Hypusination, a Metabolic Posttranslational Modification of eIF5A in Plants during Development and Environmental Stress Responses
Abstract
1. Introduction
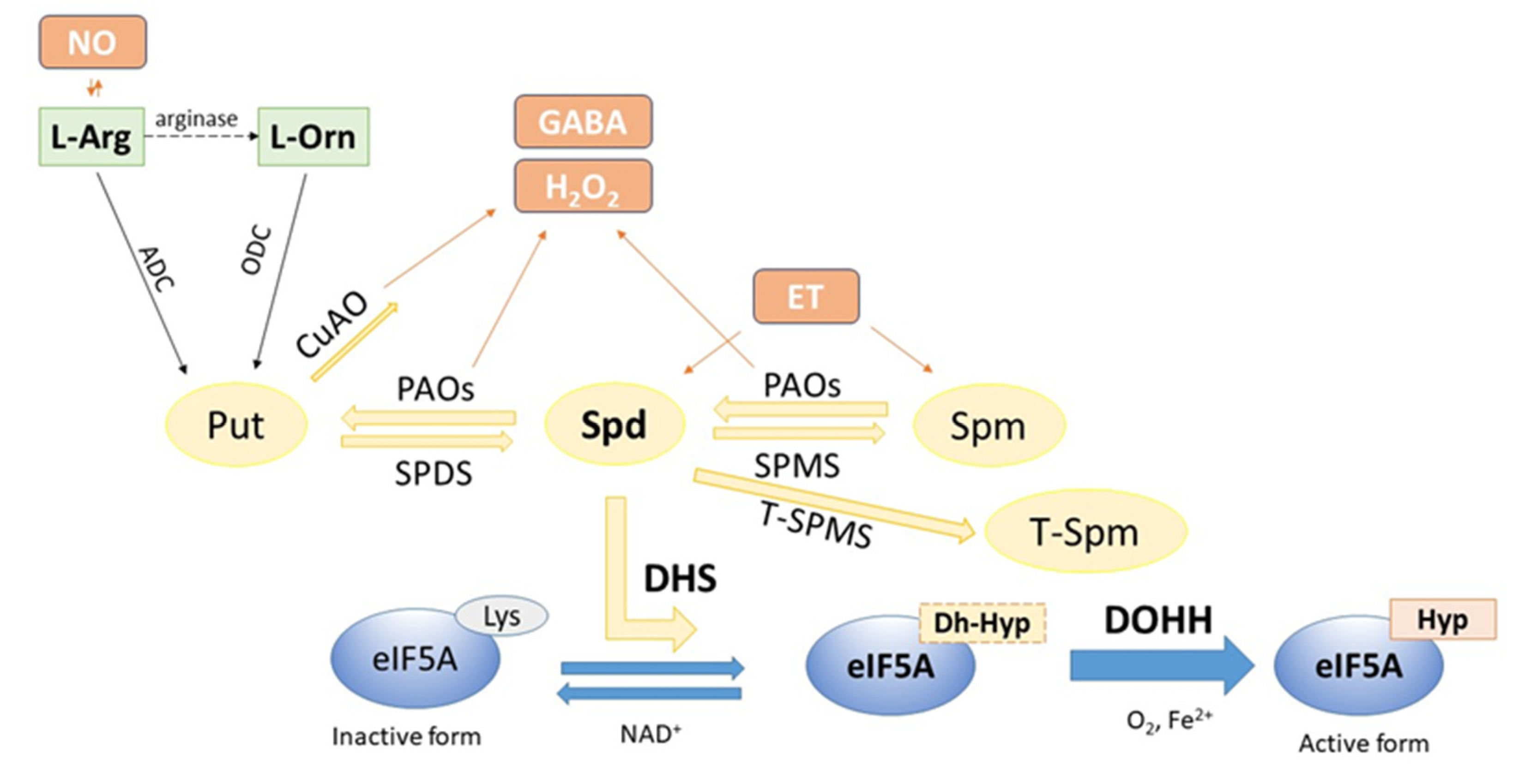
2. Polyamine-Dependent Hypusination of eIF5A in Plants
3. Two Steps of Hypusination
3.1. First Step: Synthesis of Deoxyhypusine by Deoxyhypusine Synthase (DHS)
3.2. Second Step: Synthesis of Hypusine by Deoxyhypusine Hydroxylase (DOHH)
4. Functions of eIF5A in Plants
5. Diverse Roles of eIF5A Isoforms in Plants
5.1. eIF5A-1
5.2. eIF5A-2 or FBR12
5.3. eIF5A-3
6. Potential Biotechnological Application of eIF5A-Related Processes of Plants
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Park, M.H.; Wolff, E.C. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J. Biol. Chem. 2018, 293, 18710–18718. [Google Scholar] [CrossRef]
- Belda-Palazón, B.; Almendáriz, C.; Martí, E.; Carbonell, J.; Ferrando, A. Relevance of the Axis Spermidine/eIF5A for Plant Growth and Development. Front. Plant Sci. 2016, 7, 245. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrian, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Wang, T.-W.; Lu, L.; Zhang, C.-G.; Taylor, C.; Thompson, J.E. Pleiotropic effects of suppressing deoxyhypusine synthase expression in Arabidopsis thaliana. Plant Mol. Biol. 2003, 52, 1223–1235. [Google Scholar] [CrossRef]
- Ma, F.; Liu, Z.; Wang, T.-W.; Hopkins, M.T.; Peterson, C.A.; Thompson, J.E. Arabidopsis eIF5A3 influences growth and the response to osmotic and nutrient stress. Plant Cell Environ. 2010, 33, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, B.; Jiang, C.; Ming, F. RceIF5A, encoding an eukaryotic translation initiation factor 5A in Rosa chinensis, can enhance thermotolerance, oxidative and osmotic stress resistance of Arabidopsis thaliana. Plant Mol. Biol. 2010, 75, 167–178. [Google Scholar] [CrossRef]
- Thompson, J.E.; Hopkins, M.T.; Taylor, C.; Wang, T.-W. Regulation of senescence by eukaryotic translation initiation factor 5A: Implications for plant growth and development. Trends Plant Sci. 2004, 9, 174–179. [Google Scholar] [CrossRef]
- Gutierrez, E.; Shin, B.-S.; Woolstenhulme, C.J.; Kim, J.-R.; Saini, P.; Buskirk, A.R.; Dever, T.E. eIF5A Promotes Translation of Polyproline Motifs. Mol. Cell 2013, 51, 35–45. [Google Scholar] [CrossRef]
- Schuller, A.; Wu, C.; Dever, T.E.; Buskirk, A.R.; Green, R. eIF5A Functions Globally in Translation Elongation and Termination. Mol. Cell 2017, 66, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Pelechano, V.; Alepuz, P. eIF5A facilitates translation termination globally and promotes the elongation of many non polyproline-specific tripeptide sequences. Nucleic Acids Res. 2017, 45, 7326–7338. [Google Scholar] [CrossRef] [PubMed]
- Belda-Palazã, N.B.; Nohales, M.A.; Rambla, J.L.; Aceña, J.L.; Delgado, O.; Fustero, S.; Martínez, M.C.; Granell, A.; Carbonell, J.; Ferrando, A.; et al. Biochemical quantitation of the eIF5A hypusination in Arabidopsis thaliana uncovers ABA-dependent regulation. Front. Plant Sci. 2014, 5, 202. [Google Scholar] [CrossRef]
