Physiological, Biochemical and Molecular Assessment of UV-A and UV-B Supplementation in Solanum lycopersicum
Abstract
1. Introduction
2. Results
2.1. Plant Growth
2.2. Chlorophyll a Fluorescence and Pigments
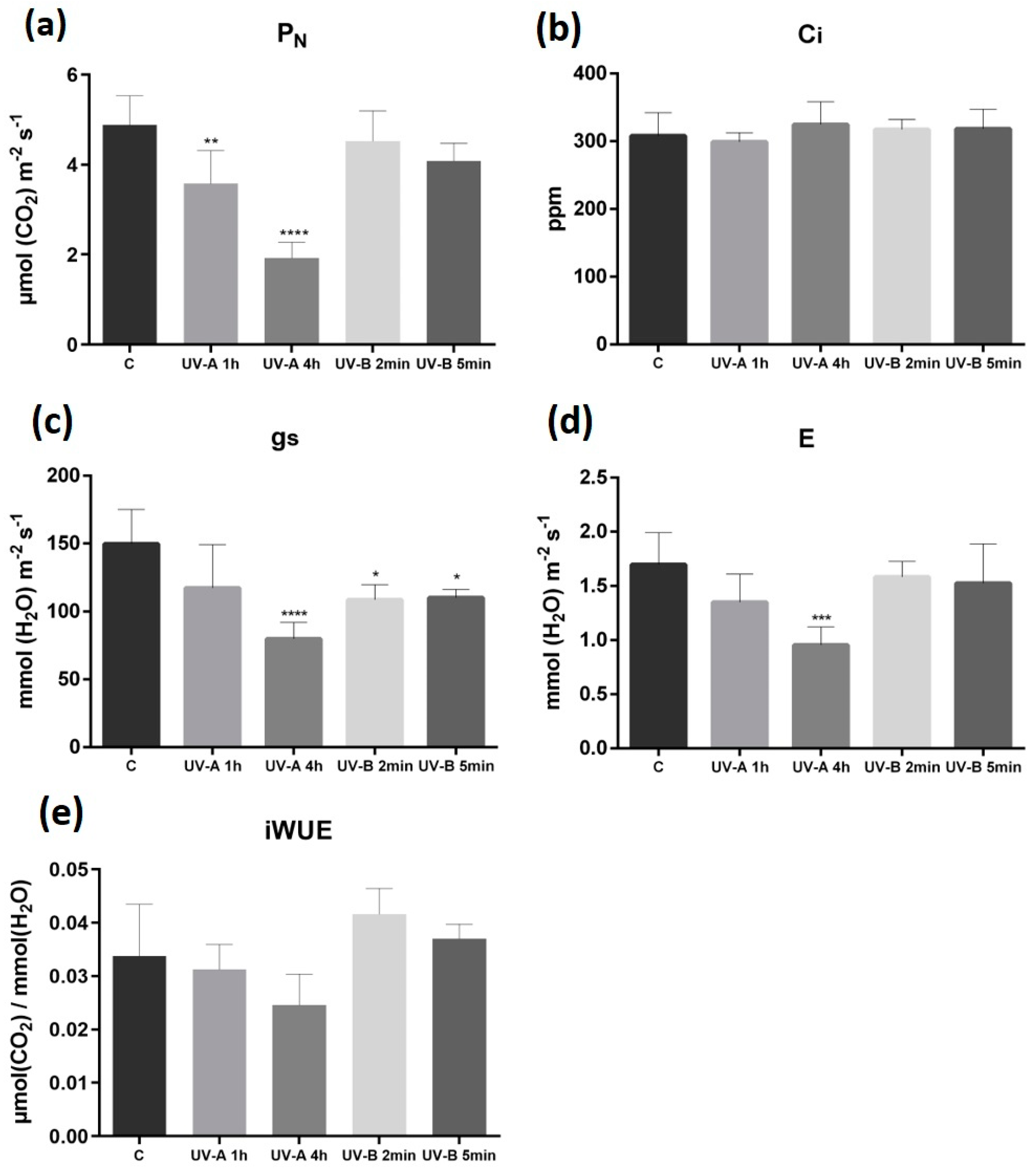
2.3. Gas Exchange, Carbohydrates, and RuBisCO
2.4. Gene Expression for RuBisCO and PSII
2.5. Multivariate Approach
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Research and Market. World-Tomato-Market Analysis, Forecast, Size, Trends and Insights; IndexBox Inc.: Walnut, CA, USA, 2020. [Google Scholar]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Torres Pineda, I.; Lee, Y.D.; Kim, Y.S.; Lee, S.M.; Park, K.S. Review of inventory data in life cycle assessment applied in production of fresh tomato in greenhouse. J. Clean. Prod. 2020, 282, 124395. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Shen, W.; Cui, J. Analyzing photosynthetic activity and growth of Solanum lycopersicum seedlings exposed to different light qualities. Acta Physiol. Plant 2014, 36, 1411–1420. [Google Scholar] [CrossRef]
- Muñoz, P.; Antón, A.; Nuñez, M.; Paranjpe, A.; Ariño, J.; Castells, X.; Rieradevall, J. Comparing the environmental impacts of greenhouse versus open-field tomato production in the Mediterranean region. Int. Symp. High Technol. Greenh. Syst. Manag. Greensys2007 2007, 801, 1591–1596. [Google Scholar] [CrossRef]
- Gil, M.; Bottini, R.; Berli, F.; Pontin, M.; Silva, M.F.; Piccoli, P. Volatile organic compounds characterized from grapevine (Vitis vinifera L. cv. Malbec) berries increase at pre-harvest and in response to UV-B radiation. Phytochemistry 2013, 96, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.; Flint, S.; Ryel, R.; Tobler, M.; Barkley, A.E.; Wargent, J. Rediscovering leaf optical properties: New insights into plant acclimation to solar UV radiation. Plant Physiol. Biochem. 2015, 93, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Melo, P.; Santos, C. Moderate UV-A supplementation benefits tomato seed and seedling invigoration: A contribution to the use of UV in seed technology. Sci. Hortic. 2018, 235, 357–366. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Martins, S.; Gonçalves, A.; Correia, C.M.; Ribeiro, C.; Dias, M.C.; Santos, C. The potential use of the UV-A and UV-B to improve tomato quality and preference for consumers. Sci. Hortic. 2019, 246, 777–784. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Economic analysis of greenhouse lighting: Light emitting diodes vs. high-intensity discharge fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Bravo, R.; Chen, G.; Kim, H.K.; Grosser, K.; van Dam, N.M.; Leiss, K.A.; Klinkhamer, P.G. Ultraviolet radiation exposure time and intensity modulate tomato resistance to herbivory through activation of jasmonic acid signaling. J. Exp. Bot. 2019, 70, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Bashri, G.; Singh, M.; Mishra, R.K.; Kumar, J.; Singh, V.P.; Prasad, S.M. Kinetin regulates UV-B-induced damage to growth, photosystem II photochemistry, and nitrogen metabolism in tomato seedlings. J. Plant Growth Regul. 2018, 37, 233–245. [Google Scholar] [CrossRef]
