar]
- Poikane, S.; Portielje, R.; Denys, L.; Elferts, D.; Kelly, M.; Kolada, A.; Mäemets, H.; Phillips, G.; Søndergaard, M.; Willby, N.; et al. Macrophyte assessment in European lakes: Diverse approaches but convergent views of ‘good’ ecological status. Ecol. Indic. 2018, 94, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, M.; Johansson, L.S.; Lauridsen, T.L.; Jørgensen, T.B.; Liboriusse, L.; Jeppesen, E. Submerged macrophytes as indicators of the ecological quality of lakes. Freshw. Biol. 2010, 55, 893–908. [Google Scholar] [CrossRef]
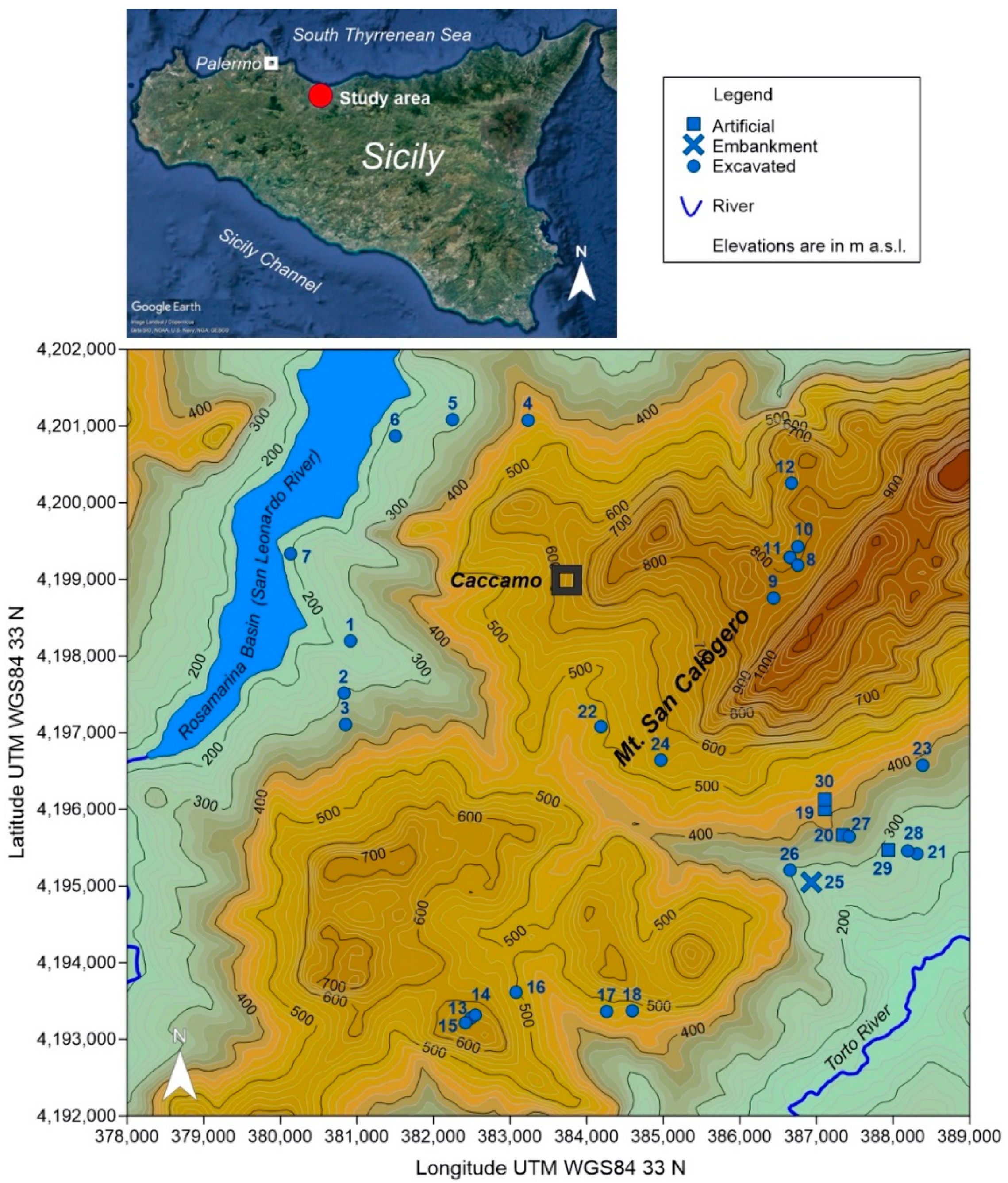

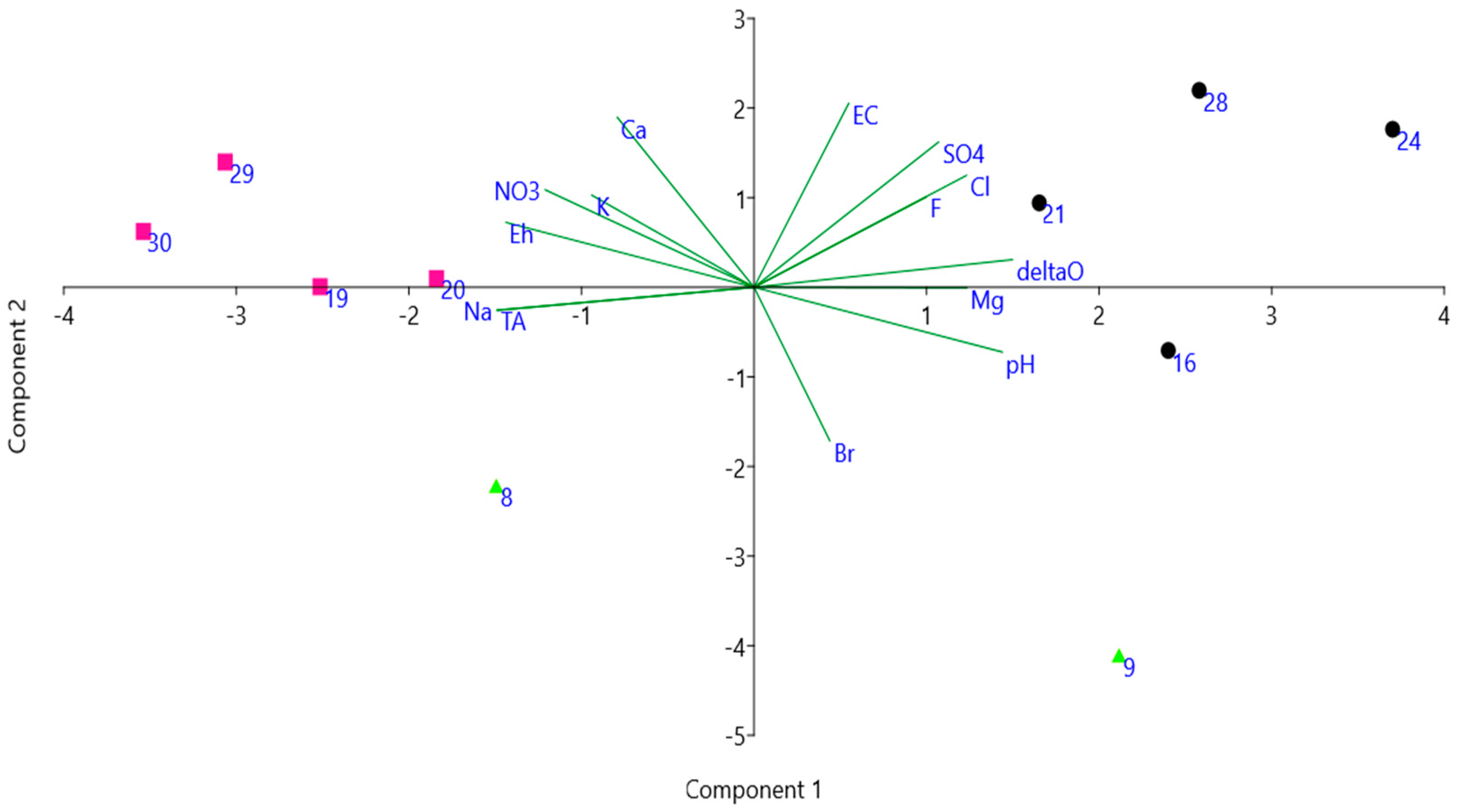
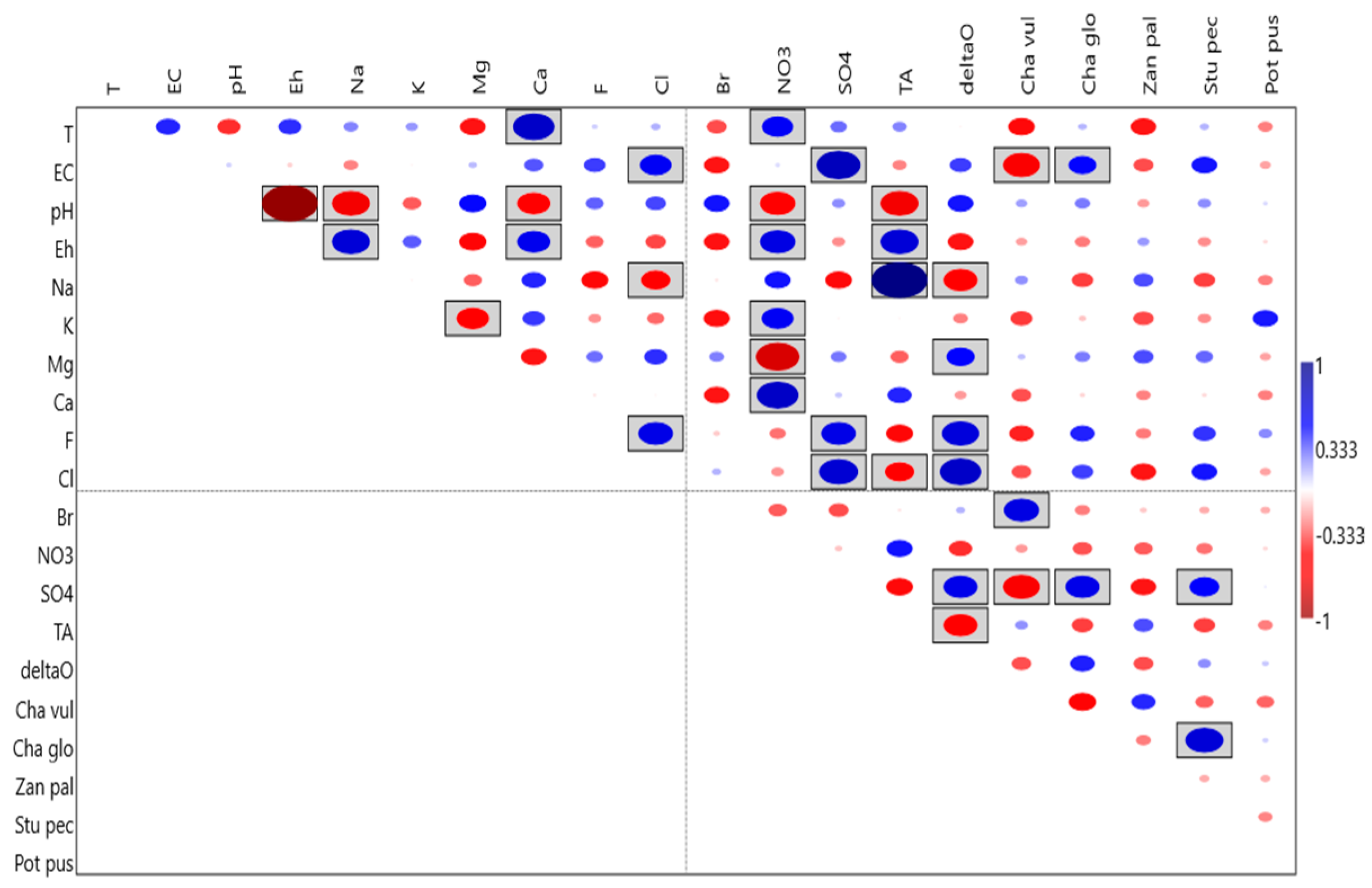
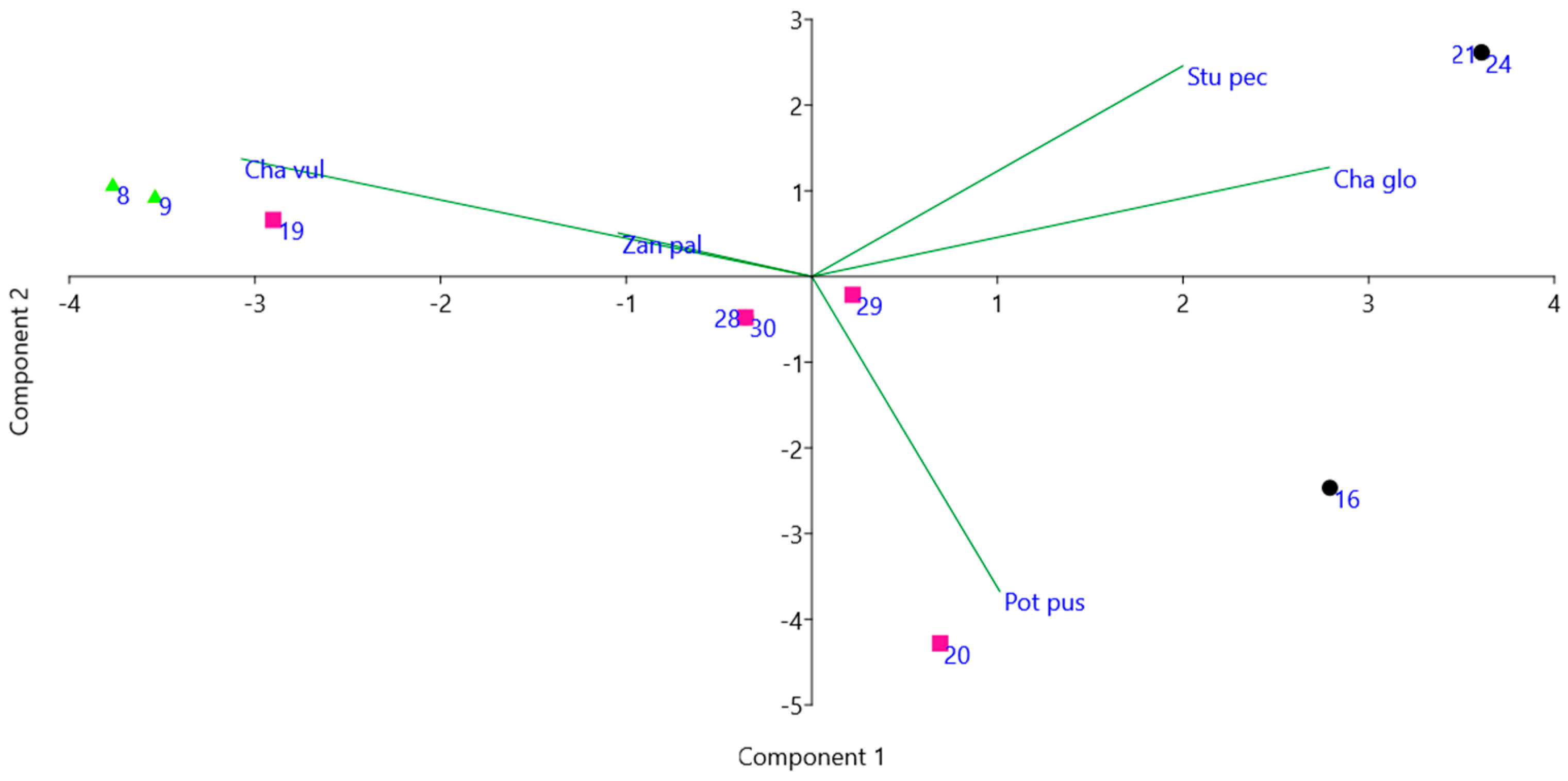
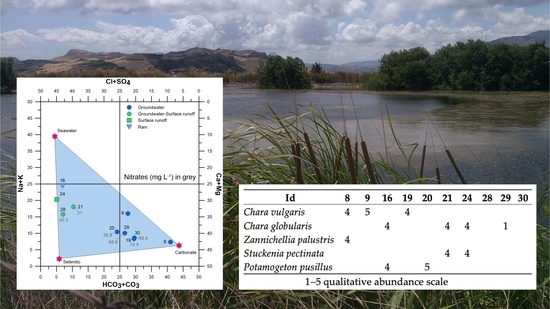
| Id | Longitude | Latitude | Elevation | Area | Water | Type | Use | Depth | Visibility | SM |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13.6451 | 37.9235 | 224 | 638 | R-S | E | IRR | D | C | 2 |
