Hydroscapes: A Useful Metric for Distinguishing Iso-/Anisohydric Behavior in Almond Cultivars
Abstract
1. Introduction
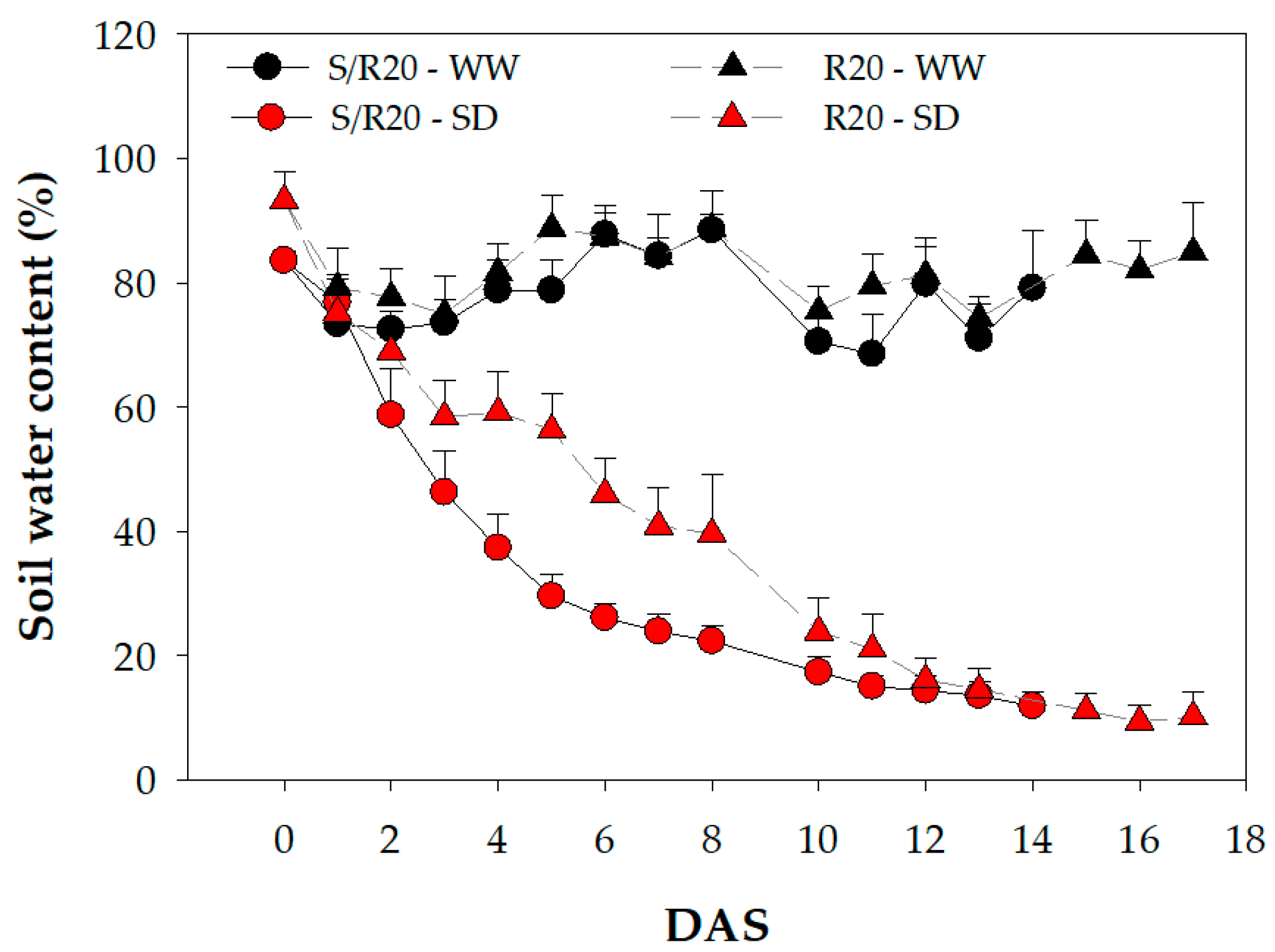
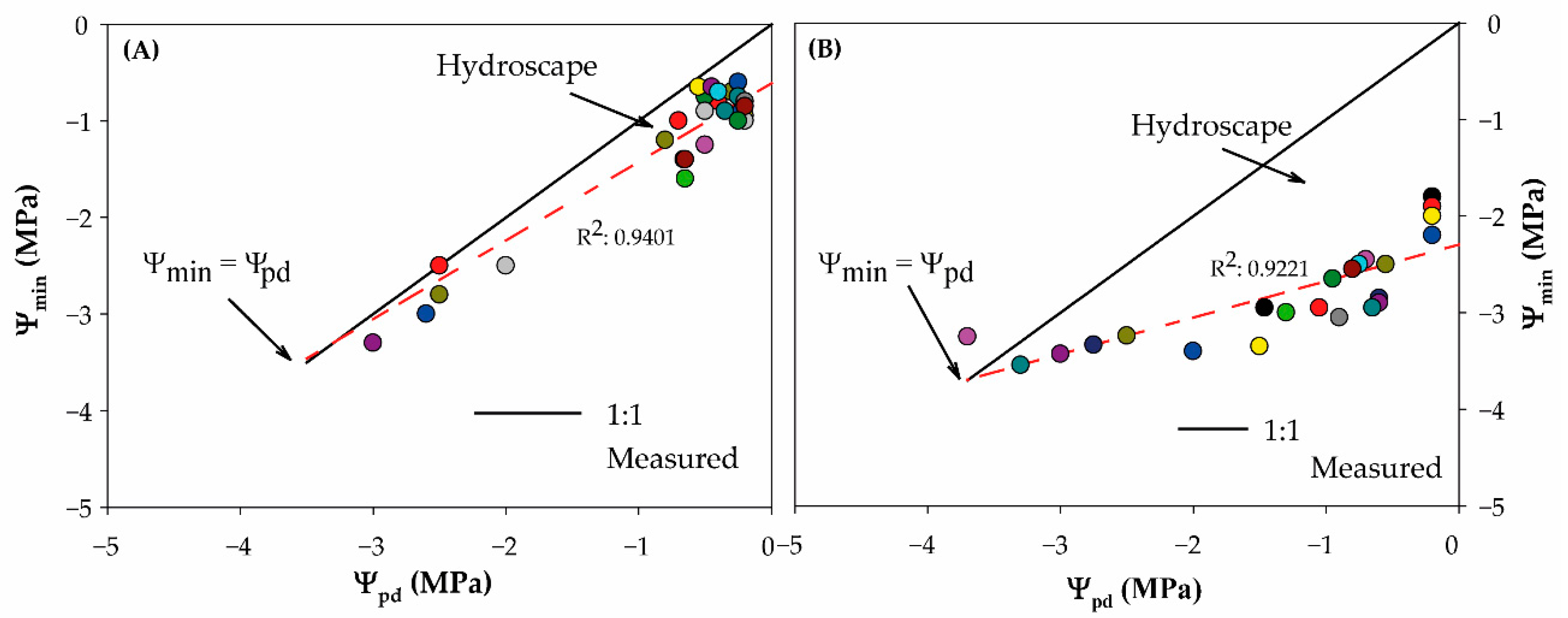
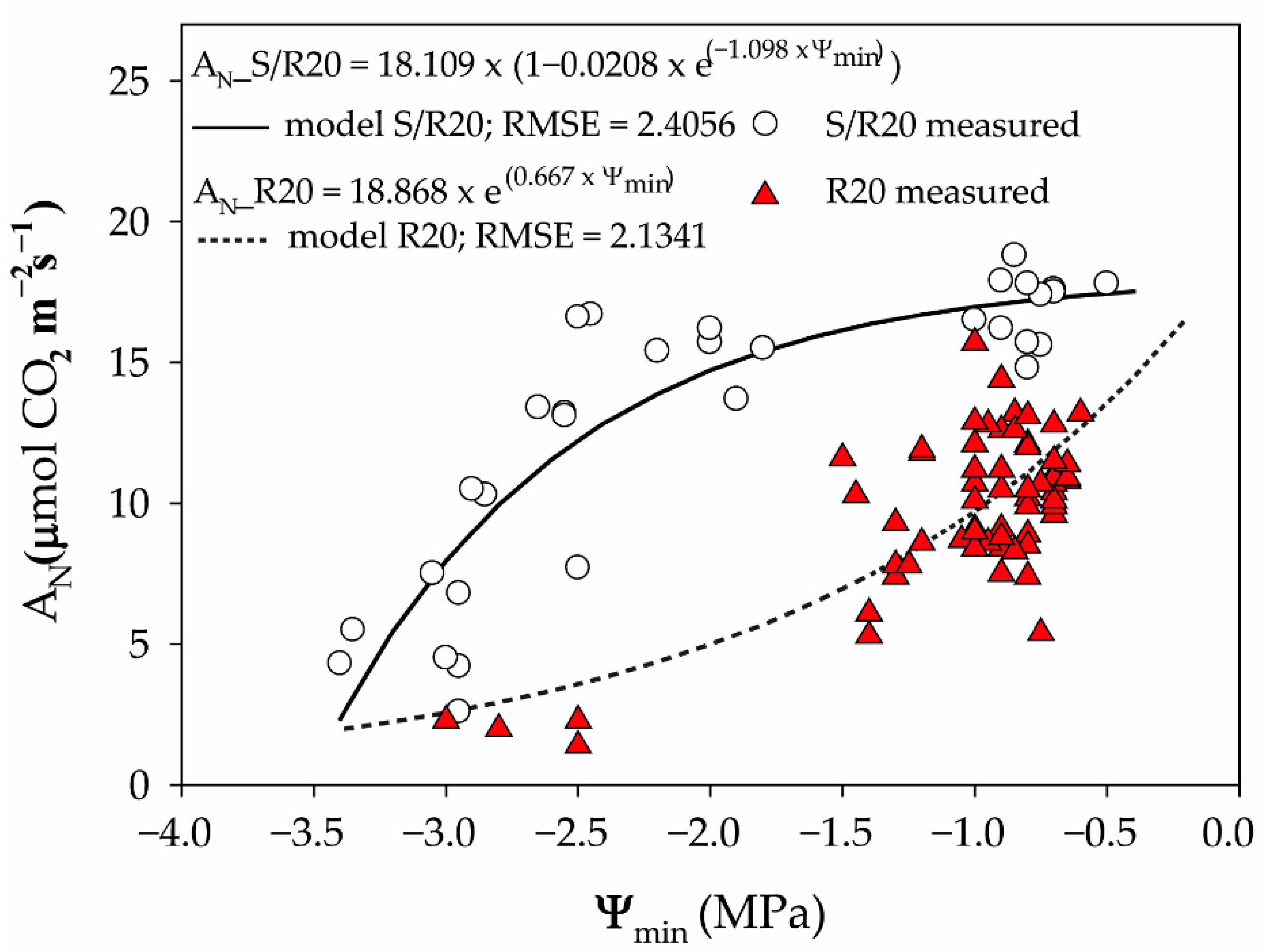
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Drought Treatment Application
3.3. Midday and Pre-Dawn Water Potential
3.4. Metric for Characterizing An-/Isohydry
3.5. Gas Exchange Measurements
3.6. Biomass and Leaf Area Measurements
3.7. Experimental Design and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheffield, J.; Wood, E.F. Global trends and variability in soil moisture and drought characteristics 1950–2000, from observation-driven simulations of terrestrial hydrologic cycle. J. Clim. 2008, 21, 432–458. [Google Scholar] [CrossRef]
- Valladares, F.; Vilagrosa, A.; Peñuelas, J.; Romá, O.; Camarero, J.J.; Corcuera, L. Estrés hídrico: Ecofisiología y escalas de sequía. In Ecología del Bosque Mediterráneo en un Mundo Cambiante, 1st ed.; Valladares, F., Ed.; Ministerio del Medio Ambiente: Madrid, Spain, 2004; pp. 163–190. [Google Scholar]
- Garreaud, R.D.; Álvarez-Garretón, C.; Barichivich, J.; Christie, D.; Galleguillos, M. The 2010–2015 megadrought in central Chile: Impacts on regional hydroclimate and vegetation. Hydrol. Earth Syst. Sci. 2017, 21, 6307–6327. [Google Scholar] [CrossRef]
- Marsal, J.; Girona, J.; Mata, M. Leaf water relation parameters in almond compared to hazelnut trees during a deficit irrigation period. J. Am. Soc. Hortic. Sci. 1997, 122, 582–587. [Google Scholar] [CrossRef]
- Camposeo, S.; Palasciano, M.; Vivaldi, G.A.; Godini, A. Effect of increasing climatic water deficit on some leaf and stomatal parameters of wild and cultivated almonds under Mediterranean conditions. Sci. Hort. 2011, 127, 234–241. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Alfonso, S.; Gonçalvez, B. Compared leaf anatomy and water relations of commercial and traditional Prunus dulcis (Mill.) cultivars under rain-fed conditions. Sci. Hort. 2018, 229, 226–232. [Google Scholar]