- Mandal, A.; Mandal, S.; Park, M.H. Genome-Wide Analyses and Functional Classification of Proline Repeat-Rich Proteins: Potential Role of eIF5A in Eukaryotic Evolution. PLoS ONE 2014, 9, e111800. [Google Scholar] [CrossRef]
- Lewandowska-Gnatowska, E.; Szymona, L.; Łebska, M.; Szczegielniak, J.; Muszyńska, G. Phosphorylation of maize eukaryotic translation initiation factor on Ser2 by catalytic subunit CK2. Mol. Cell. Biochem. 2011, 356, 241–244. [Google Scholar] [CrossRef]
- Poidevin, L.; Unal, D.; Belda-Palazón, B.; Ferrando, A. Polyamines as Quality Control Metabolites Operating at the Post-Transcriptional Level. Plants 2019, 8, 109. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef]
- Szepesi, Á. Molecular Mechanisms of Polyamines-Induced Abiotic Stress Tolerance in Plants. In Approaches for Enhancing Abiotic Stress Tolerance in Plants; Hasanuzzaman, M., Nahar, K., Fujita, M., Oku, H., Islam, T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 387–403. [Google Scholar]
- Urano, K.; Hobo, T.; Shinozaki, K. Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed devel-opment. FEBS Lett. 2005, 579, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Unique Characteristics of the Parasite Polyamine Pathway. Structure 2018, 26, 1427–1429. [Google Scholar] [CrossRef] [PubMed]
- Coni, S.; Serrao, S.M.; Yurtsever, Z.N.; Di Magno, L.; Bordone, R.; Bertani, C.; Licursi, V.; Ianniello, Z.; Infante, P.; Moretti, M.; et al. Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Manjunath, H.; Zhang, H.; Rehfeld, F.; Han, J.; Chang, T.-C.; Mendell, J.T. Suppression of Ribosomal Pausing by eIF5A Is Necessary to Maintain the Fidelity of Start Codon Selection. Cell Rep. 2019, 29, 3134–3146.e6. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.; Song, J.; Liu, J.-H. Polyamine Catabolism in Plants: A Universal Process with Diverse Functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Nishimura, K.; Murozumi, K.; Shirahata, A.; Park, M.H.; Kashiwagi, K.; Igarashi, K. Independent roles of eIF5A and polyamines in cell proliferation. Biochem. J. 2005, 385, 779–785. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. The nitric oxide donor sodium nitroprusside regulates polyamines and proline me-tabolism in leaves of Medicago truncatula plants. Free Radic. Biol. Med. 2013, 56, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef]
- Esong, Y.; Exiang, F.; Ezhang, G.; Emiao, Y.; Esong, C.-P. Abscisic Acid as an Internal Integrator of Multiple Physiological Processes Modulates Leaf Senescence Onset in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 181. [Google Scholar] [CrossRef]