| 2 | 13.6442 | 37.9174 | 309 | 93 | R | E | CWA | D | C | 2 |
| 3 | 13.6445 | 37.9137 | 349 | 24 | R | E | CWA | S | C | 1 |
| 4 | 13.6710 | 37.9498 | 438 | 379 | R | E | NUS | D | T | 0 |
| 5 | 13.6598 | 37.9497 | 280 | 408 | R | E | NUS | D | T | 2 |
| 6 | 13.6513 | 37.9477 | 220 | 224 | R-S | E | CWA | D | C | 1 |
| 7 | 13.6360 | 37.9337 | 193 | 623 | R | E | CWA | D | C | 1 |
| 8 | 13.7114 | 37.9332 | 808 | 343 | G | E | CWA | S | C | 2 |
| 9 | 13.7078 | 37.9293 | 838 | 114 | G | E | CWA | S | C | 1 |
| 10 | 13.7113 | 37.9354 | 786 | 66 | G-R | E | CWA | S | C | 2 |
| 11 | 13.7102 | 37.9341 | 785 | 48 | G-R | E | CWA | S | C | 2 |
| 12 | 13.7103 | 37.9428 | 767 | 213 | R | E | CWA | D | T | 0 |
| 13 | 13.6638 | 37.8794 | 641 | 42 | R | E | CWA | D | T | 1 |
| 14 | 13.6644 | 37.8798 | 628 | 113 | R | E | NUS | D | T | 0 |
