Abstract
Eukaryotic microalgae have been classified into several biological divisions and have evolutionarily acquired diverse morphologies, metabolisms, and life cycles. They are naturally exposed to environmental stresses that cause oxidative damage due to reactive oxygen species accumulation. To cope with environmental stresses, microalgae contain various antioxidants, including carotenoids, ascorbate (AsA), and glutathione (GSH). Carotenoids are hydrophobic pigments required for light harvesting, photoprotection, and phototaxis. AsA constitutes the AsA-GSH cycle together with GSH and is responsible for photooxidative stress defense. GSH contributes not only to ROS scavenging, but also to heavy metal detoxification and thiol-based redox regulation. The evolutionary diversity of microalgae influences the composition and biosynthetic pathways of these antioxidants. For example, α-carotene and its derivatives are specific to Chlorophyta, whereas diadinoxanthin and fucoxanthin are found in Heterokontophyta, Haptophyta, and Dinophyta. It has been suggested that AsA is biosynthesized via the plant pathway in Chlorophyta and Rhodophyta and via the Euglena pathway in Euglenophyta, Heterokontophyta, and Haptophyta. The GSH biosynthetic pathway is conserved in all biological kingdoms; however, Euglenophyta are able to synthesize an additional thiol antioxidant, trypanothione, using GSH as the substrate. In the present study, we reviewed and discussed the diversity of microalgal antioxidants, including recent findings.
1. Introduction
Eukaryotic microalgae (excluding prokaryotic microalgae in this review) are classified into various phylogenetic divisions, including Chlorophyta (e.g., Chlamydomonas reinhardtii and Chlorella vulgaris), Rhodophyta (e.g., Cyanidioschyzon merolae), Heterokontophyta (e.g., the diatom Phaeodactylum tricornutum), Haptophyta (e.g., Emiliania huxleyi), Dinophyta (e.g., Symbiodinium minutum), and Euglenophyta (e.g., Euglena gracilis) [1]; their evolution, morphology, habitat, and metabolism are extremely diverse. Although the genomes of C. reinhardtii and C. merolae were sequenced prior to the genomes of other algal species [2,3], research using limited algal species is not sufficient to understand the biology of diverse microalgae. To gain this understanding, a wide range of algal species needs to be studied. Notably, as microalgae have photosynthetic ability, fast and autotrophic growth, and various material productivity, they have recently attracted attention because their biomass can be used to produce food, fuel, and other valuable materials, and outdoor culture equipment has been developed [4,5,6]. Under outdoor culture conditions, microalgae cannot avoid fluctuating environmental stresses, such as high light, low and high temperatures, and UV irradiation. Exposure to these environmental stresses increases the accumulation of reactive oxygen species (ROS), including H2O2, superoxide radical, hydroxyl radical, and singlet oxygen, as they are the byproducts of cellular oxygenic processes. The superoxide radical is generated by the reduction of molecular oxygen in the photosynthetic electron transport chain and the respiratory chain. It is then converted to H2O2 via the reaction with superoxide dismutase (SOD). The hydroxyl radical is generated from H2O2 in the Fenton reaction with free Cu+ or Fe2+. Singlet oxygen is generated by transferring the energy of the photoexcited pigments to molecular oxygen. At appropriate levels, ROS act as signaling molecules that regulate cellular activities, but when accumulated excessively, they oxidize nucleic acids, proteins, and lipids, leading to oxidative stress damage in cells [7,8]. To avoid ROS-induced cytotoxicity, organisms have developed various antioxidants, including carotenoids, ascorbate, and glutathione. These antioxidants are the key factors in determining the environmental stress tolerance and outdoor growth efficiency of microalgae [9]. This review describes recent findings regarding the diverse biosynthetic pathways and functions of these antioxidants which act to relieve environmental stress in microalgae.
2. Carotenoids
2.1. Carotenoid Compounds
Carotenoids are isoprenoid compounds with C40 backbones, and their colors range from yellow to red. In nature, more than 750 carotenoid compounds have been structurally defined, and among them, at least 44 are found in eukaryotic microalgae. Most microalgae possess β-carotene and zeaxanthin, whereas other carotenoid compounds are extremely diverse depending on their phylogeny (Figure 1) [10,11]. Just like land plants, Chlorophyta species contain an abundance of β-carotene, lutein, neoxanthin, and violaxanthin. Specific carotenoid compounds, such as loroxanthin and siphonaxanthin, were also detected in Chlorophyta [10,11,12,13]. In macrophytic-type Rhodophyta (e.g., Porphyra umbilicalis), lutein is a major carotenoid compound, but it is absent in microphytic-type C. merolae, in which β-carotene and zeaxanthin are the predominant carotenoid compounds [14,15]. Diadinoxanthin and fucoxanthin are the major carotenoid compounds in Heterokontophyta, Haptophyta, and Dinophyta, whereas only diadinoxanthin is present in Euglenophyta [10,11,16,17,18,19]. In some microalgae (e.g., Chlorophyta species Haematococcus pluvialis and Chromochloris zofingiensis), astaxanthin synthesis is induced under various stress conditions, such as high light and high salinity [20]. Astaxanthin has also been detected in non-photosynthetic E. gracilis, suggesting that Euglenophyta can potentially synthesize this carotenoid compound [21]. In microalgae, these diverse carotenoid compounds are valuable chemotaxonomic biomarkers [10].
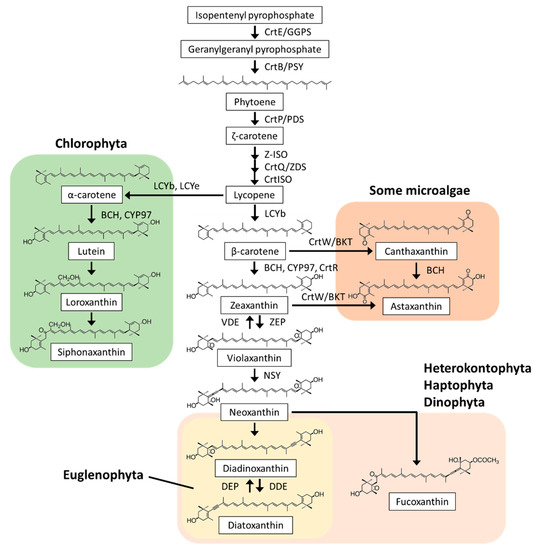
Figure 1.
Carotenoid structures and biosynthesis in microalgae. Most microalgae contain a series of carotenoid compounds, from isopentenyl pyrophosphate to violaxanthin; the exception are red algae (e.g., Cyanidioschyzon merolae), which lack violaxanthin. α-carotene, lutein, loroxanthin, and siphonaxanthin are found in Chlorophyta, diadinoxanthin and diatoxathin are found in Heterokontophyta, Haptophyta, Dinophyta, and Euglenophyta, fucoxanthin is found in Heterokontophyta, Haptophyta, and Dinophyta, and canthaxanthin and astaxanthin are found in some microalgae (e.g., Haematococcus pluvialis, Chromochloris zofingiensis, and Euglena gracilis). The synthetic genes of loroxanthin, siphonaxanthin, diadinoxanthin, and fucoxanthin have not yet been identified. Violaxanthin and zeaxanthin are interconverted by VDE and ZEP, respectively, in the violaxanthin cycle via antheraxanthin; diadinoxanthin and diatoxanthin are similarly interconverted by DDE and DEP, respectively, in the diadinoxanthin cycle. CrtE/GGPS, geranylgeranyl pyrophosphate synthase; CrtB/PSY, phytoene synthase; CrtP, PDS, phytoene desaturase; Z-ISO, ζ-carotene isomerase; CrtQ/ZDS, ζ-carotene desaturase; CrtISO, prolycopene isomerase; LCYb, lycopene β-cyclase; LCYe, lycopene ε-cyclase; BCH, CYP97, and CrtR, carotene hydroxylase; ZEP, zeaxanthin epoxidase; VDE, violaxanthin de-epoxidase; NSY, neoxanthin synthase; DDE, diadinoxanthin de-epoxidase; DEP, diatoxanthin epoxidase; CrtW/BKT, β-carotene ketolase.
2.2. Carotenoid Biosynthesis
In eukaryotic microalgae, the biosynthetic pathway from phytoene to lycopene is conserved, whereas the downstream pathway from lycopene to each end carotenoid compound is diverse, as reviewed below (Figure 1).
2.2.1. Lycopene Synthesis
Isopentenyl pyrophosphate (IPP), which is a C5 unit of the carotenoid backbone, is synthesized via the mevalonate (MVA) pathway or via the non-mevalonate (1-deoxy-D-xylulose 5-phosphate/2-C-methylerythritol 4-phosphate, DOXP/MEP) pathway. Most microalgae utilize the DOXP/MEP pathway, whereas the Euglenophyta species are exceptionally dependent on the MVA pathway [11,22]. IPP is added to farnesyl pyrophosphate (C15), produced from three IPP molecules, by geranylgeranyl pyrophosphate (GGPP, C20) synthase (CrtE, also called GGPS). Two GGPP molecules are condensed by phytoene synthase (CrtB, also called PSY) to produce phytoene (C40). Phytoene is the first carotenoid compound, and its synthesis is known to be the rate-limiting step in the carotenoid biosynthetic pathway [23]. It has been reported that mutations in the PSY gene in C. reinhardtii cause carotenoid deficiency, colorless cell appearance, and ROS accumulation [24,25], and crtB gene knockdown in E. gracilis showed a similar tendency [19].
