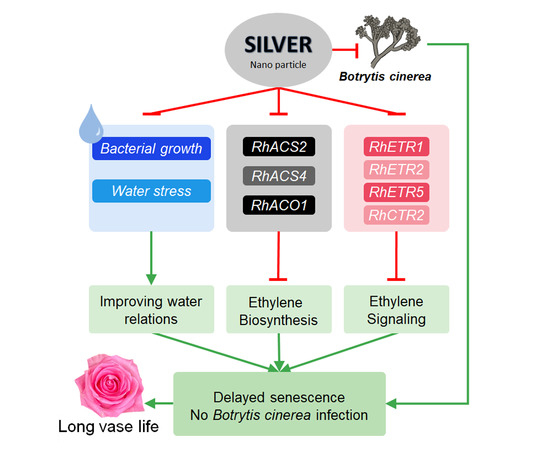
Regulation of Botrytis cinerea Infection and Gene Expression in Cut Roses by Using Nano Silver and Salicylic Acid
Abstract
1. Introduction
2. Results
2.1. B. cinerea Infection Rate, Vase Life and Flower Opening of Cut Roses
2.2. Expression Patterns of Ethylene Biosynthesis and Bcspl1 Genes
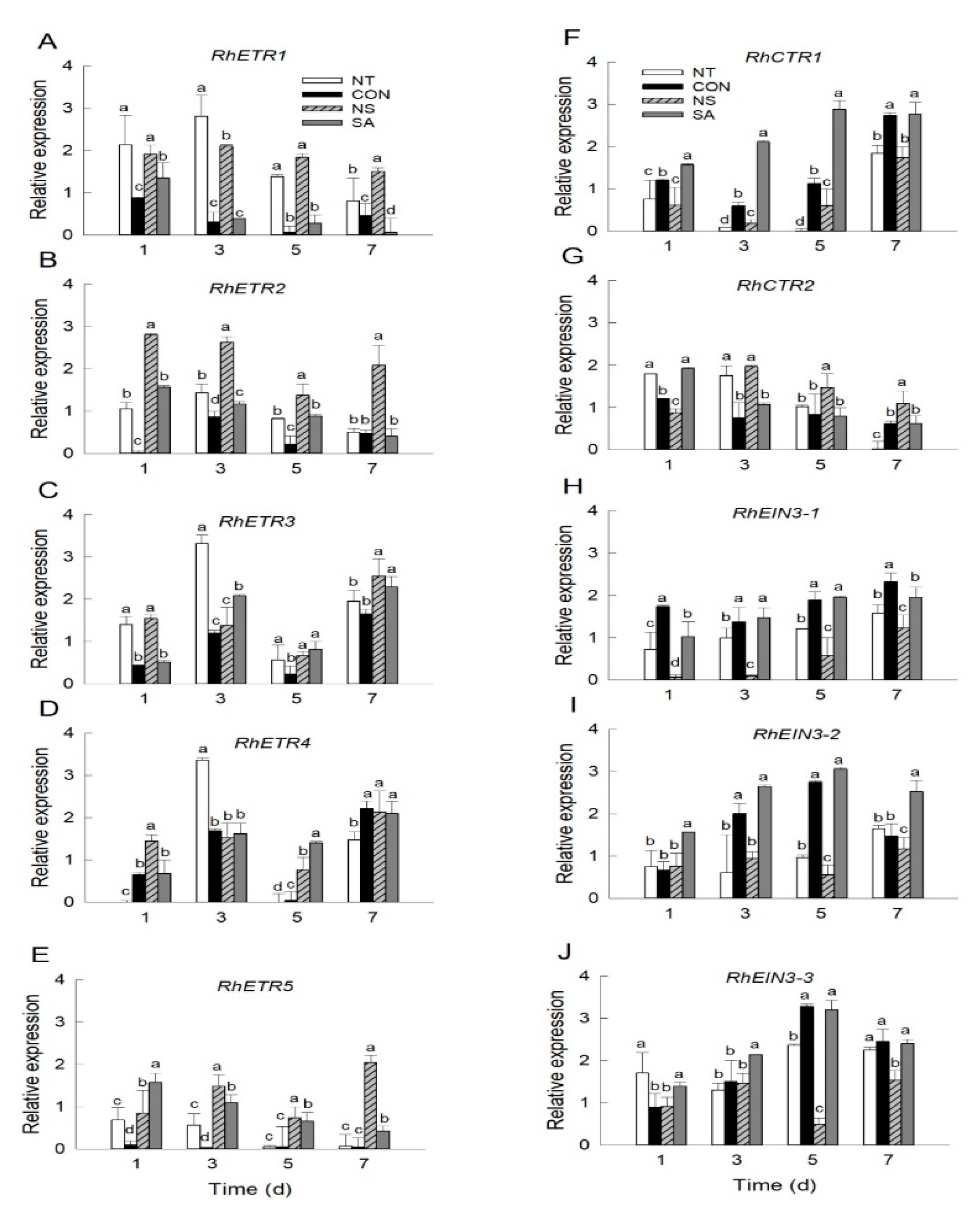
2.3. Expression Patterns of Ethylene Receptor and Signaling Genes
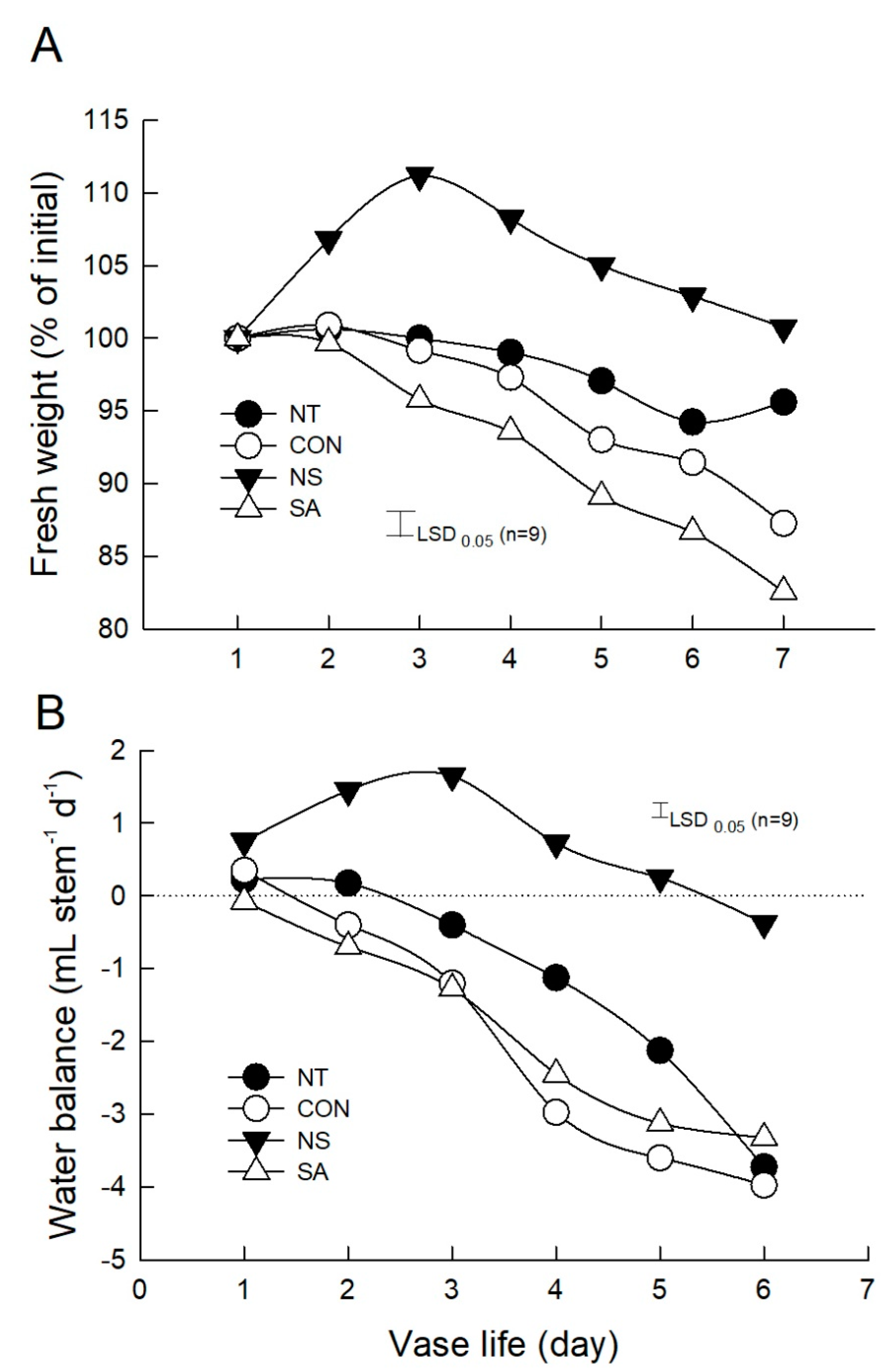
2.4. Changes in the Fv/Fm, Leaf Temperature, and Water Relations of Cut Roses
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Preparation of B. cinerea Conidial Suspension
4.3. Nano Silver, Salicylic Acid, and B. cinerea Treatments
4.4. Evaluation of B. cinerea Infection and the Morphological and Physiological Characteristics of Cut Flowers
4.5. Measurement of Leaf Temperature and Chlorophyll Fluorescence (CF) Parameters
4.6. RNA Isolation and cDNA Synthesis
4.7. qRT-PCR
4.8. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, B.; Duncan, G.H.; Harrison, J.G.; Harding, L.A.; Elad, Y.; Zimand, G. Effect of humidity on infection of rose petals by dry-inoculated conidia of Botrytis cinerea. Mycol. Res. 1995, 99, 1303–1310. [Google Scholar] [CrossRef]
- Elad, Y.; Volpin, H. The involvement of ethylene and calcium in gray mold of pelargonium, ruscus, and rose plants. Phytoparasitica 1988, 16, 119–131. [Google Scholar] [CrossRef]
- Muñoz, M.; Faust, J.E.; Schnabel, G. Characterization of Botrytis cinerea From Commercial Cut Flower Roses. Plant Dis. 2019, 103, 1577–1583. [Google Scholar] [CrossRef]