- Pou, A.; Medrano, H.; Tomás, M.; Martorell, S.; Ribas-Carbó, M.; Flexas, J. Anisohydric behavior in grapevines results in better performance under moderate water stress and recovery than isohydric behavior. Plant Soil 2012, 359, 335–349. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants. Anisohydric behavior as a stress resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef]
- Martínez-Vilalte, J.; García-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2016, 40, 962–976. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Woodruff, D.R.; Marias, D.E.; Smith, D.D.; McCulloh, K.A.; Howard, A.R.; Magedman, A.L. Mapping hydroscapes along the iso- to anisohydric continuum of stomatal regulation of plant water status. Ecol. Lett. 2016, 19, 1343–1352. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/anisohydry: A plant-environment interaction rather than a simple hydraulic trait. Trend. Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P. Daily and seasonal patterns of leaf water relations and gas exchange of regulated deficit-irrigated almond trees under semiarid conditions. Environ. Exp. Bot. 2006, 56, 158–173. [Google Scholar] [CrossRef]
- Lavoie-Lamoreux, A.; Sacco, D.; Risse, P.-A.; Lovisolo, C. Factors influencing stomatal conductance in response to water availability in grapevine: A meta-analysis. Physiol. Plant 2016, 159, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U.; Degu, A.; Fait, A.; Rachmilevitch, S. Near isohydric grapevine cultivar displays higher photosynthetic efficiency and photorespiration rates under drought stress as compared with near anisohydric grapevine cultivar. Physiol. Plant 2013, 147, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Couple-Ledru, A.; Lebon, É.; Christophe, A. Genetic variation in grapevine progeny (Vitis vinifera L. cvs GrenachexSyrah) reveals inconsistencies between maintenance of daytime leaf water potential and responses of transpiration rate under drought. J. Exp. Bot. 2014, 65, 6205–6218. [Google Scholar]
- Rouhi, V.; Samson, R.; Lemeur, R.; Van Damme, P. Photosynthetic gas exchange characteristics in three different almond species during drought stress and subsequent recovery. Environ. Exp. Bot. 2007, 59, 117–129. [Google Scholar] [CrossRef]