Subsequently, phytoene is desaturated to 9,15,9′-tri-cis-ζ-carotene by phytoene desaturase (CrtP, also called PDS), isomerized to 9,9′-di-cis ζ-carotene by ζ-carotene isomerase (Z-ISO), desaturated to 7,9,7′,9′-tetra-cis lycopene (pro-lycopene) by ζ-carotene desaturase (CrtQ, also called ZDS), and isomerized to all-trans lycopene by prolycopene isomerase (CrtISO) [10,11]. Cis-carotenes are isomerized by Z-ISO and CrtISO in the dark and non-enzymatically photoisomerized in the light [26]. The genes encoding enzymes catalyzing six carotenoids synthesis steps from IPP to lycopene are widely conserved in microalgae.
2.2.2. α-Carotene and Derivatives Synthesis
Lycopene is cyclized at both ends by lycopene cyclase (LCY). Distinct LCYb and LCYe enzymes generally form a β-ring at one end and an ε-ring at the other end of α-carotene, respectively [27]. A recent study reported that LCYb and LCYe from Dunaliella bardawil exhibit both β- and ε-cyclase activities [28]. Ostreococcus lucimarinus and its relatives have a unique gene encoding LCYb, LCYe, and a C-terminal light-harvesting complex (LHC, see Section 2.3.1) domain fusion protein in a single polypeptide [29]. α-Carotene is then hydroxylated to lutein by nonheme/di-iron carotene hydroxylase (BCH) and heme-containing cytochrome P450-type carotene hydroxylase (CYP97). BCH and CYP97A have hydroxylation activity towards the β-ring of α-carotene, and CYP97C have a hydroxylation activity towards the ε-ring of α-carotene [30,31]. The LCYb and CYP97 family enzymes are widely distributed in microalgae, whereas LCYe and CYP97C are involved in ε-ring formation and hydroxylation in Chlorophyta [32]. Therefore, the α-carotene, lutein, and downstream carotenoid compound synthetic pathways are specific to Chlorophyta. Loroxanthin, siphonaxanthin, prasinoxanthin, and monadoxanthin are considered to be synthesized from lutein, but the genes involved in their synthesis have not yet been identified.
2.2.3. β-Carotene and Derivatives Synthesis
β-Carotene is produced by β-cyclization of lycopene at both ends. LCYb generally catalyzes this reaction [33], and LCYb genes are found in all microalgae. The β-rings of β-carotene are hydroxylated by BCH, CYP97, and CrtR (which is a third-type β-carotene hydroxylase homologous to BCH) to produce zeaxanthin. This step is highly diversified in carotenoid biosynthesis. Chlorophyta species have two types of β-carotene hydroxylases, BCH and CYP97A [31,34]. The microphytic red alga C. merolae possesses the crtR gene and lacks the BCH and CYP97 genes [15]. In Heterokontophyta, Haptophyta, Dinophyta, and Euglenophyta, CYP97s (clans E, F, G, and H) are the sole β-carotene hydroxylases [32,35,36]. Lycopene β-cyclases and β-carotene hydroxylases have been demonstrated to be physiologically important for various environmental stress responses in microalgae. The halotolerant green alga D. salina upregulates the LCYb gene to accumulate β-carotene when exposed to saline, high light, and nitrogen depletion stresses [37]. In P. tricornutum, CYP97 gene expression is induced in response to high light in order to accumulate β-carotene derivatives, fucoxanthin and diatoxanthin [35]. Our reverse genetic analysis revealed that E. gracilis CYP97H1 is essential for carotenoid synthesis and chloroplast homeostasis [36].
The resulting zeaxanthin is epoxidized to violaxanthin via antheraxanthin at both β-rings by zeaxanthin epoxidase (ZEP). In accordance with their carotenoid compositions, ZEP genes are found in Chlorophyta, Heterokontophyta, Haptophyta, Dinophyta, and Euglenophyta, but not in microphytic Rhodophyta [38]. ZEP genes in chlorophytes (C. reinhardtii and C. zofingiensis) and heterokontophytes (P. tricornutum and Nannochloropsis oceanica) have been shown to be functional [39,40,41,42]. Violaxanthin is then converted to neoxanthin through catalysis by neoxanthin synthase (NSY). The gene encoding NSY has been identified as an LCY paralog in tomatoes [43], but not yet in microalgae. Based on their chemical structures, specific carotenoid compounds, such as diadinoxanthin and fucoxanthin found in Heterokontophyta, Haptophyta, Dinophyta, and Euglenophyta, are predicted to be synthesized from neoxanthin, but their synthetic pathways have not yet been clarified [10,11].
Astaxanthin is produced by hydroxylation and ketolation of β-carotene at both β-rings. The ketolation reactions are catalyzed by β-carotene ketolase (CrtW/BKT) [10,11]. The crtW genes have been identified in Chlorophyta H. pluvialis and C. zofingiensis [44,45]. It has been reported that C. zofingiensis accumulates astaxanthin under high light, nitrogen deprivation, and high salinity conditions by upregulating BCH and crtW gene expression [46,47,48].
2.3. Carotenoid Functions
2.3.1. Light Harvesting
Carotenoids bind to light-harvesting complexes (LHCs) with chlorophylls. Carotenoids in LHC promote photosynthesis by absorbing blue-green light and transferring energy to nearby chlorophylls [49]. The efficiency of this energy transfer varies depending on the carotenoid and chlorophyll compositions in the LHC. In addition to LHC, fucoxanthin-chlorophyll a/c binding proteins (FCP) in diatoms [50,51] and the peridinin-chlorophyll a protein complex (PCP) in dinoflagellates [52,53] also act as light-harvesting complexes binding specific carotenoids.
2.3.2. Photoprotection
When photoexcited, chlorophyll transitions to the triplet state, after which it generates singlet oxygen by transferring energy from triplet chlorophyll to oxygen molecules. Singlet oxygen may damage the D1 subunit of photosystem II (PSII) and inhibit the repair of this subunit, leading to photoinhibition [54,55]. Carotenoids suppress singlet oxygen generation by receiving excess energy from triplet chlorophyll and dissipating this energy as heat. Carotenoids also directly receive energy from singlet oxygen and scavenge it [56]. In fact, C. reinhardtii mutant lacking carotenoid (the FN68 strain) is sensitive to light and unable to accumulate LHCs associated with both photosystems [57], demonstrating that quenching capacities of carotenoids against triplet chlorophyll and singlet oxygen contribute to photoprotection.
2.3.3. Xanthophyll Cycles
Xanthophyll cycles control non-photochemical quenching (NPQ), which dissipates excessive light energy in the form of heat under high light conditions. There are two types of xanthophyll cycles: the violaxanthin cycle found in Chlorophyta and the diadinoxanthin cycle found in Heterokontophyta, Haptophyta, Dinophyta, and Euglenophyta. In the violaxanthin cycle, violaxanthin bound to the LHC of PSII is converted to zeaxanthin via antheraxanthin by violaxanthin de-epoxidase (VDE) under high light conditions to reduce the light harvesting efficiency (Figure 1). Under low light or dark conditions, zeaxanthin is converted back to violaxanthin via antheraxanthin by zeaxanthin epoxidase (ZEP) (Figure 1). Similarly, in the diadinoxanthin cycle, diadinoxanthin is converted to diatoxanthin by diadinoxanthin de-epoxidase (DDE) under high light conditions, and the reverse reaction is performed by diatoxanthin epoxidase (DEP) under low light or dark conditions. In Chlorophyta, C. reinhardtii VDE converts violaxanthin to zeaxanthin under high light conditions; however, it is not required for high light acclimation [39]. In contrast, C. vulgaris VDE-mediated zeaxanthin accumulation is crucial for the induction of NPQ under high light, suggesting diverse evolution of the violaxanthin cycle among Chlorophyta [58]. In diatoms, a silencing study suggested that P. tricornutum DDE, a VDE homolog, catalyzes diadinoxanthin de-epoxidation and induces NPQ under high light [59]. Moreover, it was reported that the culturing of E. gracilis at low temperatures results in photosensitivity and an increase in the ratio of diatoxanthin/diadinoxanthin, suggesting a functional diadinoxanthin cycle in Euglenophyta [60].
2.3.4. Stabilization of Lipid Membranes
In microalgae, physical properties of lipid membranes are associated with cellular processes and environmental stress tolerance. Notably, the physical properties of thylakoid membranes affect the photosynthetic activity. Hydrophobic carotenoids are incorporated into lipid membranes, and xanthophylls containing polar groups at both ends are oriented across the lipid membranes. These carotenoids modify membrane fluidity and enhance its stability [61,62]. Physiological evidence of carotenoid-mediated membrane stability has been documented in violaxanthin de-epoxidation in plants [63]. A recent study reported that diadinoxanthin de-epoxidation in the thylakoid membrane of P. tricornutum causes membrane rearrangement and confers stabilization and rigidification to membranes [64].