- Borochov, A.; Woodson, W. Physiology and Biochemistry of Flower Petal Senescence. Hortic. Rev. 2011, 11, 15–43. [Google Scholar]
- Pantelides, I.S.; Tjamos, S.E.; Paplomatas, E.J. Insights into the role of ethylene perception in tomato resistance to vascular infection by Verticillium dahliae. Plant Pathol. 2010, 59, 130–138. [Google Scholar] [CrossRef]
- Elad, Y. Involvement of ethylene in the disease caused by Botrytis cinerea on rose and carnation flowers and the possibility of control. Ann. Appl. Biol. 1988, 113, 589–598. [Google Scholar] [CrossRef]
- Benito, E.P.; ten Have, A.; van’t Klooster, J.W.; van Kan, J.A.L. Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea. Eur. J. Plant Pathol. 1998, 104, 207–220. [Google Scholar] [CrossRef]
- Díaz, J.; ten Have, A.; van Kan, J.A.L. The Role of Ethylene and Wound Signaling in Resistance of Tomato to Botrytis cinerea. Plant Physiol. 2002, 129, 1341–1351. [Google Scholar] [CrossRef]
- Chagué, V.; Danit, L.V.; Siewers, V.; Schulze-Gronover, C.; Tudzynski, P.; Tudzynski, B.; Sharon, A. Ethylene sensing and gene activation in Botrytis cinerea: A missing link in ethylene regulation of fungus-plant interactions? Mol. Plant Microbe Interact. Mpmi 2006, 19, 33–42. [Google Scholar] [CrossRef]
- Elad, Y.; Shtienberg, D. Botrytis cinerea in greenhouse vegetables: Chemical, cultural, physiological and biological controls and their integration. Integr. Pest Manag. Rev. 1995, 1, 15–29. [Google Scholar] [CrossRef]
- Chu, E.-H.; Shin, E.-J.; Park, H.-J.; Jeong, R.-D. Effect of gamma irradiation and its convergent treatment for control of postharvest Botrytis cinerea of cut roses. Radiat. Phys. Chem. 2015, 115, 22–29. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal Effects of Silver Nanoparticles (AgNPs) against Various Plant Pathogenic Fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef]