- Lee, J.; Oh, M.; Son, K. Short-Term Ultraviolet (UV)-A Light-Emitting Diode (LED) Radiation Improves Biomass and Bioactive Compounds of Kale. Front. Plant Sci. 2019, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, M.D.; Correia, C.; Silva, A.M.S.; Santos, C. UV-B radiation modulates physiology and lipophilic metabolite profile in Olea europaea. J. Plant Physiol. 2018, 222, 39–50. [Google Scholar] [CrossRef]
- Katsoulas, N.; Bari, A.; Papaioannou, C. Plant Responses to UV Blocking Greenhouse Covering Materials: A Review. Agronomy 2020, 10, 1021. [Google Scholar] [CrossRef]
- Yokawa, K.; Kagenishi, T.; Baluska, F. UV-B Induced Generation of Reactive Oxygen Species Promotes Formation of BFA-Induced Compartments in Cells of Arabidopsis Root Apices. Front. Plant Sci. 2015, 6, 1162. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Sirtautas, R.; Novičkovas, A.; Dabašinskasm, L.; Miliauskienė, J.; Vaštakaitė, V.; Bagdonavičienė, A.; et al. Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int. Agrophys. 2013, 29, 13–22. [Google Scholar] [CrossRef]
- Osorio, S.; Ruan, Y.; Fernie, A.R. An update on source-to-sink carbon partitioning in tomato. Front. Plant Sci. 2014, 5, 516. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.; Santos, C.; Costa, M.; Moutinho-Pereira, J.; Correia, C.; Dias, M.C. Plasticity of young Moringa oleifera L. plants to face water deficit and UVB radiation challenges. J. Photochem. Photobiol. B Biol. 2016, 162, 278–285. [Google Scholar] [CrossRef]
- Inostroza-Blancheteau, C.; Acevedo, P.; Loyola, R.; Arce-Johnson, P.; Alberdi, M.; Reyes-Díaz, M. Short-term UV-B radiation affects photosynthetic performance and antioxidant gene expression in highbush blueberry leaves. Plant Physiol. Biochem. 2016, 107, 301–309. [Google Scholar] [CrossRef]
- Khudyakova, A.Y.; Kreslavski, V.D.; Shmarev, A.N.; Lyubimov, V.Y.; Shirshikova, G.N.; Pashkovskiy, P.P.; Kuznetsov, V.V.; Allakhverdiev, S.I. Impact of UV-B radiation on the photosystem II activity, pro-/antioxidant balance and expression of light-activated genes in Arabidopsis thaliana hy4 mutants grown under light of different spectral composition. Photochem. Photobiol. B Biol. 2019, 194, 14–20. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.; Li, Y.; Han, R. Chloroplast proteomic analysis of Triticum aestivum L. seedlings responses to low levels of UV-B stress reveals novel molecular mechanism associated with UV-B tolerance. Environ. Sci. Pollut. Res. 2019, 26, 7143–7155. [Google Scholar] [CrossRef] [PubMed]
- Soriano, G.; Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Photosynthetically-active radiation, UV-A and UV-B, causes both common and specific damage and photoprotective responses in the model liverwort Marchantia polymorpha subsp. ruderalis. Photochem. Photobiol. Sci. 2019, 18, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.B.; Brunetti, C.; Agati, G.; Lo Iacono, C. Short-Term Pre-Harvest UV-B Supplement Enhances the Polyphenol Content and Antioxidant Capacity of Ocimum basilicum Leaves during Storage. Plants 2020, 9, 797. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; de Oliveira, J.F.