2.3.5. Eyespot Formation for Phototaxis
Carotenoids are also major components of eyespot globules found in flagellated microalgae and are essential for phototactic responses. Phototaxis is a responsive movement in which the swimming direction changes to optimize photosynthetic activity depending on the light intensity [65,66]. In C. reinhardtii, an eyespot is formed in the chloroplasts, and two carotenoid-rich layers reflect light from outside the cell and amplify the light signal received by the photoreceptor, or they shade light from inside the cell to accurately recognize the light direction [67]. The eyespot of E. gracilis is positioned in the cytosol near the base of the major flagellum, its development is independent of chloroplast development, and it has been demonstrated that its presence is required for initiating phototaxis [21,68]. These findings suggest that eyespot position and physiological function differ between Chlorophyta and Euglenophyta.
3. Ascorbate
The hydrophilic antioxidant ascorbate (AsA) accumulates at high (millimolar) concentrations in cells and plays a crucial role in photooxidative stress defense in all microalgae.
3.1. Ascorbate Biosynthesis
Photosynthetic organisms and most animals, except for humans and some others, can synthesize AsA. In photosynthetic organisms, AsA biosynthetic pathways are classified into the plant pathway (also called the D-mannose/L-galactose pathway) and the Euglena pathway (also called the D-galacturonate pathway).
3.1.1. Plant Pathway
In the plant pathway (Figure 2), D-glucose-6-phosphate (P) is stepwise converted to GDP-L-galactose via D-fructose-6-P, D-mannose-6-P, D-mannose-1-P, and GDP-D-mannose. GDP-L-galactose is then converted to L-galactose by GDP-L-galactose phosphorylase (VTC2) and L-galactose-1-P phosphatase (VTC4). L-galactose is dehydrogenated to L-galactono-1,4-lactone by L-galactose dehydrogenase (L-galDH) and finally to AsA by L-galactono-1,4-lactone dehydrogenase (GLDH) [69,70]. AsA is distributed in most cellular compartments; however, the final AsA synthesis step by GLDH occurs in the mitochondria, and the others occur in the cytosol [71,72]. Therefore, the synthesized AsA is transported from the mitochondria to the chloroplasts, where AsA is the most abundant, and to other compartments [73].
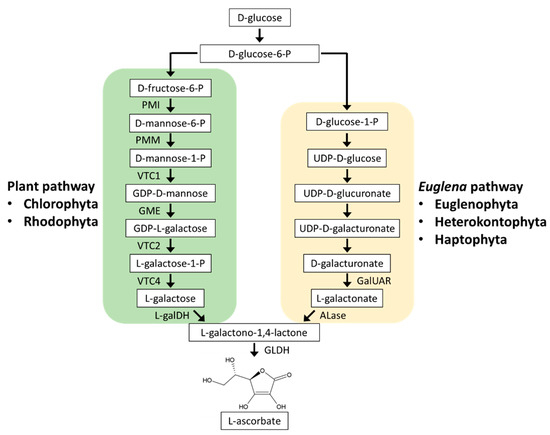
Figure 2.
Ascorbate biosynthesis in microalgae. Microalgae use two distinct pathways: plant pathway (D-mannose/L-galactose pathway) or Euglena pathway (D-galacturonate pathway). Chlorophyta and Euglenophyta have been demonstrated to use the plant pathway and Euglena pathway, respectively. Rhodophyta lacking the VTC2 homolog are predicted to use a modified plant pathway including alternative L-galactose phosphorylase instead of VTC2. Heterokontophyta and Haptophyta are predicted to use the Euglena pathway. PMI, phosphomannose isomerase; PMM, phosphomannomutase; VTC1, GDP-L-mannose pyrophosphorylase; GME, GDP-D-mannose-3’,5’-epimerase; VTC2, L-galactose phosphorylase; VTC4, L-galactose-1-P phosphatase; L-galDH, L-galactose dehydrogenase; GLDH, L-galactono-1,4-lactone dehydrogenase; GalUAR, D-galacturonic acid reductase; ALase, aldonolactonase.
All of these plant pathway genes have been identified in A. thaliana and have been reported to be conserved in Chlorophyta C. reinhardtii, V. carteri, Chlorella sp. NC64A, and Coccomyxa sp. C169 [74]. The enzymatic property of C. reinhardtii VTC2, a key enzyme of the plant pathway, was found to be similar to those of A. thaliana VTC2, and its knockdown resulted in a 90% decrease in AsA content. Moreover, in C. reinhardtii, the transition from dark to light, high light irradiation, and H2O2 treatment caused VTC2 gene upregulation and AsA accumulation [74,75]. These findings suggested that AsA synthesis via the plant pathway protects C. reinhardtii cells from photooxidative stress.
In contrast to land plants and Chlorophyta, Rhodophyta lack the VTC2 homologous gene. However, supplementation experiments of plant pathway intermediates and positional isotopic labeling approach suggested that Rhodophyta synthesized AsA via a plant-like pathway. Therefore, Rhodophyta may use a modified plant pathway by the catalysis of an unidentified enzyme that converts GDP-L-galactose to L-galactose instead of VTC2 [70].
3.1.2. Euglena Pathway
The Euglena pathway was proposed after the detection of D-galacturonate and L-galactono-1,4-lactone as AsA biosynthesis intermediates in E. gracilis (Figure 2) [76]. This pathway was then supported by genetic and biochemical characterizations of D-galacturonic acid reductase (GalUAR) and aldonolactonase (ALase) in E. gracilis [77,78]. GalUAR reduces D-galacturonate to L-galactonate, which is then converted to L-galactono-1,4-lactone by ALase. The final step that converts L-galactono-1,4-lactone to AsA by GLDH is common in both plant and Euglena pathways. The fact that growth inhibition of ALase-knockdown E. gracilis can be counteracted by supplementation with L-galactono-1,4-lactone indicated that in E. gracilis, the Euglena pathway is predominantly utilized for AsA biosynthesis [78]. The Euglena pathway-specific ALase gene is homologous to that in the diatoms P. tricornutum and Thalassiosira pseudonana, but not to that in A. thaliana, C. reinhardtii, and V. carteri, suggesting the utilization of this pathway in diatoms [78]. Genome sequencing and phylogenetic analyses predicted that Heterokontophyta other than diatoms, Haptophyta, and Cryptophyta also use the Euglena pathway [70,79,80].
In E. gracilis, light irradiation induces ALase activity and AsA accumulation [78]. The photoinduction of AsA in this algal species is specific to blue light, but not to red and green light [81]. In the diatom Skeletonema marinoi, strong blue light irradiation induces AsA synthesis along with the synthesis of photosynthetic pigments [82]. Therefore, AsA biosynthesis is considered to be sensitive to the light environment in a wide range of microalgae, regardless of whether they drive either plant or Euglena pathways.
3.2. Ascorbate Functions
3.2.1. Ascorbate Peroxidase and the Ascorbate-regenerating System
AsA is an electron donor of the ROS-scavenging enzyme ascorbate peroxidase (APX) which catalyzes the reduction of H2O2 to H2O and prevents oxidative stress damage in cells (Figure 3) [83,84]. The rate constant of APX for scavenging H2O2 (107) is much higher than that of AsA itself (up to 6); thus, APX activity allows rapid avoidance of H2O2 toxicity [8]. APX also has a reduction activity towards organic hydroperoxides, but this activity is lower than that of H2O2 [83,85], suggesting that APX is an enzyme specialized for H2O2 scavenging.
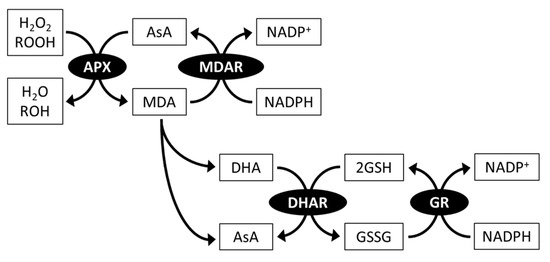
Figure 3.
Ascorbate (AsA)-glutathione (GSH) cycle. All or some of genes and activities of AsA-GSH components have been identified in all microalgal divisions. APX, ascorbate peroxidase; ROOH, organic hydroperoxides; MDA, monodehydroascorbate; MDAR, MDA reductase; DHA, dehydroascorbate; DHAR, DHA reductase; GSSG, oxidized glutathione; GR, glutathione reductase.
During the APX reaction, APX simultaneously produces monodehydroascorbate (MDA), which is a univalent oxidant of AsA. MDA is then spontaneously disproportionated to AsA and dehydroascorbate (DHA), a divalent oxidant of AsA. MDA and DHA are reduced back to AsA by MDA reductase (MDAR) using NADPH as an electron donor, and DHA reductase (DHAR) using glutathione (GSH) as an electron donor, respectively. The resulting oxidized form of glutathione (GSSG) is reduced back to GSH by glutathione reductase (GR) using NADPH as an electron donor. This AsA-regenerating system is termed the AsA-GSH cycle and is essential for maintaining AsA redox homeostasis (Figure 3) [84,86].