- Parkash, J.; Vaidya, T.; Kirti, S.; Dutt, S. Translation initiation factor 5A in Picrorhiza is up-regulated during leaf senescence and in response to abscisic acid. Gene 2014, 542, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chen, Q.; Feng, J.; Zhang, J.; Yang, X.; Zuo, J. Functional Characterization of the Arabidopsis Eukaryotic Translation Initiation Factor 5A-2 That Plays a Crucial Role in Plant Growth and Development by Regulating Cell Division, Cell Growth, and Cell Death. Plant Physiol. 2007, 144, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Duguay, J.; Ma, F.; Wang, T.-W.; Tshin, R.; Hopkins, M.T.; McNamara, L.; Thompson, J.E. Modulation of eIF5A1 expression alters xylem abundance in Arabidopsis thaliana. J. Exp. Bot. 2008, 59, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.K.; Tabor, C.W.; Tabor, H. Spermidine but not spermine is essential for hypusine biosynthesis and growth in Saccharomyces cerevisiae: Spermine is converted to spermidine in vivo by the FMS1-amine oxidase. Proc. Natl. Acad. Sci. USA 2003, 100, 13869–13874. [Google Scholar] [CrossRef]
- Pagnussat, G.; Yu, H.-J.; Ngo, Q.A.; Rajani, S.; Mayalagu, S.; Johnson, C.S.; Capron, A.; Xie, L.-F.; Ye, D.; Sundaresan, V. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 2005, 132, 603–614. [Google Scholar] [CrossRef]
- Liu, J.; Chang, X.; Ding, B.; Zhong, S.; Peng, L.; Wei, Q.; Meng, J.; Yu, Y. PhDHS Is Involved in Chloroplast Development in Petunia. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Duguay, J.; Jamal, S.; Liu, Z.; Wang, T.-W.; Thompson, J.E. Leaf-specific suppression of deoxyhypusine synthase in Arabidopsis thaliana enhances growth without negative pleiotropic effects. J. Plant Physiol. 2007, 164, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-W.; Wu, W.; Zhang, C.-G.; Nowack, L.M.; Liu, Z.; Thompson, J.E. Antisense suppression of deoxyhypusine synthase by vacuum-infiltration ofAgrobacteriumenhances growth and seed yield of canola. Physiol. Plant. 2005, 124, 493–503. [Google Scholar] [CrossRef]
- Wang, T.-W.; Lu, L.; Wang, D.; Thompson, J.E. Isolation and Characterization of Senescence-induced cDNAs Encoding Deoxyhypusine Synthase and Eucaryotic Translation Initiation Factor 5A from Tomato. J. Biol. Chem. 2001, 276, 17541–17549. [Google Scholar] [CrossRef] [PubMed]
- Ober, D.; Gibas, L.; Witte, L.; Hartmann, T. Evidence for general occurrence of homospermidine in plants and its supposed origin as by-product of deoxyhypusine synthase. Phytochemistry 2003, 62, 339–344. [Google Scholar] [CrossRef]
- Ober, D.; Hartmann, T. Deoxyhypusine Synthase from Tobacco. J. Biol. Chem. 1999, 274, 32040–32047. [Google Scholar] [CrossRef] [PubMed]
- Reimann, A.; Nurhayati, N.; Backenköhler, A.; Ober, D. Repeated evolution of the pyrrolizidine alkaloid-mediated defense system in separate angiosperm lineages. Plant Cell 2004, 16, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Wątor, E.; Wilk, P.; Grudnik, P. Half Way to Hypusine—Structural Basis for Substrate Recognition by Human Deoxyhypusine Synthase. Biomolecules 2020, 10, 522. [Google Scholar] [CrossRef]