- Naing, A.H.; Win, N.M.; Han, J.S.; Lim, K.B.; Kim, C.K. Role of Nano-silver and the Bacterial Strain Enterobacter cloacae in Increasing Vase Life of Cut Carnation ‘Omea’. Front. Plant Sci. 2017, 8, 1590. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Naing, A.H.; Ai, T.N.; Han, J.-S.; Kang, I.-K.; Kim, C.K. Synergistic Effect of Nano-Sliver with Sucrose on Extending Vase Life of the Carnation cv. Edun. Front. Plant Sci. 2017, 8, 1601. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Dieryckx, C.; Gaudin, V.; Dupuy, J.W.; Bonneu, M.; Girard, V.; Job, D. Beyond plant defense: Insights on the potential of salicylic and methylsalicylic acid to contain growth of the phytopathogen Botrytis cinerea. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.; Li, X.; Ma, L.; Gu, Q.; Wu, H.; Liu, J.; Borriss, R.; Wu, Z.; Gao, X. Stomatal Closure and SA-, JA/ET-Signaling Pathways Are Essential for Bacillus amyloliquefaciens FZB42 to Restrict Leaf Disease Caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Ma, N.; Cai, L.; Lu, W.; Tan, H.; Gao, J. Exogenous ethylene influences flower opening of cut roses (Rosa hybrida) by regulating the genes encoding ethylene biosynthesis enzymes. Sci. China. Ser. C Life Sci. 2005, 48, 434–444. [Google Scholar] [CrossRef]
- In, B.-C.; Ha, S.T.T.; Lee, Y.S.; Lim, J.H. Relationships between the longevity, water relations, ethylene sensitivity, and gene expression of cut roses. Postharvest Biol. Technol. 2017, 131, 74–83. [Google Scholar] [CrossRef]
