Wind Exposure Regulates Water Oxygenation in Densely Vegetated Shallow Lakes
Abstract
1. Introduction
2. Results
2.1. Diel Variations in Vegetated Stands
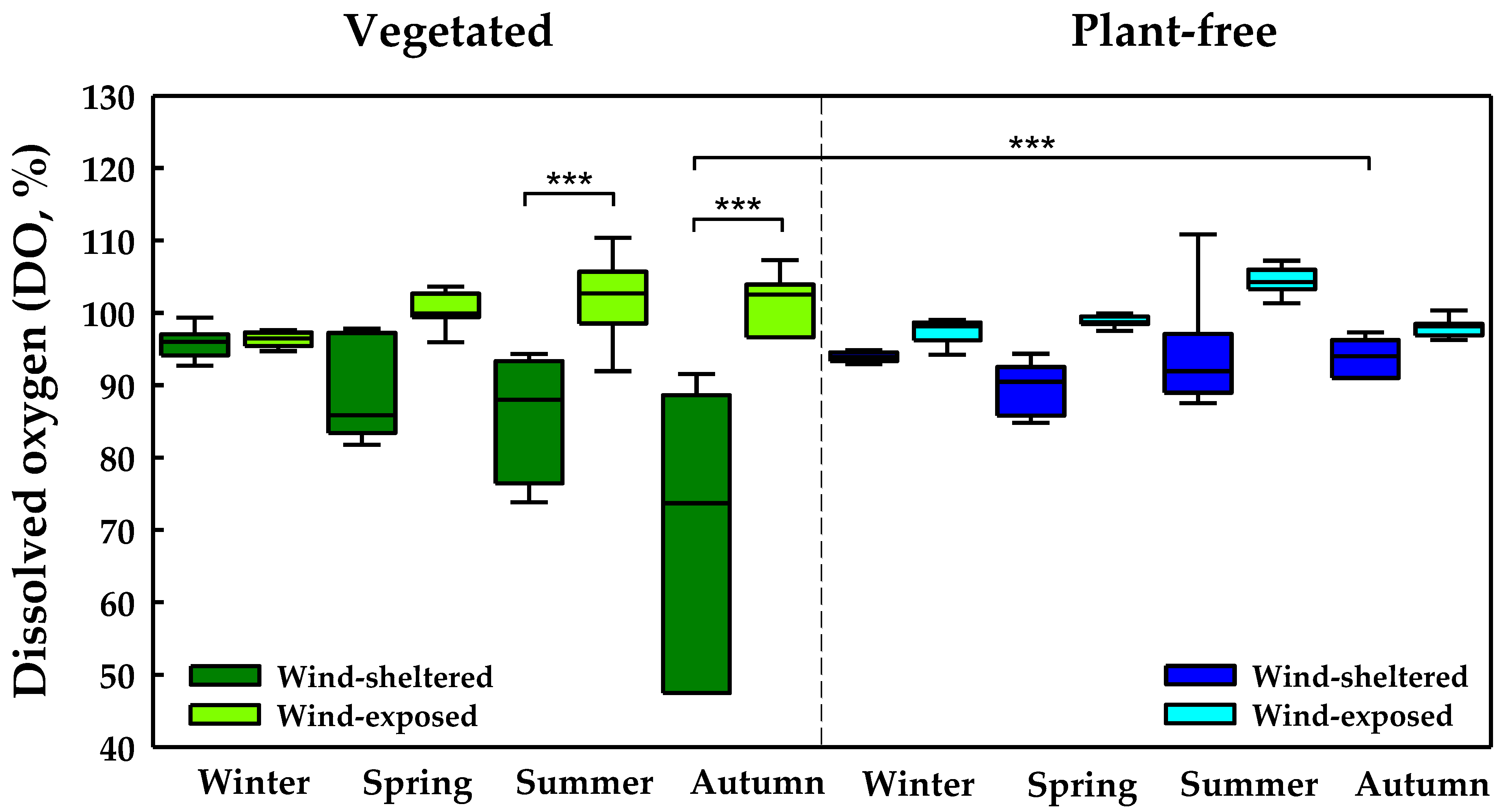
2.2. Wind-Sheltered vs. Wind-Exposed Sites
2.3. Dependence of DO Saturation on Plant Biomass and Sedimentary OM
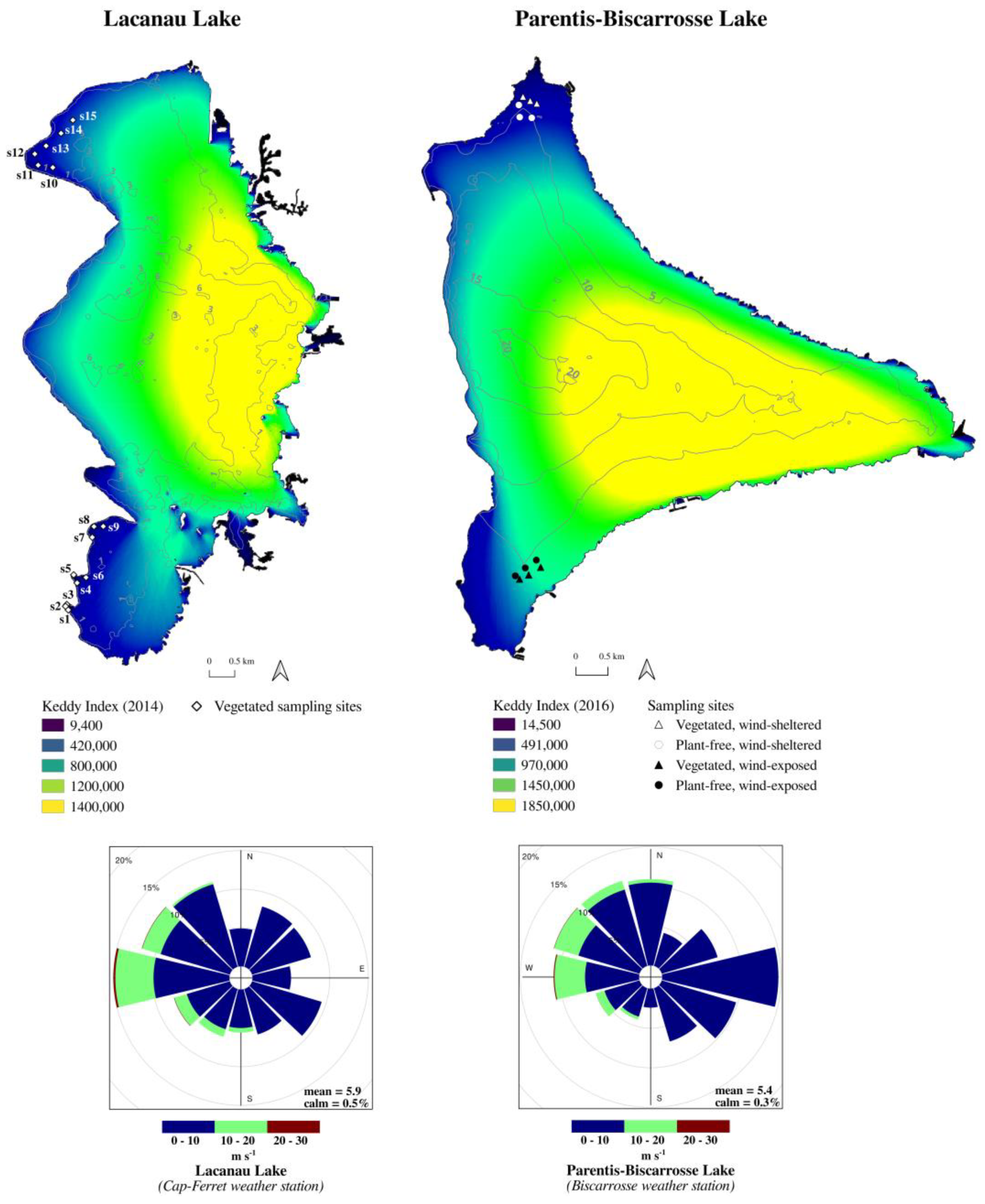
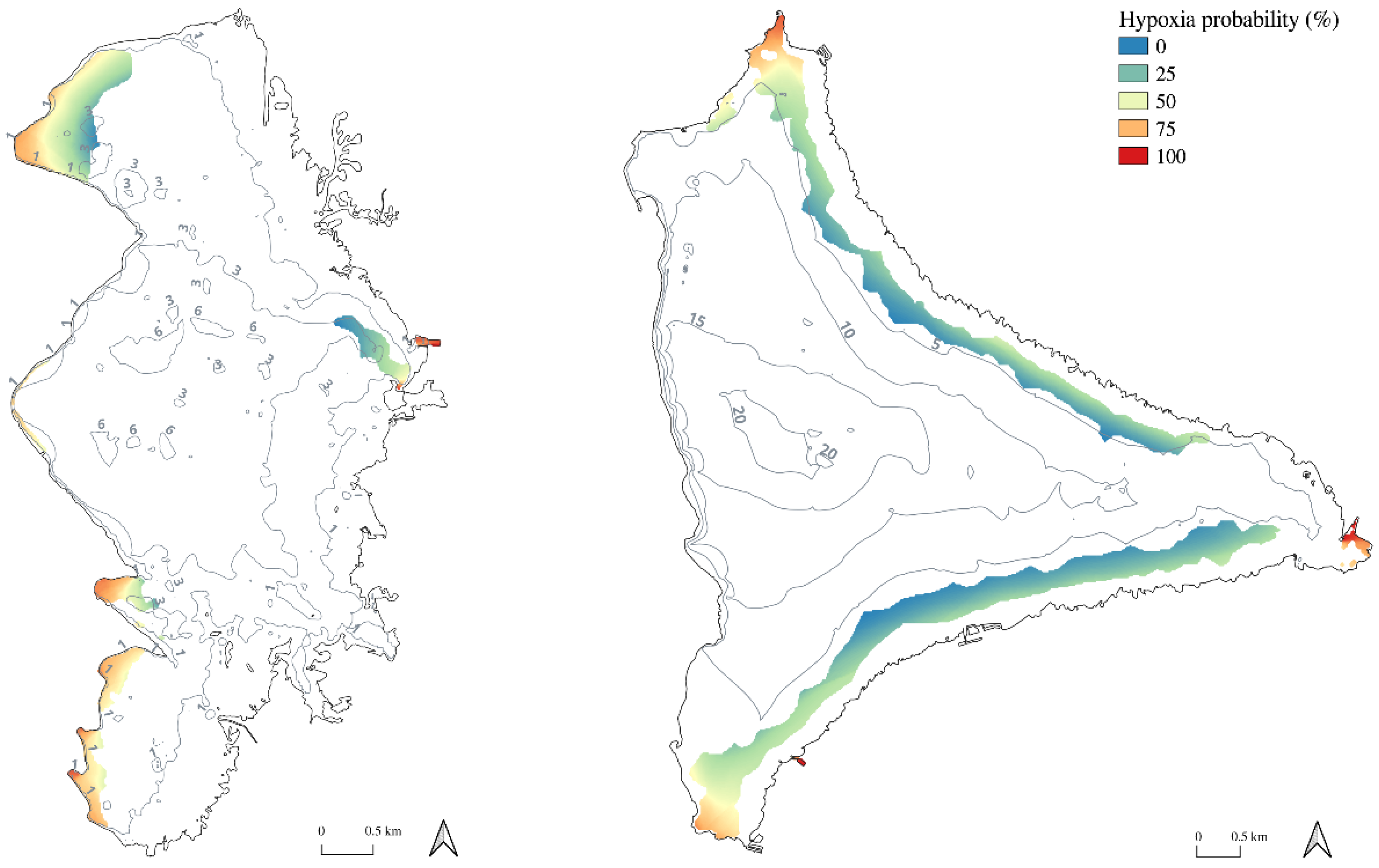
2.4. Dependence of DO on Wind Exposure and Hypoxia Risk Map Production
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Field Campaigns
4.3. Wind Exposure Calculations
4.4. Coupling DO and Wind Exposure
4.5. Hypoxia Risk Map Production
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caraco, N.F.; Cole, J.J. Contrasting impacts of a native and alien macrophyte on dissolved oxygen in a large river. Ecol. Appl. 2002, 12, 1496–1509. [Google Scholar] [CrossRef]