- Thompson, G.M.; Cano, V.S.P.; Valentini, S.R. Mapping eIF5A binding sites for Dys1 and Lia1: In vivo evidence for regulation of eIF5A hypusination. FEBS Lett. 2003, 555, 464–468. [Google Scholar] [CrossRef]
- Chen, M.; Gai, Z.; Okada, C.; Ye, Y.; Yu, J.; Yao, M. Flexible NAD+ Binding in Deoxyhypusine Synthase Reflects the Dynamic Hypusine Modification of Translation Factor IF5A. Int. J. Mol. Sci. 2020, 21, 5509. [Google Scholar] [CrossRef]
- Von Koschitzky, I.; Gerhardt, H.; Lämmerhofer, M.; Kohout, M.; Gehringer, M.; Laufer, S.; Pink, M.; Schmitz-Spanke, S.; Strube, C.; Kaiser, A. New insights into novel inhibitors against deoxyhypusine hydroxylase from plasmodium falciparum: Compounds with an iron chelating potential. Amino Acids 2015, 47, 1155–1166. [Google Scholar] [CrossRef]
- Frey, A.G.; Nandal, A.; Park, J.H.; Smith, P.M.; Yabe, T.; Ryu, M.-S.; Ghosh, M.C.; Lee, J.; Rouault, T.A.; Park, M.H.; et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc. Natl. Acad. Sci. USA 2014, 111, 8031–8036. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Hung, L.-W.; Yokota, H.; Kim, R.; Kim, S.-H. Crystal structures of eukaryotic translation initiation factor 5A from Methanococcus jannaschii at 1.8 A resolution. Proc. Natl. Acad. Sci. USA 1998, 95, 10419–10424. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Chen, K.Y. Hypusine Is Required for a Sequence-specific Interaction of Eukaryotic Initiation Factor 5A with Postsystematic Evolution of Ligands by Exponential Enrichment RNA. J. Biol. Chem. 2001, 276, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Parreiras-E-Silva, L.T.; Gomes, M.D.; Oliveira, E.B.; Costa-Neto, C.M. The N-terminal region of eukaryotic translation initiation factor 5A signals to nuclear localization of the protein. Biochem. Biophys. Res. Commun. 2007, 362, 393–398. [Google Scholar] [CrossRef]
- Maier, B.; Ogihara, T.; Trace, A.P.; Tersey, S.A.; Robbins, R.D.; Chakrabarti, S.K.; Nunemaker, C.S.; Stull, N.D.; Taylor, C.A.; Thompson, J.E.; et al. The unique hypusine modification of eIF5A promotes islet β cell inflammation and dysfunction in mice. J. Clin. Investig. 2010, 120, 2156–2170. [Google Scholar] [CrossRef]
- Wu, G.-Q.; Xu, Y.-M.; Lau, A.T.Y. Recent insights into eukaryotic translation initiation factors 5A1 and 5A2 and their roles in human health and disease. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Łebska, M.; Ciesielski, A.; Szymona, L.; Godecka, L.; Lewandowska-Gnatowska, E.; Szczegielniak, J.; Muszyńska, G. Phosphorylation of Maize Eukaryotic Translation Initiation Factor 5A (eIF5A) by Casein Kinase 2: Identification of phosphorylated residue and influence on intracellular localization of eIF5A. J. Biol. Chem. 2010, 285, 6217–6226. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Miura, E.; Lucas, W.J.; Ham, B.-K.; Cheng, H.-W.; Lee, Y.-J. Pumpkin eIF5A isoforms interact with components of the translational machinery in the cucurbit sieve tube system. Plant J. 2010, 64, 536–550. [Google Scholar] [CrossRef]