- Lund, S.T.; Stall, R.E.; Klee, H.J. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 1998, 10, 371–382. [Google Scholar] [CrossRef]
- Veloso, J.; Van Kan, J.A.L. Many shades of grey in Botrytis-Host plant interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef]
- Tudzynski, P.; Sharon, A. Fungal pathogenicity genes. Appl. Mycol. Biotechnol. 2003, 3, 187–212. [Google Scholar]
- Wolpert, T.J.; Dunkle, L.D.; Ciuffetti, L.M. Host-selective toxins and avirulence determinants: What’s in a name? Annu. Rev. Phytopathol. 2002, 40, 251–285. [Google Scholar] [CrossRef]
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Rodríguez, F.I.; Esch, J.J.; Hall, A.E.; Binder, B.M.; Schaller, G.E.; Bleecker, A.B. A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 1999, 283, 996–998. [Google Scholar] [CrossRef]
- Melotto, M.; Zhang, L.; Oblessuc, P.R.; He, S.Y. Stomatal Defense a Decade Later. Plant Physiol. 2017, 174, 561–571. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Torres, P.S.; Vojnov, A.A. Stomata and pathogens: Warfare at the gates. Plant Signal. Behav. 2009, 4, 1114–1116. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Steiner, U.; Dehne, H.-W.; Lindenthal, M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J. Exp. Bot. 2006, 57, 2121–2132. [Google Scholar] [CrossRef]
- Prodhan, M.Y.; Munemasa, S.; Nahar, M.N.-E.-N.; Nakamura, Y.; Murata, Y. Guard Cell Salicylic Acid Signaling Is Integrated into Abscisic Acid Signaling via the Ca2+/CPK-Dependent Pathway. Plant Physiol. 2018, 178, 441–450. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Scholes, J.D. Chlorophyll fluorescence imaging of plant-pathogen interactions. Protoplasma 2010, 247, 163–175. [Google Scholar] [CrossRef]