- Pokorný, J.; Rejmánková, E. Oxygen regime in a fishpond with duckweeds (Lemnaceae) and Ceratophyllum. Aquat. Bot. 1983, 17, 125–137. [Google Scholar] [CrossRef]
- Pelicice, F.M.; Agostinho, A.A.; Thomaz, S.M. Fish assemblages associated with Egeria in a tropical reservoir: Investigating the effects of plant biomass and diel period. Acta Oecologica 2005, 27, 9–16. [Google Scholar] [CrossRef]
- Sorrell, B.K.; Downes, M.T.; Stanger, C.L. Methanotrophic bacteria and their activity on submerged aquatic macrophytes. Aquat. Bot. 2002, 72, 107–119. [Google Scholar] [CrossRef]
- Yoshida, N.; Iguchi, H.; Yurimoto, H.; Murakami, A.; Sakai, Y. Aquatic plant surface as a niche for methanotrophs. Front. Microbiol. 2014, 5, 30. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Prahl, C.; Stokholm, H. Oxygen release from roots of submerged aquatic macrophytes. Oikos 1982, 38, 349–354. [Google Scholar] [CrossRef]
- Sorrell, B.K.; Dromgoole, F.I. Oxygen transport in the submerged freshwater macrophyte Egeria densa Planch. I. Oxygen production, storage and release. Aquat. Bot. 1987, 28, 63–80. [Google Scholar] [CrossRef]
- Ribaudo, C.; Bartoli, M.; Racchetti, E.; Longhi, D.; Viaroli, P. Seasonal fluxes of O2, DIC and CH4 in sediments with Vallisneria spiralis: Indications for radial oxygen loss. Aquat. Bot. 2011, 94, 134–142. [Google Scholar] [CrossRef]
- Haroon, M.F.; Hu, S.; Shi, Y.; Imelfort, M.; Keller, J.; Hugenholtz, P.; Yuan, Z.; Tyson, G.W. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 2013, 500, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Tucker, J.C.; Debusk, W.F. The role of Egeria in removing nitrogen and phosphorus from nutrient enriched waters. J. Aquat. Plant Manag. 1987, 25, 14–19. [Google Scholar]
- Drexler, J.Z.; Khanna, S.; Lacy, J.R. Carbon storage and sediment trapping by Egeria densa Planch, a globally invasive, freshwater macrophyte. Sci. Total Environ. 2020, 755, 142602. [Google Scholar] [CrossRef]
- Barko, J.W.; Godshalk, G.L.; Carter, V.; Rybicki, N.B. Effects of Submersed Aquatic Macrophytes on Physical and Chemical Properties of Surrounding Water; Aquatic Plant Control Research Program Technical Report A-88-1 I; Depart Army, U.S. Army Corps of Engineers: Washington, DC, USA, 1988; p. 28.