- Wang, L.; Huang, G.-Q.; Sun, Y.; Li, Y.; Yao, W.-J.; Jiang, T.-B. Cloning and expression analysis of eIF-5A gene in Apocynum venetum. Biotechnol. Biotechnol. Equip. 2016, 30, 677–684. [Google Scholar] [CrossRef]
- Schroeder, S.; Hofer, S.J.; Zimmermann, A.; Pechlaner, R.; Dammbrueck, C.; Pendl, T.; Marcello, G.M.; Pogatschnigg, V.; Bergmann, M.; Müller, M.; et al. Dietary spermidine improves cognitive function. Cell Rep. 2021, 35, 108985. [Google Scholar] [CrossRef] [PubMed]
- Faundes, V.; Jennings, M.D.; Crilly, S.; Legraie, S.; Withers, S.E.; Cuvertino, S.; Davies, S.J.; Douglas, A.G.L.; Fry, A.E.; Harrison, V.; et al. Impaired eIF5A function causes a Mendelian disorder that is partially rescued in model systems by spermidine. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Carmona-Gutierrez, D.; Madeo, F. Spermidine supplementation in rare translation associated disorders. Cell Stress 2021, 5, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Arsovski, A.A.; Yu, K.; Wang, A. Deep sequencing leads to the identification of eukaryotic translation initiation factor 5A as a key element in Rsv1 -mediated lethal systemic hypersensitive response to Soybean mosaic virus infection in soybean. Mol. Plant Pathol. 2016, 18, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.A.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Cordts, S.; Lörz, H. A transcript encoding translation initiation factor eIF-5A is stored in unfertilized egg cells of maize. Plant Mol. Biol. 1999, 39, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.T.; Lampi, Y.; Wang, T.-W.; Liu, Z.; Thompson, J.E. Eukaryotic Translation Initiation Factor 5A Is Involved in Pathogen-Induced Cell Death and Development of Disease Symptoms in Arabidopsis. Plant Physiol. 2008, 148, 479–489. [Google Scholar] [CrossRef]
- Wang, T.-W.; Zhang, C.-G.; Wu, W.; Nowack, L.M.; Madey, E.; Thompson, J.E. Antisense Suppression of Deoxyhypusine Synthase in Tomato Delays Fruit Softening and Alters Growth and Development. Plant Physiol. 2005, 138, 1372–1382. [Google Scholar] [CrossRef]
- Puleston, D.J.; Buck, M.; Geltink, R.K.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019, 30, 352–363.e8. [Google Scholar] [CrossRef]
- Ko, J.; Prassinos, C.; Han, K. Developmental and seasonal expression of PtaHB1, a Populus gene encoding a class III HD-Zip protein, is closely associated with secondary growth and inversely correlated with the level of microRNA ( miR166 ). New Phytol. 2006, 169, 469–478. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Wang, C.; Wang, Y. Characterization of a eukaryotic translation initiation factor 5A homolog from Tamarix androssowii involved in plant abiotic stress tolerance. BMC Plant Biol. 2012, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, C.X.; Wang, C.; Wang, Y. A eukaryotic translation initiation factor 5A from Tamarix androssowii (Tamarisk), TaeIF5A1, can form a homodimer and interact with other proteins. Plant Omics J. 2014, 7, 468–473. [Google Scholar]