The number and localization (including predictions) of some microalgae AsA-GSH cycle enzymes have been documented. C. reinhardtii contains three APX isoforms; APX1 and APX2 were predicted to be dual-targeted in chloroplasts and mitochondria, and APX4 in chloroplasts [87]. Single MDAR and DHAR enzymes are present in C. reinhardtii, and they are probably located in the cytosol [88,89]. C. reinhardtii GRs are composed of two isoforms [90]. E. gracilis contains APX, MDAR, DHAR, and GR enzymes as a single isoform, all of which are localized in the cytosol [91,92,93]. Therefore, AsA regeneration is functional only in the cytosol, at least in C. reinhardtii and E. gracilis. In other microalgal species, two APX isoforms in the cytosol and chloroplasts of C. merolae and four APX isoforms in the cytosol and peroxisomes of Galdieria sulphuraria have been reported [94]. In contrast to microalgae, A. thaliana contains more AsA-GSH cycle enzyme sets, which are composed of eight APX, five MDAR, three DHAR, and two GR isoforms and are widely distributed in the cytosol, chloroplasts, mitochondria, and peroxisomes [95]. Microalgae that live in water environments are less exposed to oxygen and light, which stimulates ROS generation than that in land plants. Thus, it can be presumed that in microalgae, the number and localization of AsA-GSH cycle enzymes were more limited than those in land plants during evolution.
A recent study reported that in C. reinhardtii, the expression of APX genes is induced under high light stress, and a knockdown of chloroplastic APX4 caused sensitivity to photo-oxidative stress [87]. Moreover, overexpression and knockdown of MDAR and DHAR genes in C. reinhardtii resulted in tolerance and sensitivity to high light stress, respectively [88,89]. In E. gracilis, APX-knockdown cells showed high H2O2 accumulation [91]. These findings demonstrated that the microalgal AsA-GSH cycle plays a key role in photooxidative stress defense.
3.2.2. Reductant for Xanthophyll Cycles
Furthermore, AsA is used as a reductant of VDE and DDE reactions in xanthophyll cycles and is thus required for maintaining appropriate NPQ levels in photosynthetic organisms (see Section 2.3.3.) [96]. It has been reported that in C. vulgaris and P. tricornutum, VDE enzymes are active in the presence of AsA in vitro [58,97]. However, a recent study using C. reinhardtii demonstrated that AsA deficiency caused by vtc2 knockout does not limit violaxanthin de-epoxidation and NPQ induction [98]. Therefore, the role of AsA as a reductant in the xanthophyll cycle of microalgae remains controversial.
4. Glutathione
GSH is a low molecular weight thiol tripeptide found in all organisms. It is composed of Glu, Cys, and Gly, plays an important role as a hydrophilic antioxidant and thiol-based redox regulator, and is essential for the survival of microalgae. It is also used for the biosynthesis of phytochelatins and trypanothione (GSH derivatives).
4.1. Glutathione Biosynthesis
GSH is synthesized in two ATP-dependent steps catalyzed by γ-glutamylcysteine synthetase (GSH1, also abbreviated as γECS) and glutathione synthetase (GSH2, also abbreviated as GS). In the first step, GSH1 ligates Cys with Glu to produce γEC. In the second step, Gly is ligated to γ-EC by GSH2 to yield GSH [99] (Figure 4). Two GSH biosynthesis genes are conserved in all biological kingdoms. Genetic and physiological analyses using A. thaliana mutants have demonstrated that both GSH1 and GSH2 are essential for the development of plant roots and seedlings [100,101]. One study reported that glutathione synthesis in E. gracilis grown in the dark was photoinduced post-transcriptionally [102]. In Chlorophyta, glutathione synthesis is downregulated by cold and superoxide generator treatment in D. viridis [103] and by high light in C. reinhardtii [89]. These findings suggested that microalgae acclimate to environmental stresses by altering cellular glutathione levels. However, to our knowledge, the glutathione synthetic genes in microalgae have not yet been characterized, and thus, the physiological significance of glutathione synthesis is poorly understood.
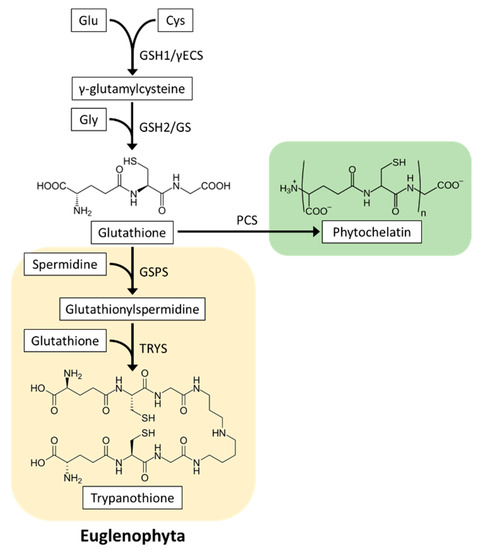
Figure 4.
Structure and biosynthesis of glutathione, phytochelatins, and trypanothione in microalgae. Glutathione biosynthesis is conserved in all biological kingdoms. Phytochelatin biosynthesis occurs in most microalgae, whereas among photosynthetic organisms, trypanothione is detected only in Euglenophyta. GSH1/γECS, γ-glutamylcysteine synthetase; GSH2/GS, glutathione synthetase; PCS, phytochelatin synthase; GSPS, glutathionylspermidine synthetase; TRYS, trypanothione synthetase.
4.2. Glutathione Functions
4.2.1. Glutathione Peroxidase
Glutathione peroxidase (GPX) is an antioxidant enzyme that reduces H2O2, organic hydroperoxides and lipid peroxides, and detoxifies them using GSH or thioredoxin (Trx) as electron donors. During the GPX reaction, GSSG and oxidized Trx are reduced by GR and NADPH-dependent Trx reductase (NTR) (Figure 5A). GPX is classified into two types: enzyme-containing selenocysteine (SeCys) at the catalytic site and enzyme without SeCys [104,105]. C. reinhardtii contains five genes encoding GPXs, including both SeCys-containing (GPX1 and GPX2) and non-selenium GPXs (GPX3, GPX4, and GPX5). These GPX enzymes are predicted to be distributed in cellular compartments, including the cytosol, chloroplasts, and mitochondria [106,107]. To date, their functional characterization has been focused on C. reinhardtii GPX5, which uses Trx as an electron donor, and its gene expression is responsive to high light and singlet oxygen generators [108]. Knockout of GPX5 in C. reinhardtii causes ROS accumulation and thereby arrests growth, suggesting the crucial role of GPX5 as an antioxidant enzyme [109]. However, little is known about the physiological functions of GSH-dependent GPX in C. reinhardtii. Chlorella sp. NJ-18 contains two genes encoding non-selenium GPXs, which use Trx as an electron donor. These GPX genes are upregulated in response to singlet oxygen generator treatment and UV-B irradiation [110]. Unlike non-selenium GPXs from Chlorophyta, non-selenium GPX isolated from E. gracilis uses GSH as an electron donor [111]. In addition to GSH-dependent GPX, the transcriptome data of E. gracilis indicated the existence of three putative Trx-dependent GPXs [112].
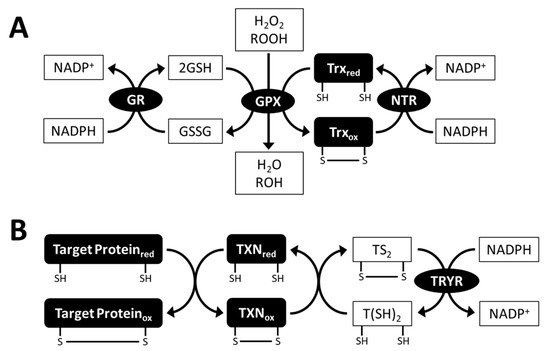
Figure 5.
Glutathione peroxidase (GPX) reaction (A) and trypanothione (T(SH)2) system (B). GPX uses glutathione (GSH) or thioredoxin (Trx) as electron donors. T(SH)2 system is biochemically and physiologically characterized in trypanosomatids, and Euglena gracilis contains a set of genes encoding the components of the T(SH)2 system. GSSG, oxidized glutathione; GR, glutathione reductase; NTR, NADPH-dependent thioredoxin reductase; TS2, oxidized trypanothione; TRYR, trypanothione reductase; TXN, tryparedoxin; red, reduced form; ox, oxidized form.
4.2.2. Ascorbate Regeneration
As described in Section 3.1.1. GSH is involved in AsA regeneration by providing an electron donor for DHAR. In addition, GSH itself contributes to the non-enzymatic reduction of DHA to AsA in high pH environments. A recent study using A. thaliana demonstrated that DHAR activity and GSH content cooperatively act as DHA reductants under high light stress conditions [113]. Non-enzymatic DHA regeneration by GSH is assumed to be functional in microalgae.