| 15 | 13.6630 | 37.8788 | 649 | 43 | R | E | CWA | D | T | 1 |
| 16 | 13.6704 | 37.8825 | 500 | 129 | R | E | IRR | D | C | 2 |
| 17 | 13.6914 | 37.9100 | 472 | 273 | R | E | NUS | D | T | 0 |
| 18 | 13.6877 | 37.8805 | 469 | 422 | R | E | NUS | D | T | 0 |
| 19 | 13.7159 | 37.9045 | 409 | 64 | G | A | IRR | D | C | 1 |
| 20 | 13.7186 | 37.9015 | 371 | 16 | G | A | NUS | S | C | 1 |
| 21 | 13.7297 | 37.8995 | 249 | 527 | G-S | E | IRR | D | C | 2 |
| 22 | 13.6825 | 37.9139 | 486 | 1034 | S | E | IRR | D | C | 1 |
| 23 | 13.7303 | 37.9099 | 387 | 401 | R | E | IRR | D | T | 2 |
| 24 | 13.6914 | 37.9100 | 564 | 2395 | S | E | IRR | D | C | 2 |
| 25 | 13.7140 | 37.8959 | 255 | 54 | S | D | NUS | S | C | 1 |
| 26 | 13.7108 | 37.8973 | 296 | 208 | S | E | IRR | S | C | 1 |
| 27 | 13.7195 | 37.9014 | 359 | 233 | G | E | IRR | D | T | 0 |