- Hyodo, H.; Yamakawa, S.; Takeda, Y.; Tsuduki, M.; Yokota, A.; Nishitani, K.; Kohchi, T. Active gene expression of a xyloglucan endotransglucosylase/hydrolase gene, XTH9, in inflorescence apices is related to cell elongation in Arabidopsis thaliana. Plant Mol. Biol. 2003, 52, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Valentini, S.R.; Casolari, J.M.; Oliveira, C.; A Silver, P.; E McBride, A. Genetic Interactions of Yeast Eukaryotic Translation Initiation Factor 5A (eIF5A) Reveal Connections to Poly(A)-Binding Protein and Protein Kinase C Signaling. Genetics 2002, 160, 393–405. [Google Scholar] [CrossRef]
- Li, T.; Belda-Palazón, B.; Ferrando, A.; Alepuz, P. Fertility and Polarized Cell Growth Depends on eIF5A for Translation of Polyproline-Rich Formins in Saccharomyces cerevisiae. Genetics 2014, 197, 1191–1200. [Google Scholar] [CrossRef]
- Muñoz-Soriano, V.; Domingo-Muelas, A.; Li, T.; Gamero, E.; Bizy, A.; Fariñas, I.; Alepuz, P.; Paricio, N. Evolutionary conserved role of eukaryotic translation factor eIF5A in the regulation of actin-nucleating formins. Sci. Rep. 2017, 7, 9580. [Google Scholar] [CrossRef]
- Chamot, D.; Kuhlemeier, C. Differential expression of genes encoding the hypusine-containing translation initiation factor, elF-5A, in tobacco. Nucleic Acids Res. 1992, 20, 665–669. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T.; Renaut, J. Plant Abiotic Stress Proteomics: The Major Factors Determining Alterations in Cellular Proteome. Front. Plant Sci. 2018, 9, 122. [Google Scholar] [CrossRef]
- Maršálová, L.; Vítámvás, P.; Hynek, R.; Prášil, I.T.; Kosová, K. Proteomic Response of Hordeum vulgare cv. Tadmor and Hordeum marinum to Salinity Stress: Similarities and Differences between a Glycophyte and a Halophyte. Front. Plant Sci. 2016, 7, 1154. [Google Scholar] [CrossRef]
- Ren, B.; Chen, Q.; Hong, S.; Zhao, W.; Feng, J.; Feng, H.; Zuo, J. The Arabidopsis Eukaryotic Translation Initiation Factor eIF5A-2 Regulates Root Protoxylem Development by Modulating Cytokinin Signaling. Plant Cell 2013, 25, 3841–3857. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Ding, Z.-J.; Chen, L.; Yan, J.-Y.; Li, G.-X.; Zheng, S.-J. An eukaryotic translation initiation factor, AteIF5A-2, affects cadmium accumulation and sensitivity in Arabidopsis. J. Integr. Plant Biol. 2015, 57, 848–858. [Google Scholar] [CrossRef]
- Chou, W.-C.; Huang, Y.-W.; Tsay, W.-S.; Chiang, T.-Y.; Huang, D.-D.; Huang, H.-J. Expression of genes encoding the rice translation initiation factor, eIF5A, is involved in developmental and environmental responses. Physiol. Plant. 2004, 121, 50–57. [Google Scholar] [CrossRef]