4.2.3. Heavy Metal Detoxification
Exposure of microalgae to heavy metal ions, such as cadmium, copper, and zinc, causes ROS production and cytotoxicity. GSH and phytochelatins (PCs), which are GSH polymers found in most microalgae, bind to heavy metal ions and detoxify them [114,115]. The general formula of PCs is represented as (γGlu-Cys)n-Gly (PCn), and microalgae can synthesize those ranging from PC2 to PC6 [116,117,118,119,120]. PC synthesis is catalyzed by phytochelatin synthase (PCS), which binds two molecules of GSH to produce PC2 or GSH and PCn to PCn+1 (Figure 4); therefore, cellular levels of GSH and its precursor γEC are the key factors in PC synthesis induction. In addition, PCS is activated in the presence of heavy metal ions and promotes PC synthesis [121]. It has been reported that a wide range of microalgae (Chlorophyta C. reinhardtii and D. tertiolecta, Rhodophyta C. merolae, diatoms P. tricornutum and Thalassiosira weissflogii, and Euglenophyta E. gracilis) markedly induced γEC, GSH, and PC synthesis and resisted heavy metal toxicity when exposed to cadmium [116,117,118,119,120,122,123,124,125]. Moreover, the heterologous expression of PCS genes from C. merolae and E. gracilis in yeast confers Cd2+ tolerance [118,126]. These findings explain the physiological importance of GSH and PC accumulation in microalgae for heavy metal detoxification.
4.2.4. Glutathione Derivative Trypanothione
Tyrpanothione (N1,N8-bis(glutathionyl)spermidine, T(SH)2) is a specific thiol-based antioxidant found in Euglenophyta and phylogenetically related trypanosomatid parasites [127]. Its biosynthesis is catalyzed by two distinct enzymes: glutathionylspermidine (GSP) synthetase (GSPS) conjugates the first GSH molecule to spermidine, and trypanothione synthetase (TRYS) adds the second GSH molecule to GSP (Figure 4) [128]. Transcriptome data showed that E. gracilis contains two highly homologous genes to GSPS and TRYS genes from trypanosomatid Crithidia fasciculata [112]; however, these genes have not yet been functionally characterized.
In trypanosomatids, the T(SH)2 system, which consists of T(SH)2, trypanothione reductase (TRYR), and Trx family protein tryparedoxin (TXN), activates target proteins via a dithiol/disulfide exchange reaction (Figure 5B) [128]. Target proteins of the trypanothione system include peroxiredoxin, which is a thiol peroxidase involved in oxidative stress defense. In addition, T(SH)2 is able to reduce DHA, thus contributing to APX-dependent ROS scavenging [129]. It has been demonstrated that the T(SH)2 system plays a crucial role in the survival of parasites exposed to oxidative stress in the host [130,131]. In E. gracilis, the TRYR enzyme was purified from algal cells and biochemically characterized [132]. Genes encoding putative TRYR and TXN were identified in the E. gracilis transcriptome data [112]. Moreover, knockdown of TRYR genes in E. gracilis inhibited growth, suggesting a functional T(SH)2 system in this algal species [133].
4.2.5. Glutathione-Mediated Redox Regulations
GSH is also known to be involved in redox regulation of photosynthesis and the cell cycle. A previous study using C. reinhardtii identified 10 Calvin cycle enzymes that underwent protein S-glutathionylation, which is a post-translational modification in which GSH is added to the Cys residue of protein under oxidative stress conditions. Among them, the activities of phosphoribulokinase (PRK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were demonstrated to be modified by S-glutathionylation [134].
Cell cycle progression is regulated by nuclear GSH. It has been reported that the cell cycle was arrested at the G1 checkpoint in tobacco cell suspension cultures depleted of GSH levels [100,135]. However, these GSH-mediated redox regulations in microalgae are not fully understood at present, and thus, further investigation is necessary.
5. Conclusion and Future Perspectives
In all organisms, antioxidant biosynthesis and functions are key factors that determine environmental stress tolerance and cellular process maintenance. Microalgae have evolved antioxidant biosynthesis and function depending on their phylogenetic diversity. As a result, specific carotenoid compounds, such as diadinoxanthin, fucoxanthin, and astaxanthin, as well as the glutathione derivative trypanothione, and many distinct biosynthetic pathways occur in microalgae. This is essential for understanding the cellular metabolism and evolutionary processes of microalgae; however, the findings obtained to date may not be sufficient. Recently, the application of transgenic and genome editing technologies to study microalgae has enabled the modification of their metabolism. Modifications of antioxidant biosynthesis encouraged microalgae researchers to produce high levels of antioxidants and confer resistance to environmental stress. Importantly, as specific carotenoids, such as astaxanthin and fucoxanthin, are known to be effective in maintaining health and preventing disease in humans, carotenoid biosynthesis in microalgae has been actively studied as an attractive target for metabolic modification. In order to understand the evolution and physiology of antioxidants in microalgae and to be able to flexibly design them, future studies should further elucidate their pathways, regulatory mechanisms, and functions.
Author Contributions
Conceptualization, S.T., K.M. and K.S.; writing—original draft preparation, S.T.; writing—review and editing, K.M. and K.S.; visualization, S.T.; supervision, K.M. and K.S.; project administration, K.M. and K.S.; funding acquisition, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Japan Science and Technology Agency (JST)-OPERA Program.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Misumi, O.; Matsuzaki, M.; Nozaki, H.; Miyagishima, S.Y.; Mori, T.; Nishida, K.; Yagisawa, F.; Yoshida, Y.; Kuroiwa, H.; Kuroiwa, T. Cyanidioschyzon merolae genome. A tool for facilitating comparable studies on organelle biogenesis in photosynthetic eukaryotes. Plant Physiol. 2005, 137, 567–585. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Jerney, J.; Spilling, K. Large Scale Cultivation of Microalgae: Open and Closed Systems. In Biofuels from Algae; Spilling, K., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–8. [Google Scholar]
- Harada, R.; Nomura, T.; Yamada, K.; Mochida, K.; Suzuki, K. Genetic engineering strategies for Euglena gracilis and its industrial contribution to sustainable development goals: A review. Front. Bioeng. Biotechnol. 2020, 8, 790. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnol. Adv. 2017, 35, 597–618. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in Phototrophic Microalgae: Distributions and Biosynthesis. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Zepka, L.Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 19–42. [Google Scholar]
- Grossman, A.R.; Lohr, M.; Im, C.S. Chlamydomonas reinhardtii in the landscape of pigments. Annu. Rev. Genet. 2004, 38, 119–173. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008, 26, 631–638. [Google Scholar] [CrossRef]
- Yang, L.E.; Huang, X.Q.; Hang, Y.; Deng, Y.Y.; Lu, Q.Q.; Lu, S. The P450-type carotene hydroxylase PuCHY1 from Porphyra suggests the evolution of carotenoid metabolism in red algae. J. Integr. Plant Biol. 2014, 56, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X., Jr.; Lee, H.; Gantt, E. Carotenoid biosynthesis in the primitive red alga Cyanidioschyzon merolae. Eukaryot. Cell 2007, 6, 533–545. [Google Scholar] [CrossRef]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Büchel, C.; Sandmann, G. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.L.; Brunet, C.; Rodríguez, F. Pigment variations in Emiliania huxleyi (CCMP370) as a response to changes in light intensity or quality. Environ. Microbiol. 2016, 18, 4412–4425. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Soshino, M.; Takaichi, S.; Ishikawa, T.; Nagata, N.; Asahina, M.; Shinomura, T. Suppression of the phytoene synthase gene (EgcrtB) alters carotenoid content and intracellular structure of Euglena gracilis. BMC Plant Biol. 2017, 17, 125. [Google Scholar] [CrossRef]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef]
- Tamaki, S.; Tanno, Y.; Kato, S.; Ozasa, K.; Wakazaki, M.; Sato, M.; Toyooka, K.; Maoka, T.; Ishikawa, T.; Maeda, M.; et al. Carotenoid accumulation in the eyespot apparatus required for phototaxis is independent of chloroplast development in Euglena gracilis. Plant Sci. 2020, 298, 110564. [Google Scholar] [CrossRef]
- Bertrand, M. Carotenoid biosynthesis in diatoms. Photosynth. Res. 2010, 106, 89–102. [Google Scholar] [CrossRef]