- Frodge, J.D.; Thomas, G.L.; Pauley, G.B. Effects of canopy formation by floating and submergent aquatic macrophytes on the water quality of two shallow Pacific Northwest lakes. Aquat. Bot. 1990, 38, 231–248. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Andersen, M.R.; Martinsen, K.T.; Borum, J.; Kristensen, E.; Kragh, T. Shallow plant-dominated lakes–extreme environmental variability, carbon cycling and ecological species challenges. Ann. Bot. 2019, 124, 355–366. [Google Scholar] [CrossRef]
- Moore, T.P.; Clearwater, S.J.; Duggan, I.C.; Collier, K.J. Invasive macrophytes induce context-specific effects on oxygen, pH, and temperature in a hydropeaking reservoir. River Res. Appl. 2020, 36, 1717–1729. [Google Scholar] [CrossRef]
- Herb, W.R.; Stefan, H.G. Dynamics of vertical mixing in a shallow lake with submersed macrophytes. Water Resour. Res. 2005, 41. [Google Scholar] [CrossRef]
- Coates, M.J.; Folkard, A.M. The effects of littoral zone vegetation on turbulent mixing in lakes. Ecol. Model. 2009, 220, 2714–2726. [Google Scholar] [CrossRef]
- Andersen, M.R.; Sand-Jensen, K.; Woolway, R.I.; Jones, I.D. Profound daily vertical stratification and mixing in a small, shallow, wind-exposed lake with submerged macrophytes. Aquat. Sci. 2017, 79, 395–406. [Google Scholar] [CrossRef]
- Ribaudo, C.; Tison-Rosebery, J.; Buquet, D.; Jan, G.; Jamoneau, A.; Abril, G.; Anschutz, P.; Bertrin, V. Invasive aquatic plants as ecosystem engineers in an Oligo-Mesotrophic shallow lake. Front. Plant Sci. 2018, 9, 1781. [Google Scholar] [CrossRef] [PubMed]
- Vilas, M.P.; Marti, C.L.; Oldham, C.E.; Hipsey, M.R. Macrophyte-induced thermal stratification in a shallow urban lake promotes conditions suitable for nitrogen-fixing cyanobacteria. Hydrobiologia 2018, 806, 411–426. [Google Scholar] [CrossRef]
- Zhang, X.; Nepf, H.M. Thermally driven exchange flow between open water and an aquatic canopy. J. Fluid Mech. 2009, 632, 227. [Google Scholar] [CrossRef]
- Ulloa, H.N.; Constantinescu, G.; Chang, K.; Horna-Munoz, D.; Hames, O.; Wüest, A. Horizontal transport under wind-induced resonance in stratified waterbodies. Phys. Rev. Fluids 2020, 5, 054503. [Google Scholar] [CrossRef]
- Lin, Y.T. Wind effect on diurnal thermally driven flow in vegetated nearshore of a lake. Environ. Fluid Mech. 2015, 15, 161–178. [Google Scholar] [CrossRef]
- Ulloa, H.N.; Constantinescu, G.; Chang, K.; Horna-Munoz, D.; Steiner, O.S.; Bouffard, D.; Wüest, A. Hydrodynamics of a periodically wind-forced small and narrow stratified basin: A large-eddy simulation experiment. Environ. Fluid Mech. 2019, 19, 667–698. [Google Scholar] [CrossRef]
- Torma, P.; Wu, C.H. Temperature and circulation dynamics in a small and shallow lake: Effects of weak stratification and littoral submerged macrophytes. Water 2019, 11, 128. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef]
- Matzek, V.; Covino, J.; Funk, J.L.; Saunders, M. Closing the knowing–doing gap in invasive plant management: Accessibility and interdisciplinarity of scientific research. Conserv. Lett. 2014, 7, 208–215. [Google Scholar] [CrossRef]