| 28 | 13.7283 | 37.8998 | 260 | 891 | G | E | IRR | D | T | 0 |
| 29 | 13.7254 | 37.8998 | 287 | 2 | G | A | DRT | S | C | 1 |
| 30 | 13.7159 | 37.9057 | 423 | 4 | G | A | DRT | S | C | 0 |
| Id | 8 | 9 | 16 | 19 | 20 | 21 | 24 | 28 | 29 | 30 |
|---|---|---|---|---|---|---|---|---|---|---|
| T | 10.9 | 12.8 | 13.1 | 15.4 | 14.0 | 16.2 | 14.3 | 15.6 | 19.3 | 18.5 |
| EC | 572 | 452 | 834 | 805 | 720 | 1012 | 1363 | 1152 | 1052 | 832 |
| pH | 7.51 | 8.59 | 8.40 | 7.80 | 7.51 | 8.02 | 8.31 | 8.03 | 7.00 | 6.98 |
| Eh | −50 | −108 | −98 | −66 | −50 | −77 | −93 | −78 | −23 | −22 |
| Na+ | 5.40 | 2.72 | 1.15 | 4.86 | 3.47 | 2.32 | 1.62 | 1.73 | 5.87 | 5.03 |
| K+ | 0.09 | 0.06 | 0.16 | 0.22 | 0.24 | 0.11 | 0.14 | 0.13 | 0.16 | 0.22 |
| Mg2+ | 2.24 | 2.14 | 1.59 | 0.66 | 0.71 | 1.56 | 3.87 | 2.07 | 1.07 | 0.66 |
| Ca2+ | 3.14 | 1.24 | 2.70 | 6.97 | 5.56 | 5.73 | 5.54 | 6.77 | 8.75 | 7.15 |