- Rodríguez-Villalón, A.; Gas, E.; Rodríguez-Concepción, M. Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown Arabidopsis seedlings. Plant J. 2009, 60, 424–435. [Google Scholar] [CrossRef]
- Inwood, W.; Yoshihara, C.; Zalpuri, R.; Kim, K.; Kustu, S. The ultrastructure of a Chlamydomonas reinhardtii mutant strain lacking phytoene synthase resembles that of a colorless alga. Mol. Plant 2008, 1, 925–937. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Couso, I.; Crespo, J.L. Carotenoid deficiency triggers autophagy in the model green alga Chlamydomonas reinhardtii. Autophagy 2012, 8, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, J.; Sandmann, G. ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 2005, 220, 785–793. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Pogson, B.; Sun, Z.; McDonald, K.A.; DellaPenna, D.; Gantt, E. Functional analysis of the β and ε lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 1996, 8, 1613–1626. [Google Scholar]
- Liang, M.; Liang, Z.; Chen, H.; Jiang, J. The bifunctional identification of both lycopene β- and ε-cyclases from the lutein-rich Dunaliella bardawil. Enzyme Microb. Technol. 2019, 131, 109426. [Google Scholar] [CrossRef]
- Blatt, A.; Bauch, M.E.; Pörschke, Y.; Lohr, M. A lycopene β-cyclase/lycopene ε-cyclase/light-harvesting complex-fusion protein from the green alga Ostreococcus lucimarinus can be modified to produce α-carotene and β-carotene at different ratios. Plant J. 2015, 82, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Smith, J.J.; Tian, L.; Dellapenna, D. The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol. 2009, 50, 463–479. [Google Scholar] [CrossRef]
- Liang, M.; Xie, H.; Chen, H.; Liang, Z.; Jiang, J. Functional identification of two types of carotene hydroxylases from the green alga Dunaliella bardawil rich in lutein. ACS Synth. Biol. 2020, 9, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yu, X.; Wang, Y.; Cui, Y.; Li, X.; Liu, Z.; Qin, S. Evolutionary origins, molecular cloning and expression of carotenoid hydroxylases in eukaryotic photosynthetic algae. BMC Genomics. 2013, 14, 457. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Sun, Z.; Chamovitz, D.; Hirschberg, J.; Gantt, E. Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium (Synechococcus sp.) strain PCC7942. Plant Cell 1994, 6, 1107–1121. [Google Scholar] [PubMed]
- Linden, H. Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochim. Biophys. Acta 1999, 1446, 203–212. [Google Scholar] [CrossRef]
- Cui, H.; Ma, H.; Cui, Y.; Zhu, X.; Qin, S.; Li, R. Cloning, identification and functional characterization of two cytochrome P450 carotenoids hydroxylases from the diatom Phaeodactylum tricornutum. J. Biosci. Bioeng. 2019, 128, 755–765. [Google Scholar] [CrossRef]
- Tamaki, S.; Kato, S.; Shinomura, T.; Ishikawa, T.; Imaishi, H. Physiological role of β-carotene monohydroxylase (CYP97H1) in carotenoid biosynthesis in Euglena gracilis. Plant Sci. 2019, 278, 80–87. [Google Scholar] [CrossRef]
- Ramos, A.; Coesel, S.; Marques, A.; Rodrigues, M.; Baumgartner, A.; Noronha, J.; Rauter, A.; Brenig, B.; Varela, J. Isolation and characterization of a stress-inducible Dunaliella salina Lcy-β gene encoding a functional lycopene β-cyclase. Appl. Microbiol. Biotechnol. 2008, 79, 819–828. [Google Scholar] [CrossRef]
- Dautermann, O.; Lohr, M. A functional zeaxanthin epoxidase from red algae shedding light on the evolution of light-harvesting carotenoids and the xanthophyll cycle in photosynthetic eukaryotes. Plant J. 2017, 92, 879–891. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Bjorkman, O.; Grossman, A.R. Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell 1997, 9, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Couso, I.; Cordero, B.F.; Vargas, M.A.; Rodríguez, H. Efficient heterologous transformation of Chlamydomonas reinhardtii npq2 mutant with the zeaxanthin epoxidase gene isolated and characterized from Chlorella zofingiensis. Mar. Drugs 2012, 10, 1955–1976. [Google Scholar] [CrossRef] [PubMed]
- Eilers, U.; Dietzel, L.; Breitenbach, J.; Büchel, C.; Sandmann, G. Identification of genes coding for functional zeaxanthin epoxidases in the diatom Phaeodactylum tricornutum. J. Plant Physiol. 2016, 192, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, L.; Erickson, E.; Lyska, D.; Niyogi, K.K. Transient expression in Nicotiana benthamiana for rapid functional analysis of genes involved in non-photochemical quenching and carotenoid biosynthesis. Plant J. 2016, 88, 375–386. [Google Scholar] [CrossRef]
- Bouvier, F.; D’harlingue, A.; Backhaus, R.A.; Kumagai, M.H.; Camara, B. Identification of neoxanthin synthase as a carotenoid cyclase paralog. Eur. J. Biochem. 2000, 267, 6346–6352. [Google Scholar] [CrossRef]
- Kajiwara, S.; Kakizono, T.; Saito, T.; Kondo, K.; Ohtani, T.; Nishio, N.; Nagai, S.; Misawa, N. Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli. Plant Mol. Biol. 1995, 29, 343–352. [Google Scholar] [CrossRef]
- Huang, J.C.; Wang, Y.; Sandmann, G.; Chen, F. Isolation and characterization of a carotenoid oxygenase gene from Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2006, 71, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Couso, I.; Leon, R.; Rodriguez, H.; Vargas, M.A. Isolation and characterization of a lycopene ε-cyclase gene of Chlorella (Chromochloris) zofingiensis. Regulation of the carotenogenic pathway by nitrogen and light. Mar. Drugs 2012, 10, 2069–2088. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, M.; Mao, X.; Kou, Y.; Liu, J. Time-resolved carotenoid profiling and transcriptomic analysis reveal mechanism of carotenogenesis for astaxanthin synthesis in the oleaginous green alga Chromochloris zofingiensis. Biotechnol. Biofuels 2019, 12, 287. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, Y.; Wang, X.; Liu, J. Novel insights into salinity-induced lipogenesis and carotenogenesis in the oleaginous astaxanthin-producing alga Chromochloris zofingiensis: A multi-omics study. Biotechnol. Biofuels 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Green, B.R.; Durnford, D.G. The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 685–714. [Google Scholar] [CrossRef] [PubMed]
- Nagao, R.; Yokono, M.; Teshigahara, A.; Akimoto, S.; Tomo, T. Light-harvesting ability of the fucoxanthin chlorophyll a/c-binding protein associated with photosystem II from the Diatom Chaetoceros gracilis as revealed by picosecond time-resolved fluorescence spectroscopy. J. Phys. Chem. B 2014, 118, 5093–5100. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, S.; Pi, X.; Kuang, T.; Sui, S.; Shen, J. Structural features of the diatom photosystem II-light-harvesting antenna complex. FEBS J. 2020, 287, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Kodama, M.; Nomura, S.; Kobayashi, M.; Nozawa, T.; Katoh, T.; Mimuro, M. A novel peridinin-chlorophyll a protein (PCP) from the marine dinoflagellate Alexandrium cohorticula: A high pigment content and plural spectral forms of peridinin and chlorophyll a. FEBS Lett. 1994, 356, 367–371. [Google Scholar] [CrossRef]
- Schulte, T.; Johanning, S.; Hofmann, E. Structure and function of native and refolded peridinin-chlorophyll-proteins from dinoflagellates. Eur. J. Cell Biol. 2010, 89, 990–997. [Google Scholar] [CrossRef]
- Murata, N.; Nishiyama, Y. ATP is a driving force in the repair of photosystem II during photoinhibition. Plant Cell Environ. 2018, 41, 285–299. [Google Scholar] [CrossRef]
- Li, L.; Aro, E.; Millar, A.H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef]
- Santabarbara, S.; Casazza, A.P.; Ali, K.; Economou, C.K.; Wannathong, T.; Zito, F.; Redding, K.E.; Rappaport, F.; Purton, S. The requirement for carotenoids in the assembly and function of the photosynthetic complexes in Chlamydomonas reinhardtii. Plant Physiol. 2013, 161, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, L.; Bellamoli, F.; de la Cruz Valbuena, G.; Perozeni, F.; D’Andrea, C.; Cerullo, G.; Cazzaniga, S.; Ballottari, M. Evolutionary divergence of photoprotection in the green algal lineage: A plant-like violaxanthin de-epoxidase enzyme activates the xanthophyll cycle in the green alga Chlorella vulgaris modulating photoprotection. New Phytol. 2020, 228, 136–150. [Google Scholar] [CrossRef]
- Lavaud, J.; Materna, A.C.; Sturm, S.; Vugrinec, S.; Kroth, P.G. Silencing of the violaxanthin de-epoxidase gene in the diatom Phaeodactylum tricornutum reduces diatoxanthin synthesis and non-photochemical quenching. PLoS ONE 2012, 7, e36806. [Google Scholar] [CrossRef]