- Shahenshah; Isoda, A. Effects of Water Stress on Leaf Temperature and Chlorophyll Fluorescence Parameters in Cotton and Peanut. Plant Prod. Sci. 2010, 13, 269–278. [Google Scholar] [CrossRef]
- Rousseau, C.; Belin, E.; Bove, E.; Rousseau, D.; Fabre, F.; Berruyer, R.; Guillaumès, J.; Manceau, C.; Jacques, M.-A.; Boureau, T. High throughput quantitative phenotyping of plant resistance using chlorophyll fluorescence image analysis. Plant Methods 2013, 9, 17. [Google Scholar] [CrossRef]
- Kim, J.H.; Bhandari, S.R.; Chae, S.Y.; Cho, M.C.; Lee, J.G. Application of maximum quantum yield, a parameter of chlorophyll fluorescence, for early determination of bacterial wilt in tomato seedlings. Hortic. Environ. Biotechnol. 2019, 60, 821–829. [Google Scholar] [CrossRef]
- Pineda, M.; Soukupová, J.; Matouš, K.; Nedbal, L.; Barón, M. Conventional and combinatorial chlorophyll fluorescence imaging of tobamovirus-infected plants. Photosynthetica 2008, 46, 441–451. [Google Scholar] [CrossRef]
- Rossi, F.R.; Krapp, A.R.; Bisaro, F.; Maiale, S.J.; Pieckenstain, F.L.; Carrillo, N. Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea. Plant J. Cell Mol. Biol. 2017, 92, 761–773. [Google Scholar] [CrossRef]
- Kuckenberg, J.; Tartachnyk, I.; Noga, G. Temporal and spatial changes of chlorophyll fluorescence as a basis for early and precise detection of leaf rust and powdery mildew infections in wheat leaves. Precis. Agric. 2009, 10, 34–44. [Google Scholar] [CrossRef]