- Isaakidis, A.; Sotiropoulos, T.; Almaliotis, D.; Therios, I.; Stylianidis, D. Response to severe water stress of the almond (Prunus amygdalus) “Ferragnes” grafted on eight rootstocks. N. Z. J. Crop. Hortic. Sci. 2004, 32, 355–362. [Google Scholar] [CrossRef]
- Gomes-Laranjo, J.; Coutinho, J.P.; Galhano, V.; Cordeiro, V. Responses of five almond cultivars to irrigation: Photosynthesis and leaf water potential. Agric. Water Manag. 2006, 83, 261–265. [Google Scholar] [CrossRef]
- Yadollahi, A.; Arzani, K.; Ebadi, A.; Wirthensohn, M.; Karimi, S. The response of different almond genotypes to moderate and severe water stress in order to screen for drought tolerance. Sci. Hort. 2011, 129, 403–413. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.M.; Hutton, R.J.; Clarke, S.J.; Hutchinson, P.A.; Somers, A. Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiol. 2012, 32, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.C.; Smith, D.D.; Woodruff, D.R.; Marias, D.E.; McCulloh, K.A.; Howard, A.R.; Magedman, A.L. Stomatal kinetics and photosynthetic gas exchange along a continuum of isohydric to anisohydric regulation of plant water status. Plant Cell Environ. 2017, 40, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Blacjman, C.J.; Peters, J.M.R.; Choat, B.; Rymer, P.D.; Medlyn, B.E.; Tissue, D.T. More than iso/anisohydry: Hydroscapes integrate plant water use and drought tolerance traits in 10 Eucalyptus species from contrasting climates. Funct. Ecol. 2019, 33, 1035–1049. [Google Scholar] [CrossRef]
- Domec, J.C.; Johnson, D.M. Does homeostasis or disturbance of homeostasis in minimum leaf water potential explain the isohydric versus anisohydric behavior of Vitis vinifera L. cultivars? Tree Physiol. 2012, 32, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Poni, S.; Bernizzoni, F.; Civardi, S. Response of “Sangiovese” grapevines to partial root-zone drying: Gas-exchange, growth and grape composition. Sci. Hort. 2007, 114, 96–103. [Google Scholar] [CrossRef]