- Kato, S.; Tanno, Y.; Takaichi, S.; Shinomura, T. Low temperature stress alters the expression of phytoene desaturase genes (crtP1 and crtP2) and the ζ-carotene desaturase gene (crtQ) together with the cellular carotenoid content of Euglena gracilis. Plant Cell Physiol. 2019, 60, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 2005, 1740, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Tardy, F.; Havaux, M. Thylakoid membrane fluidity and thermostability during the operation of the xanthophyll cycle in higher-plant chloroplasts. Biochim. Biophys. Acta 1997, 1330, 179–193. [Google Scholar] [CrossRef]
- Bojko, M.; Olchawa-Pajor, M.; Goss, R.; Schaller-Laudel, S.; Strzałka, K.; Latowski, D. Diadinoxanthin de-epoxidation as important factor in the short-term stabilization of diatom photosynthetic membranes exposed to different temperatures. Plant Cell Environ. 2019, 42, 1270–1286. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, G. The green algal eyespot apparatus: A primordial visual system and more? Curr. Genet. 2009, 55, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Colley, N.J.; Nilsson, D. Photoreception in phytoplankton. Integr. Comp. Biol. 2016, 56, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Ueki, N.; Ide, T.; Mochiji, S.; Kobayashi, Y.; Tokutsu, R.; Ohnishi, N.; Yamaguchi, K.; Shigenobu, S.; Tanaka, K.; Minagawa, J.; et al. Eyespot-dependent determination of the phototactic sign in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2016, 113, 5299–5304. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Ozasa, K.; Maeda, M.; Tanno, Y.; Tamaki, S.; Higuchi-Takeuchi, M.; Numata, K.; Kodama, Y.; Sato, M.; Toyooka, K.; et al. Carotenoids in the eyespot apparatus are required for triggering phototaxis in Euglena gracilis. Plant J. 2020, 101, 1091–1102. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Wheeler, G.; Ishikawa, T.; Pornsaksit, V.; Smirnoff, N. Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. Elife 2015, 4, e06369. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Pastori, G.M.; Foyer, C.H. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol. 2000, 123, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, Y.; Yoshimura, K.; Takeda, T.; Shigeoka, S. Molecular characterization of tobacco mitochondrial L-galactono-γ-lactone dehydrogenase and its expression in Escherichia coli. Plant Cell Physiol. 2000, 41, 666–675. [Google Scholar] [CrossRef]
- Miyaji, T.; Kuromori, T.; Takeuchi, Y.; Yamaji, N.; Yokosho, K.; Shimazawa, A.; Sugimoto, E.; Omote, H.; Ma, J.F.; Shinozaki, K.; et al. AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat. Commun. 2015, 6, 5928. [Google Scholar] [CrossRef] [PubMed]
- Urzica, E.I.; Adler, L.N.; Page, M.D.; Linster, C.L.; Arbing, M.A.; Casero, D.; Pellegrini, M.; Merchant, S.S.; Clarke, S.G. Impact of oxidative stress on ascorbate biosynthesis in Chlamydomonas via regulation of the VTC2 gene encoding a GDP-L-galactose phosphorylase. J. Biol. Chem. 2012, 287, 14234–14245. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Meireles, A.; Neupert, J.; Zsigmond, L.; Rosado-Souza, L.; Kovács, L.; Nagy, V.; Galambos, A.; Fernie, A.R.; Bock, R.; Tóth, S.Z. Regulation of ascorbate biosynthesis in green algae has evolved to enable rapid stress-induced response via the VTC2 gene encoding GDP-l-galactose phosphorylase. New Phytol. 2017, 214, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Nakano, Y.; Kitaoka, S. The biosynthetic pathway of L-ascorbic acid in Euglena gracilis Z. J. Nutr. Sci. Vitaminol. 1979, 25, 299–307. [Google Scholar] [CrossRef]
- Ishikawa, T.; Masumoto, I.; Iwasa, N.; Nishikawa, H.; Sawa, Y.; Shibata, H.; Nakamura, A.; Yabuta, Y.; Shigeoka, S. Functional characterization of D-galacturonic acid reductase, a key enzyme of the ascorbate biosynthesis pathway, from Euglena gracilis. Biosci. Biotechnol. Biochem. 2006, 70, 2720–2726. [Google Scholar] [CrossRef][Green Version]
- Ishikawa, T.; Nishikawa, H.; Gao, Y.; Sawa, Y.; Shibata, H.; Yabuta, Y.; Maruta, T.; Shigeoka, S. The pathway via D-galacturonate/L-galactonate is significant for ascorbate biosynthesis in Euglena gracilis: Identification and functional characterization of aldonolactonase. J. Biol. Chem. 2008, 283, 31133–31141. [Google Scholar] [CrossRef] [PubMed]
- Helsper, J.P.; Kagan, L.; Hilby, C.L.; Maynard, T.M.; Loewus, F.A. L-Ascorbic acid biosynthesis in Ochromonas danica. Plant Physiol. 1982, 69, 465–468. [Google Scholar] [CrossRef]
- Grün, M.; Loewus, F.A. L-Ascorbic-acid biosynthesis in the euryhaline diatom Cyclotella cryptica. Planta 1984, 160, 6–11. [Google Scholar] [CrossRef]
- Shigeoka, S.; Yokota, A.; Nakano, Y.; Kitaoka, S. The effect of illumination on the L-ascorbic acid content in Euglena gracilis z. Agric. Biol. Chem. 1979, 43, 2053–2058. [Google Scholar] [CrossRef]
- Smerilli, A.; Orefice, I.; Corato, F.; Olea, A.G.; Ruban, A.V.; Brunet, C. Photoprotective and antioxidant responses to light spectrum and intensity variations in the coastal diatom Skeletonema marinoi. Environ. Microbiol. 2017, 19, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Nakano, Y.; Kitaoka, S. Purification and some properties of L-ascorbic-acid-specific peroxidase in Euglena gracilis Z. Arch. Biochem. Biophys. 1980, 201, 121–127. [Google Scholar] [CrossRef]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takeda, T.; Kohno, H.; Shigeoka, S. Molecular characterization of Euglena ascorbate peroxidase using monoclonal antibody. Biochim. Biophys. Acta 1996, 1290, 69–75. [Google Scholar] [CrossRef]
- Asada, K. The Role of Ascorbate Peroxidase and Monodehydroascorbate Reductase in H2O2 Scavenging in Plants. In Oxidative Stress and the Molecular Biology of Antioxidant Defenses; Scandalios, J.G., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1997; pp. 715–735. [Google Scholar]
- Kuo, E.Y.H.; Cai, M.S.; Lee, T.M. Ascorbate peroxidase 4 plays a role in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Sci. Rep. 2020, 10, 13287. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.L.; Lin, T.H.; Chen, C.C.; Cheng, T.X.; Chang, H.Y.; Lee, T.M. Monodehydroascorbate reductase plays a role in the tolerance of Chlamydomonas reinhardtii to photooxidative stress. Plant Cell Physiol. 2019, 60, 2167–2179. [Google Scholar] [CrossRef]
- Lin, S.T.; Chiou, C.W.; Chu, Y.L.; Hsiao, Y.; Tseng, Y.F.; Chen, Y.C.; Chen, H.J.; Chang, H.Y.; Lee, T.M. Enhanced ascorbate regeneration via dehydroascorbate reductase confers tolerance to photo-oxidative stress in Chlamydomonas reinhardtii. Plant Cell Physiol. 2016, 57, 2104–2121. [Google Scholar] [CrossRef]
- Lin, T.H.; Rao, M.Y.; Lu, H.W.; Chiou, C.W.; Lin, S.T.; Chao, H.W.; Zheng, Z.L.; Cheng, H.C.; Lee, T.M. A role for glutathione reductase and glutathione in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Physiol. Plant. 2018, 162, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Tajima, N.; Nishikawa, H.; Gao, Y.; Madhusudhan, R.; Shibata, H.; Sawa, Y.; Shigeoka, S. Euglena gracilis ascorbate peroxidase forms an intramolecular dimeric structure: Its unique molecular characterization. Biochem. J. 2010, 426, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Yasumoto, R.; Onishi, T.; Nakano, Y.; Kitaoka, S. Properties of monodehydroascirbate reductase and dehydroascorbate reductase and their participation in the regeneration of ascorbate in Euglena gracilis. J. Gen. Microbiol. 1987, 133, 227–232. [Google Scholar]
- Shigeoka, S.; Onishi, T.; Nakano, Y.; Kitaoka, S. Characterization and physiological function of glutathione reductase in Euglena gracilis z. Biochem. J. 1987, 242, 511–515. [Google Scholar] [CrossRef]
- Hirooka, S.; Misumi, O.; Yoshida, M.; Mori, T.; Nishida, K.; Yagisawa, F.; Yoshida, Y.; Fujiwara, T.; Kuroiwa, H.; Kuroiwa, T. Expression of the Cyanidioschyzon merolae stromal ascorbate peroxidase in Arabidopsis thaliana enhances thermotolerance. Plant Cell Rep. 2009, 28, 188193. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef] [PubMed]
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef]
- Bojko, M.; Olchawa-Pajor, M.; Tuleja, U.; Kuczyńska, P.; Strzałka, W.; Latowski, D.; Strzałka, K. Expression of three diadinoxanthin de-epoxidase genes of Phaeodacylum tricornutum in Escherichia coli Origami b and BL21 strain. Acta Biochim. Pol. 2013, 60, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Meireles, A.; Tóth, D.; Kovács, L.; Neupert, J.; Tóth, S.Z. Ascorbate deficiency does not limit nonphotochemical quenching in Chlamydomonas reinhardtii. Plant Physiol. 2020, 182, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Vernoux, T.; Wilson, R.C.; Seeley, K.A.; Reichheld, J.P.; Muroy, S.; Brown, S.; Maughan, S.C.; Cobbett, C.S.; Van Montagu, M.; Inzé, D.; et al. The root meristemless1/cadmium sensitive2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 2000, 12, 97–110. [Google Scholar] [CrossRef]