- Young, S.L.; Kettenring, K.M. The social-ecological system driving effective invasive plant management: Two case studies of non-native Phragmites. J. Environ. Manag. 2020, 267, 110612. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, S.; Ginelli, L. The victorious battles of the lost war against aquatic invasive plants. ‘Fluid’ categorisation and multiple forms of ordinary commitment. Trans. Inst. Br. Geogr. 2021. [Google Scholar] [CrossRef]
- Cole, J.J.; Pace, M.L.; Carpenter, S.R.; Kitchell, J.F. Persistence of net heterotrophy in lakes during nutrient addition and food web manipulations. Limnol. Oceanogr. 2000, 45, 1718–1730. [Google Scholar] [CrossRef]
- Bini, L.M.; Thomaz, S.M.; Carvalho, P. Limnological effects of Egeria najas planchon (Hydrocharitaceae) in the arms of Itaipu Reservoir (Brazil, Paraguay). Limnology 2010, 11, 39–47. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J.; Wang, W.; Yang, L.; Yin, C. Methane fluxes from the littoral zone of hypereutrophic Taihu Lake, China. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef]
- Brothers, S.; Kazanjian, G.; Köhler, J.; Scharfenberger, U.; Hilt, S. Convective mixing and high littoral production established systematic errors in the diel oxygen curves of a shallow, eutrophic lake. Limnol. Oceanogr. Methods 2017, 15, 429–435. [Google Scholar] [CrossRef]
- Bertrin, V.; Boutry, S.; Jan, G.; Ducasse, G.; Grigoletto, F.; Ribaudo, C. Effects of wind-induced sediment resuspension on distribution and morphological traits of aquatic weeds in shallow lakes. J. Limnol. 2017, 76, 84–96. [Google Scholar] [CrossRef]
- Tastet, J.P.; Lalanne, R.; Maurin, B.; Dubos, B. Geological and archaeological chronology of a late Holocene coastal enclosure: The Sanguinet lake (SW France). Geoarchaeology 2008, 23, 131–149. [Google Scholar] [CrossRef]
- Tamayo, M.; Olden, J. Forecasting the vulnerability of lakes to aquatic plant invasions. Invasive Plant Sci. Manag. 2014, 7, 32–45. [Google Scholar] [CrossRef]
- Bertrin, V.; Boutry, S.; Alard, D.; Haury, J.; Jan, G.; Moreira, S.; Ribaudo, C. Prediction of macrophyte distribution: The role of natural versus anthropogenic physical disturbances. Appl. Veg. Sci. 2018, 21, 395–410. [Google Scholar] [CrossRef]
- Deeds, J.; Amirbahman, A.; Norton, S.A.; Suitor, D.G.; Bacon, L.C. Predicting anoxia in low-nutrient temperate lakes. Ecol. Appl. 2021. [Google Scholar] [CrossRef]
- Carper, G.L.; Bachmann, R.W. Wind resuspension of sediments in a prairie lake. Can. J. Fish. Aquat. Sci. 1984, 41, 1763–1767. [Google Scholar] [CrossRef]
- Schutten, J.; Dainty, J.; Davy, A. Wave-induced hydraulic forces on submerged aquatic plants in shallow lakes. Ann. Bot. 2004, 93, 333–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keddy, P.A. Quantifying within-lake gradients of wave energy: Interrelationships of wave energy, substrate particle size and shoreline plants in axe lake, Ontario. Aquat. Bot. 1982, 14, 41–58. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 23 May 2021).
- Jalil, A.; Li, Y.; Du, W.; Wang, W.; Wang, J.; Gao, X.; Khan, H.O.S.; Pan, B.; Acharya, K. The role of wind field induced flow velocities in destratification and hypoxia reduction at Meiling Bay of large shallow Lake Taihu, China. Environ. Pollut. 2018, 232, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Chow, G.C. Tests of equality between sets of coefficients in two linear regressions. Econom. J. Econom. Soc. 1960, 591–605. [Google Scholar] [CrossRef]