- Ha, S.T.T.; Kim, Y.T.; In, B.C. Assessment of the preservative solutions for reducing Botrytis cinerea infection in cut roses. Flower Res. J. 2020, 28, 279–284. [Google Scholar] [CrossRef]
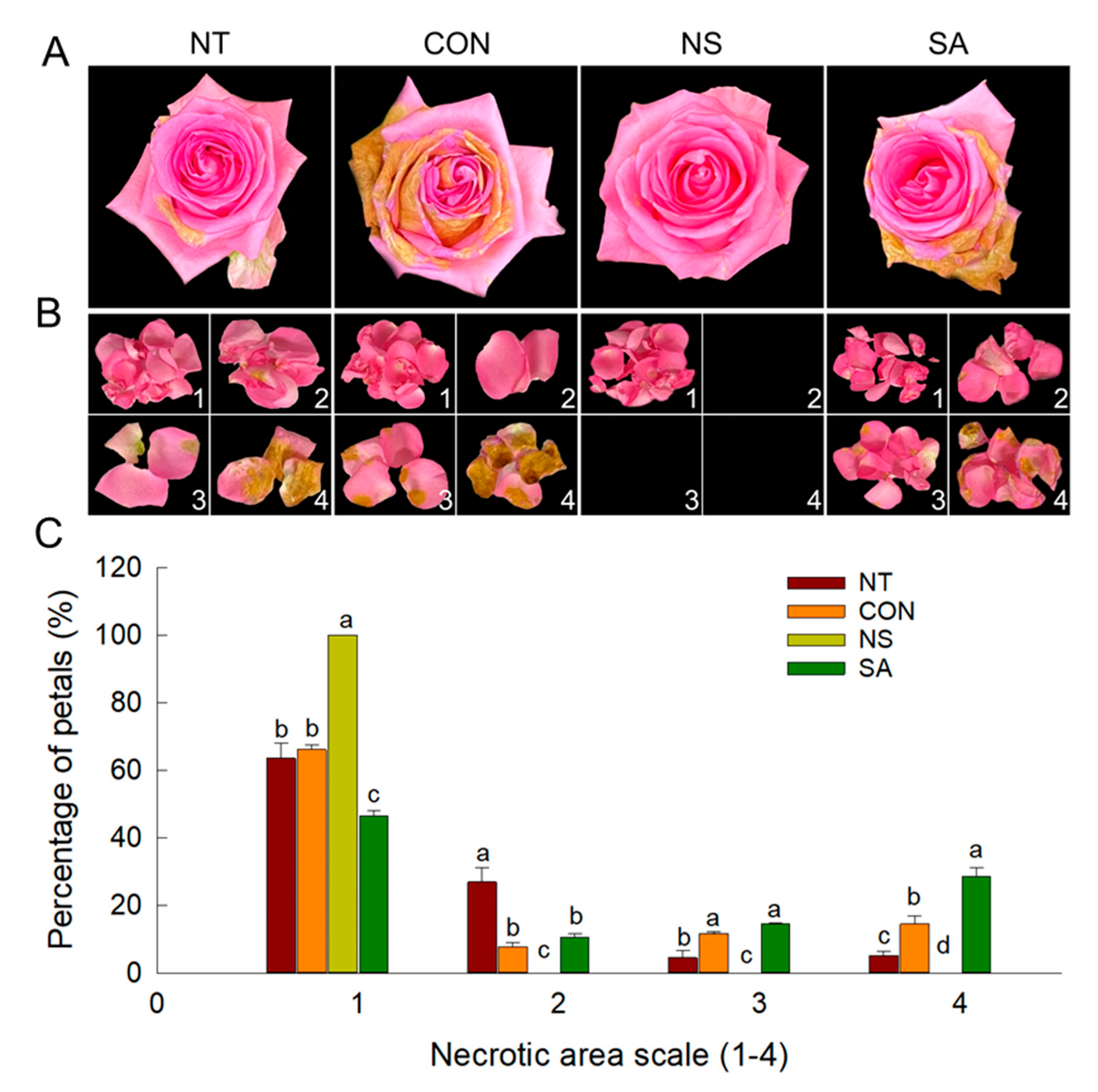


| Treatment | B. cinerea Incidence (%) ˣ | Vase Life (Days) | Maximum Flower Diameter (%) ʸ |
|---|---|---|---|
| Non-treatment (NT) | 80.0 ± 0.0 b ᶻ | 8.6 ± 0.3 b | 121.1 ± 1.3 ab |
| Control (CON) | 100.0 ± 0.0 a | 5.5 ± 0.4 c | 109.3 ± 1.9 c |
| Nano silver (NS) | 0.0 ± 0.0 c | 11.9 ± 0.8 a | 125.7 ± 2.3 a |
| Salicylic acid (SA) | 100 ± 0.0 a | 6.2 ± 0.4 c | 108.6 ± 5.8 c |
| Gene (Accession Number) | Forward Primer | Reverse Primer | Size |
|---|---|---|---|
| RhACS1 (AY378152.1) | 5′-CAGTGAGAAAGGGGAGCTTG-3′ | 5′-TGTATTGAACCGGGATGGTT-3′ | 102 |
| RhACS2 (AY803737.1) | 5′-GCGAACAGGGGTACAACTTC-3′ | 5′-GGGTTTGAGGGGTTGGTAAT-3′ | 147 |
| RhACS3 (AY803738.1) | 5′-CAGTGAGAAAGGGGAGCTTG-3′ | 5′-AACCATCCCGGTTCAATACA-3′ | 142 |
| RhACS4 (AY525068.1) | 5′-GCTTCCAACTTGGGATCAAA-3′ | 5′-GCTCCATGAAACTTGCCATT-3′ | 100 |
| RhACO1 (AF441282.1) | 5′CGTTCTACAACCCAGGCAAT-3′ | 5′-TTGAGGCCTGCATAGAGCTT-3′ | 130 |
| RhETR1 (AY953869.1) | 5′-TGACTGGCCTGATGTCTCTG-3′ | 5′-GGCAACTGGTGAAAAGGAAA-3′ | 158 |
| RhETR2 (AF127220.1) | 5′-CTGCGTTAGAGCAGCAACTG-3′ | 5′-GGAATTCGGCGATATCTTCA-3′ | 131 |
| RhETR3 (AY953392.1) | 5′-CCATGAGTTGAAAGGGAGGA-3′ | 5′-GGCTCACCAAAATCACCACT-3′ | 156 |
| RhETR4 (AF159172.1) | 5′-TTGAAGTCGTTGCAGACCAG-3′ | 5′-TCATGACAGCAAGGAAGTCG-3′ | 168 |
| RhETR5 (AF441283.1) | 5′-TGTGTGGAGCGACACATCTT-3′ | 5′-TGAGGGCAGTAGCACATGAC-3′ | 120 |
| RhEIN3-1 (AF443783) | 5′-TGCTGAAGATGATGGAGGTG-3′ | 5′-GCAGGGCCATTCTTATCAAA-3′ | 142 |