- Barba-Aliaga, M.; Villarroel-Vicente, C.; Stanciu, A.; Corman, A.; Martínez-Pastor, M.T.; Alepuz, P. Yeast Translation Elongation Factor eIF5A Expression Is Regulated by Nutrient Availability through Different Signalling Pathways. Int. J. Mol. Sci. 2020, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Schmidt, W. The enigma of eIF5A in the iron deficiency response of Arabidopsis. Plant Signal. Behav. 2011, 6, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.P.; Finley, J.L.; Latour, Y.L.; Asim, M.; Smith, T.M.; Verriere, T.G.; Barry, D.P.; Allaman, M.M.; Delagado, A.G.; Rose, K.L.; et al. Hypusination Orchestrates the Antimicrobial Response of Macrophages. Cell Rep. 2020, 33, 108510. [Google Scholar] [CrossRef]
- Martinez-Rocha, A.L.; Woriedh, M.; Chemnitz, J.; Willingmann, P.; Kröger, C.; Hadeler, B.; Hauber, J.; Schäfer, W. Posttranslational hypusination of the eukaryotic translation initiation factor-5A regulates Fusarium graminearum virulence. Sci. Rep. 2016, 6, 24698. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.; Schemmerling, B.; Ober, D. CRISPR/Cas9-Mediated Genome Editing in Comfrey (Symphytum officinale) Hairy Roots Results in the Complete Eradication of Pyrrolizidine Alkaloids. Molecules 2021, 26, 1498. [Google Scholar] [CrossRef]
- Park, M.H.; Mandal, A.; Mandal, S.; Wolff, E.C. A new non-radioactive deoxyhypusine synthase assay adaptable to high throughput screening. Amino Acids 2017, 49, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
| Protein Isoform | Synonyms | Protein Accession No. | Biological Process | Ref. |
|---|---|---|---|---|
| eIF5A-1 | Atelf5A-1 eif-5A eif5A, elf5A-1 eukaryotic elongation factor 5A, eukaryotic elongation factor 5A-1 | AT1G13950 | shuttle protein translocating mRNA from the nucleus to cytoplasmic ribosomes Xylem formation | [29,33] |
| eIF5A-2 | Atelf5A-2, elf5A-2 eukaryotic elongation factor 5A-2, FBR12 fumonisin B1-resistant12 | AT1G26630 | Involved in programmed cell death triggered as a response to pseudomonas syringae infection. The mRNA is cell-to-cell mobile | [58,71] |
| eIF5A-3 | Atelf5A-3, elf5A-3, eukaryotic elongation factor 5A-3 | AT1G69410 | Growth during osmotic and nutrient stress | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pálfi, P.; Bakacsy, L.; Kovács, H.; Szepesi, Á. Hypusination, a Metabolic Posttranslational Modification of eIF5A in Plants during Development and Environmental Stress Responses. Plants 2021, 10, 1261. https://doi.org/10.3390/plants10071261
Pálfi P, Bakacsy L, Kovács H, Szepesi Á. Hypusination, a Metabolic Posttranslational Modification of eIF5A in Plants during Development and Environmental Stress Responses. Plants. 2021; 10(7):1261. https://doi.org/10.3390/plants10071261
Chicago/Turabian StylePálfi, Péter, László Bakacsy, Henrietta Kovács, and Ágnes Szepesi. 2021. "Hypusination, a Metabolic Posttranslational Modification of eIF5A in Plants during Development and Environmental Stress Responses" Plants 10, no. 7: 1261. https://doi.org/10.3390/plants10071261
APA StylePálfi, P., Bakacsy, L., Kovács, H., & Szepesi, Á. (2021). Hypusination, a Metabolic Posttranslational Modification of eIF5A in Plants during Development and Environmental Stress Responses. Plants, 10(7), 1261. https://doi.org/10.3390/plants10071261