- Bourne, A.E.; Creek, D.; Peters, M.J.; Ellsworth, D.S.; Choat, B. Species climate range influences hydraulic and stomatal traits in Eucalyptus species. Ann. Bot. 2017, 120, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.; Szota, C.; Arndt, S.K. Does turgor loss point characterize drought response in dryland plants? Plant Cell Environ. 2017, 40, 1500–1511. [Google Scholar] [CrossRef]
- Li, X.; Blackman, C.J.; Choat, B.; Duursma, R.A.; Rymer, P.D.; Medlyn, B.E.; Tissue, D.T. Tree hydraulic traits are coordinated and strongly linked to climate-of-origin across a rainfall gradient. Plant Cell Environ. 2018, 41, 646–660. [Google Scholar] [CrossRef]
- Jiménez, S.; Dridi, J.; Gutiérrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef]
- Karimi, S.A.; Yadollahi, K.; Arzani, A.; Imani, A.; Aghaalikhani, M. Gas exchange response of almond genotypes to water stress. Photosynthetica 2015, 53, 29–34. [Google Scholar] [CrossRef]
- Niinemets, U.; Cescatti, A.; Rodeghiero, M.; Tosens, T. Lean internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaves species. Plant Cell Environ. 2005, 28, 1552–1566. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drpught-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Maldini, C.; Acevedo, M.; Dumroese, R.K.; González, M.; Cartes, E. Intraspecific variation in drought response of three populations of Cryptocarya alba and Persea lingue, two native species from Mediterranean central Chile. Front. Plant Sci. 2020, 11, 1042. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 432–458. [Google Scholar] [CrossRef]
- Duursma, R.A.; Choat, B. fitplc: An R package to fit hydraulic vulnerability curves. J. Plant Hydraul. 2017, 4, e002. [Google Scholar] [CrossRef]
- Moualeu-Ngangue, D.P.; Chen, T.-W.; Stützel, H. A new method to estimate photosynthetic parameters through net assimilation rate-intercellular space CO2 concentration (A-Ci) curve and chlorophyll fluorescence measurement. New Phytol. 2017, 213, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S. Biochemical Models of Leaf Photosynthesis, 1st ed.; CSIRO Publishing: Collingwood, Australia, 2000. [Google Scholar]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; von Caemmerer, S.; Long, S.P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitation for photosynthesis in vivo. Plant Physiol. 2002, 130, 1992–1998. [Google Scholar] [PubMed]