| T (°C) | pH Units | DO (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | df, Residuals | p-Value | df, Residuals | p-Value | df, Residuals | p-Value | ||
| Diel variation | 1, 204 | <0.001 | 1, 204 | n.s. | 1, 383 | n.s. | ||
| Season | 2, 204 | <0.001 | 2, 204 | <0.001 | 2, 383 | <0.001 | ||
| Diel × Season | 2, 204 | n.s. | 2, 204 | n.s. | 2, 383 | n.s. | ||
| CO2 (%) | CH4 (µM) | NH4+ (µM) | NO3− (µM) | |||||
| df, Residuals | p-Value | df, Residuals | p-Value | df, Residuals | p-Value | df, Residuals | p-Value | |
| Diel variation | 1, 347 | n.s. | 1, 415 | n.s. | 1, 410 | <0.05 | 1, 394 | n.s. |
| Season | 2, 347 | <0.001 | 2, 415 | <0.001 | 2, 410 | <0.05 | 2, 394 | <0.001 |
| Diel × Season | 2, 347 | n.s. | 2, 415 | n.s. | 2, 410 | n.s. | 2, 394 | n.s. |
| T (°C) | pH Units | DO (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | df, Residuals | p-Value | df, Residuals | p-Value | df, Residuals | p-Value | ||
| Wind exposure | 1, 8 | <0.001 | 1, 8 | <0.001 | 1, 8 | <0.001 | ||
| Plant presence | 1, 8 | n.s. | 1, 8 | n.s. | 1, 8 | n.s. | ||
| Season | 3, 24 | <0.001 | 3, 24 | <0.001 | 3, 72 | <0.05 | ||
| Wind × Plant | 1, 8 | n.s. | 1, 8 | <0.05 | 1, 8 | <0.05 | ||
| Wind × Seas | 3, 24 | <0.001 | 3, 24 | <0.001 | 3, 72 | <0.001 | ||
| Plant × Seas | 3, 24 | n.s. | 3, 24 | n.s. | 3, 72 | <0.05 | ||
| Wind × Plant × Seas | 3, 24 | n.s. | 3, 24 | n.s. | 3, 72 | <0.001 | ||
| CO2 (%) | CH4 (µM) | NH4+ (µM) | NO3− (µM) | |||||
| df, Residuals | p-Value | df, Residuals | p-Value | df, Residuals | p-Value | df, Residuals | p-Value | |
| Wind exposure | 1, 8 | <0.001 | 1, 8 | <0.001 | 1, 8 | n.s. | 1, 8 | <0.05 |
| Plant presence | 1, 8 | n.s. | 1, 8 | n.s. | 1, 8 | n.s. | 1, 8 | n.s. |
| Season | 3, 72 | <0.001 | 3, 72 | <0.001 | 3, 72 | <0.001 | 3, 72 | <0.001 |
| Wind × Plant | 1, 8 | <0.05 | 1, 8 | n.s. | 1, 8 | n.s. | 1, 8 | <0.05 |
| Wind × Seas | 3, 72 | <0.001 | 3, 72 | <0.001 | 3, 72 | <0.001 | 3, 72 | <0.001 |
| Plant × Seas | 3, 72 | <0.001 | 3, 72 | <0.05 | 3, 72 | n.s. | 3, 72 | n.s. |
| Wind × Plant × Seas | 3, 72 | <0.001 | 3, 72 | <0.05 | 3, 72 | n.s. | 3, 72 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribaudo, C.; Tison-Rosebery, J.; Eon, M.; Jan, G.; Bertrin, V. Wind Exposure Regulates Water Oxygenation in Densely Vegetated Shallow Lakes. Plants 2021, 10, 1269. https://doi.org/10.3390/plants10071269
Ribaudo C, Tison-Rosebery J, Eon M, Jan G, Bertrin V. Wind Exposure Regulates Water Oxygenation in Densely Vegetated Shallow Lakes. Plants. 2021; 10(7):1269. https://doi.org/10.3390/plants10071269
Chicago/Turabian StyleRibaudo, Cristina, Juliette Tison-Rosebery, Mélissa Eon, Gwilherm Jan, and Vincent Bertrin. 2021. "Wind Exposure Regulates Water Oxygenation in Densely Vegetated Shallow Lakes" Plants 10, no. 7: 1269. https://doi.org/10.3390/plants10071269
APA StyleRibaudo, C., Tison-Rosebery, J., Eon, M., Jan, G., & Bertrin, V. (2021). Wind Exposure Regulates Water Oxygenation in Densely Vegetated Shallow Lakes. Plants, 10(7), 1269. https://doi.org/10.3390/plants10071269