| F− | 0.01 | 0.02 | 0.03 | b.d.l. | 0.03 | 0.07 | 0.03 | 0.09 | 0.02 | b.d.l. |
| Cl− | 1.05 | 1.84 | 1.73 | 1.29 | 1.34 | 2.93 | 3.23 | 3.27 | 1.78 | 1.33 |
| Br− | b.d.l. | 0.05 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| NO3− | b.d.l. | b.d.l. | b.d.l. | 69.4 | 50.8 | 19.2 | b.d.l. | 45.3 | 68.8 | 74.4 |
| SO42− | 0.13 | 0.37 | 5.42 | 2.06 | 2.39 | 5.95 | 11.0 | 7.28 | 3.39 | 2.17 |
| PO43− | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 4.75 | 0.95 |
| CO32− + HCO3− | 5.40 | 2.72 | 1.15 | 4.86 | 3.47 | 2.32 | 1.62 | 1.73 | 5.87 | 5.03 |
| δ18O | −6.15 | −1.64 | 0.88 | −6.33 | −5.98 | −3.38 | 0.49 | 1.26 | −5.85 | −6.13 |
| Cha vul | 4 | 5 | 4 | |||||||
| Cha glo | 4 | 4 | 4 | 1 | ||||||
| Zan pal | 4 | |||||||||
| Stu pec | 4 | 4 | ||||||||
| Pot pus | 4 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzeca, P.; Troia, A.; Madonia, P. Aquatic Macrophytes Occurrence in Mediterranean Farm Ponds: Preliminary Investigations in North-Western Sicily (Italy). Plants 2021, 10, 1292. https://doi.org/10.3390/plants10071292
Panzeca P, Troia A, Madonia P. Aquatic Macrophytes Occurrence in Mediterranean Farm Ponds: Preliminary Investigations in North-Western Sicily (Italy). Plants. 2021; 10(7):1292. https://doi.org/10.3390/plants10071292
Chicago/Turabian StylePanzeca, Patrizia, Angelo Troia, and Paolo Madonia. 2021. "Aquatic Macrophytes Occurrence in Mediterranean Farm Ponds: Preliminary Investigations in North-Western Sicily (Italy)" Plants 10, no. 7: 1292. https://doi.org/10.3390/plants10071292
APA StylePanzeca, P., Troia, A., & Madonia, P. (2021). Aquatic Macrophytes Occurrence in Mediterranean Farm Ponds: Preliminary Investigations in North-Western Sicily (Italy). Plants, 10(7), 1292. https://doi.org/10.3390/plants10071292