| Trait | Cultivars | |
|---|---|---|
| S/R20 | R20 | |
| σ (Mpa MPa−1) | 0.38 | 0.81 |
| Hydroscape (Mpa2) | 4.25 | 0.95 |
| Pgs90 (−Mpa) | 3.37 [3.14; 3.74] | 1.63 [1.46; 2.56] |
| ΔΨ (−MPa) | 2.19 ± 0.07 a | 1.54 ± 0.04 b |
| Airr (µmol m−2s−1) | 14.51 ± 0.97 a | 10.80 ± 0.16 b |
| gsirr (mmol m−2s−1) | 389.51 ± 39.41 a | 283.52 ± 12.70 b |
| Vcmax (µmol CO2 m−2s−1) | J (µmol m−2s−1) | gm (mmol m−2s−1) | ||
|---|---|---|---|---|
| WW | SD | |||
| Cultivar (C) | ||||
| S/R20 | 149.57 ± 33.46 a | 106.22 ± 13.54 a | 208.6 ± 0.094 a | 34.8 ± 0.074 b |
| R20 | 74.40 ± 3.53 b | 81.75 ± 5.79 b | 77.3 ± 0.023 b | 39.1 ± 0.015 b |
| Treatment (T) | ||||
| WW | 108.36 ± 9.44 a | 112.96 ± 5.24 a | - | - |
| SD | 72.44 ± 8.88 b | 68.83 ± 5.91 b | - | - |
| Source of variation | p-values | |||
| C | 0.0034 | 0.0054 | 0.0187 | |
| T | 0.0014 | <0.0001 | 0.0004 | |
| C × T | 0.0708 | 0.1322 | 0.0128 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Maldini, C.; Acevedo, M.; Pinto, M. Hydroscapes: A Useful Metric for Distinguishing Iso-/Anisohydric Behavior in Almond Cultivars. Plants 2021, 10, 1249. https://doi.org/10.3390/plants10061249
Álvarez-Maldini C, Acevedo M, Pinto M. Hydroscapes: A Useful Metric for Distinguishing Iso-/Anisohydric Behavior in Almond Cultivars. Plants. 2021; 10(6):1249. https://doi.org/10.3390/plants10061249
Chicago/Turabian StyleÁlvarez-Maldini, Carolina, Manuel Acevedo, and Manuel Pinto. 2021. "Hydroscapes: A Useful Metric for Distinguishing Iso-/Anisohydric Behavior in Almond Cultivars" Plants 10, no. 6: 1249. https://doi.org/10.3390/plants10061249
APA StyleÁlvarez-Maldini, C., Acevedo, M., & Pinto, M. (2021). Hydroscapes: A Useful Metric for Distinguishing Iso-/Anisohydric Behavior in Almond Cultivars. Plants, 10(6), 1249. https://doi.org/10.3390/plants10061249