| RhEIN3-2 (AY919867.1) | 5′-ATTGAACTTGGCCAATCAGG-3′ | 5′-GCAGTCATCTTGTCCTGCAA-3′ | 168 |
| RhEIN3-3 (KC484653.1) | 5′-GCCAGTGGATCTTTGGTGAT-3′ | 5′-ACTTGAAGCCCTTCCCTCAT-3′ | 149 |
| RhCTR1 (AY032953.1) | 5′-GGCTCTGATGTTGCTGTGAA-3′ | 5′-CAAGTTTGGGGGCTTTGTAA-3′ | 150 |
| RhCTR2 (AY029067.1) | 5′-CGAGCAACCCCACTATTGTT-3′ | 5′-TTATGTTTCAAGCGCGACAG-3′ | 109 |
| RhACT1 (KC514918.1) | 5′-GTTCCCAGGAATCGCTGATA-3′ | 5′-ATCCTCCGATCCAAACACTG-3′ | 116 |
| Bcspl1 (XM024691684.1) | 5′-CCTACGACGTTGGCTACGAT-3′ | 5′-CCTCAAGAACTTCCCCAACA-3′ | 123 |
| BcactA(XM024697950.1) | 5′-GCACCACCCGAGAGAAAATA-3′ | 5′-AAGAGTACGACGAGTCCGGA-3′ | 169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.T.T.; Kim, Y.-T.; Jeon, Y.H.; Choi, H.W.; In, B.-C. Regulation of Botrytis cinerea Infection and Gene Expression in Cut Roses by Using Nano Silver and Salicylic Acid. Plants 2021, 10, 1241. https://doi.org/10.3390/plants10061241
Ha STT, Kim Y-T, Jeon YH, Choi HW, In B-C. Regulation of Botrytis cinerea Infection and Gene Expression in Cut Roses by Using Nano Silver and Salicylic Acid. Plants. 2021; 10(6):1241. https://doi.org/10.3390/plants10061241
Chicago/Turabian StyleHa, Suong Tuyet Thi, Yong-Tae Kim, Yong Ho Jeon, Hyong Woo Choi, and Byung-Chun In. 2021. "Regulation of Botrytis cinerea Infection and Gene Expression in Cut Roses by Using Nano Silver and Salicylic Acid" Plants 10, no. 6: 1241. https://doi.org/10.3390/plants10061241
APA StyleHa, S. T. T., Kim, Y.-T., Jeon, Y. H., Choi, H. W., & In, B.-C. (2021). Regulation of Botrytis cinerea Infection and Gene Expression in Cut Roses by Using Nano Silver and Salicylic Acid. Plants, 10(6), 1241. https://doi.org/10.3390/plants10061241