- Pasternak, M.; Lim, B.; Wirtz, M.; Hell, R.; Cobbett, C.S.; Meyer, A.J. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J. 2008, 53, 999–1012. [Google Scholar] [CrossRef]
- Shigeoka, S.; Onishi, T.; Nakano, Y.; Kitaoka, S. Photoinduced biosynthesis of glutathione in Euglena gracilis. Agric. Biol. Chem. 1987, 51, 2257–2258. [Google Scholar] [CrossRef]
- Haghjou, M.M.; Colville, L.; Smirnoff, N. The induction of menadione stress tolerance in the marine microalga, Dunaliella viridis, through cold pretreatment and modulation of the ascorbate and glutathione pools. Plant Physiol. Biochem. 2014, 84, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.; Gregolin, C.; Ursini, F. Phospholipid hydroperoxide glutathione peroxidase. Methods Enzymol. 1990, 186, 448–457. [Google Scholar] [CrossRef]
- Navrot, N.; Collin, V.; Gualberto, J.; Gelhaye, E.; Hirasawa, M.; Rey, P.; Knaff, D.B.; Issakidis, E.; Jacquot, J.P.; Rouhier, N. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol. 2006, 142, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Takeda, T.; Hanaoka, T. Characterization and immunological properties of selenium-containing glutathione peroxidase induced by selenite in Chlamydomonas reinhardtii. Biochem J. 1991, 275, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Dayer, R.; Fischer, B.B.; Eggen, R.I.L.; Lemaire, S.D. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhardtii. Genetics 2008, 179, 41–57. [Google Scholar] [CrossRef]
- Fischer, B.B.; Dayer, R.; Schwarzenbach, Y.; Lemaire, S.D.; Behra, R.; Liedtke, A.; Eggen, R.I.L. Function and regulation of the glutathione peroxidase homologous gene GPXH/GPX5 in Chlamydomonas reinhardtii. Plant Mol. Biol. 2009, 71, 569–583. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, B.; Miao, R.; Deng, X.; Duan, Y.; Cheng, Y.; Zhang, W.; Shi, M.; Huang, K.; Xia, X.Q. Transcriptomic and physiological responses to oxidative stress in a Chlamydomonas reinhardtii glutathione peroxidase mutant. Genes 2020, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X. Molecular cloning and functional analyses of glutathione peroxidase homologous genes from (Chlorella sp.) NJ-18. Gene 2012, 501, 17–23. [Google Scholar] [CrossRef]
- Overbaugh, J.M.; Fall, R. Characterization of a selenium-independent glutathione peroxidase from Euglena gracilis. Plant Physiol. 1985, 77, 437–442. [Google Scholar] [CrossRef]
- Ishikawa, T.; Tamaki, S.; Maruta, T.; Shigeoka, S. Biochemistry and Physiology of Reactive Oxygen Species in Euglena. In Euglena: Biochemistry, Cell and Molecular Biology; Schwartzbach, S.D., Shigeoka, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 47–64. [Google Scholar]
- Terai, Y.; Ueno, H.; Ogawa, T.; Sawa, Y.; Miyagi, A.; Kawai-Yamada, M.; Ishikawa, T.; Maruta, T. Dehydroascorbate reductases and glutathione set a threshold for high-light-induced ascorbate accumulation. Plant Physiol. 2020, 183, 112–122. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, H.; Chang, Z.; Jiang, Z.; Yin, W. The effect of cadmium on the growth and antioxidant response for freshwater algae Chlorella vulgaris. SpringerPlus 2016, 5, 1290. [Google Scholar] [CrossRef]
- Moreno-Sánchez, R.; Rodríguez-Enríquez, S.; Jasso-Chávez, R.; Saavedra, E.; García-García, J.D. Biochemistry and Physiology of Heavy Metal Resistance and Accumulation in Euglena. In Euglena: Biochemistry, Cell and Molecular Biology; Schwartzbach, S.D., Shigeoka, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 91–121. [Google Scholar]
- Bräutigam, A.; Schaumlöffel, D.; Preud’Homme, H.; Thondorf, I.; Wesenberg, D. Physiological characterization of cadmium-exposed Chlamydomonas reinhardtii. Plant Cell Environ. 2011, 34, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Hirayanagi, N.; Iwabe, O.; Namba, T.; Tagawa, M.; Miyamoto, S.; Miyasaka, H.; Takagi, M.; Hirata, K.; Miyamoto, K. Regulation of phytochelatin synthesis by zinc and cadmium in marine green alga, Dunaliella tertiolecta. Phytochemistry 2003, 62, 453–459. [Google Scholar] [CrossRef]
- Osaki, Y.; Shirabe, T.; Tamura, S.; Yoshimura, E. A functional putative phytochelatin synthase from the primitive red alga Cyanidioschyzon merolae. Biosci. Biotechnol. Biochem. 2008, 72, 3306–3309. [Google Scholar] [CrossRef][Green Version]
- Kawakami, S.K.; Gledhill, M.; Achterberg, E.P. Effects of metal combinations on the production of phytochelatins and glutathione by the marine diatom Phaeodactylum tricornutum. Biometals 2006, 19, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cózatl, D.G.; Moreno-Sánchez, R. Cd2+ transport and storage in the chloroplast of Euglena gracilis. Biochim. Biophys. Acta 2005, 1706, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Osaki, Y.; Shirabe, T.; Nakanishi, H.; Wakagi, T.; Yoshimura, E. Characterization of phytochelatin synthase produced by the primitive red alga Cyanidioschyzon merolae. Metallomics 2009, 1, 353–358. [Google Scholar] [CrossRef]
- Stoiber, T.L.; Shafer, M.M.; Armstrong, D.E. Differential effects of copper and cadmium exposure on toxicity endpoints and gene expression in Chlamydomonas reinhardtii. Environ. Toxicol. Chem. 2010, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Ahner, B.A.; Wei, L.P.; Oleson, J.R.; Ogura, N. Glutathione and other low molecular weight thiols in marine phytoplankton under metal stress. Mar. Ecol. Prog. Ser. 2002, 232, 93–103. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Z.; Zhang, W.; Tan, Q.; Zhang, L.; Ge, X.; Chen, M. Quantitative relationship between cadmium uptake and the kinetics of phytochelatin induction by cadmium in a marine diatom. Sci. Rep. 2016, 6, 35935. [Google Scholar] [CrossRef]
- Mendoza-Cozatl, D.; Devars, S.; Loza-Tavera, H.; Moreno-Sánchez, R. Cadmium accumulation in the chloroplast of Euglena gracilis. Physiol. Plant. 2002, 115, 276–283. [Google Scholar] [CrossRef]
- García-García, J.D.; Girard, L.; Hernández, G.; Saavedra, E.; Pardo, J.P.; Rodríguez-Zavala, J.S.; Encalada, R.; Reyes-Prieto, A.; Mendoza-Cózatl, D.G.; Moreno-Sánchez, R. Zn-bis-glutathionate is the best co-substrate of the monomeric phytochelatin synthase from the photosynthetic heavy metal-hyperaccumulator Euglena gracilis. Metallomics 2014, 6, 604–616. [Google Scholar] [CrossRef]
- O’Neill, E.C.; Trick, M.; Hill, L.; Rejzek, M.; Dusi, R.G.; Hamilton, C.J.; Zimba, P.V.; Henrissat, B.; Field, R.A. The transcriptome of Euglena gracilis reveals unexpected metabolic capabilities for carbohydrate and natural product biochemistry. Mol. Biosyst. 2015, 11, 2808–2820. [Google Scholar] [CrossRef]
- Manta, B.; Comini, M.; Medeiros, A.; Hugo, M.; Trujillo, M.; Radi, R. Trypanothione: A unique bis-glutathionyl derivative in trypanosomatids. Biochim. Biophys. Acta 2013, 1830, 3199–3216. [Google Scholar] [CrossRef] [PubMed]
- Krauth-Siegel, R.L.; Lüdemann, H. Reduction of dehydroascorbate by trypanothione. Mol. Biochem. Parasitol. 1996, 80, 203–208. [Google Scholar] [CrossRef]
- Dumas, C.; Ouellette, M.; Tovar, J.; Cunningham, M.L.; Fairlamb, A.H.; Tamar, S.; Olivier, M.; Papadopoulou, B. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997, 16, 2590–2598. [Google Scholar] [CrossRef]
- Wyllie, S.; Oza, S.L.; Patterson, S.; Spinks, D.; Thompson, S.; Fairlamb, A.H. Dissecting the essentiality of the bifunctional trypanothione synthetase-amidase in Trypanosoma brucei using chemical and genetic methods. Mol. Microbiol. 2009, 74, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Montrichard, F.; Le Guen, F.; Laval-Martin, D.L.; Davioud-Charvet, E. Evidence for the co-existence of glutathione reductase and trypanothione reductase in the non-trypanosomatid Euglenozoa: Euglena gracilis Z. FEBS Lett. 1999, 442, 29–33. [Google Scholar] [CrossRef]
- Tamaki, S. Functional analysis of enzymes involved in thiol based redox metabolism in Euglena gracilis. Ph.D. Thesis, Tottori University, Tottori, Japan, 11 September 2015. [Google Scholar]
- Zaffagnini, M.; Bedhomme, M.; Groni, H.; Marchand, C.H.; Puppo, C.; Gontero, B.; Cassier-Chauvat, C.; Decottignies, P.; Lemaire, S.D. Glutathionylation in the photosynthetic model organism Chlamydomonas reinhardtii: A proteomic survey. Mol. Cell. Proteomics 2012, 11, M111.014142. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Pellny, T.K.; Locato, V.; Hull, J.; De Gara, L. Analysis of Redox Relationships in the Plant Cell Cycle: Determination of Ascorbate, Glutathione, and Poly(ADPribose)Polymerase (PARP) in Plant Cell Cultures. In Redox-Mediated Signal Transduction; Hancock, J.T., Conway, M.T., Eds.; Springer: Cham, Switzerland, 2019; pp. 165–181. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).