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Huché-Thélier, L.; Crespel, L.; Gourrierec, J.L.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations-Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Yadav, A.; Bakshi, A.; Yadukrishnan, P.; Lingwan, M.; Dolde, U.; Wenkel, S.; Masakapalli, S.; Datta, S. The B-Box-Containing MicroProtein miP1a/BBX31 Regulates Photomorphogenesis and UV-B Protection. Plant Physiol. 2019, 179, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhang, Y.; Zhang, Y.; Zou, J.; Yang, Q.; Li, T. Ultraviolet-A radiation stimulates growth of indoor cultivated tomato (Solanum lycopersicum) seedlings. HortScience 2018, 53, 1429–1433. [Google Scholar] [CrossRef]
- Qian, M.; Kalbina, I.; Rosenqvist, E.; Jansen, M.A.; Teng, Y.; Strid, Å. UV regulates the expression of phenylpropanoid biosynthesis genes in cucumber (Cucumis sativus L.) in an organ and spectrum dependent manner. Photochem. Photobiol. Sci. 2019, 8, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Wargent, J.; Nelson, B.; McGhie, T.; Barnes, P. Acclimation to UV-B radiation and visible light in Lactuca sativa involves up-regulation of photosynthetic performance and orchestration of metabolome-wide responses. Plant Cell Environ. 2015, 38, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Brunetti, C.; Fini, A.; Agati, G.; Ferrini, F.; Gori, A.; Tattini, M. UV radiation promotes flavonoid biosynthesis, while negatively affecting the biosynthesis and the de-epoxidation of xanthophylls: Consequence for photoprotection? Environ. Exp. Bot. 2016, 127, 14–25. [Google Scholar] [CrossRef]
- Machado, F.; Dias, C.; Pinho, P.; Araújo, A.; Pinto, D.; Correia, C.; Santos, C. Photosynthetic performance and volatile organic compounds profile in Eucalyptus globulus after UVB radiation. Environ. Exp. Bot. 2017, 140, 141–149. [Google Scholar] [CrossRef]
- Hou, H.; Najafpour, M.M.; Moore, G.F.; Allakhverdiev, S.I. Photosynthesis: Structures, Mechanisms and Applications; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-48873-8. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Mackerness, S.H.; Surplus, S.L.; Jordan, B.R.; Thomas, B. Ultraviolet-B effects on transcript levels for photosynthetic genes are not mediated through carbohydrate metabolism. Plant Cell Environ. 1997, 20, 1431–1437. [Google Scholar] [CrossRef]
- Kiss, E.; Kós, P.B.; Chen, M.; Vass, I. A unique regulation of the expression of the psbA, psbD, and psbE genes, encoding the 01, 02 and cytochrome b559 subunits of the PSII complex in the chlorophyll d containing cyanobacterium Acaryochloris marina. Biochim. Biophys. Acta 2012, 1817, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.Z.; Moumeni, A.; Komatsu, S. Abiotic stresses: Insight into gene regulation and protein expression in photosynthetic pathways of plants. Int. J. Mol. Sci. 2015, 16, 20392–20416. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Komatsu, S.; Zhu, W.; Zhang, L.; Li, X.; Cui, L.; Tian, J. Response and Defense Mechanisms of Taxus chinensis Leaves Under UV-A Radiation are Revealed Using Comparative Proteomics and Metabolomics Analyses. Plant Cell Physiol. 2016, 57, 1839–1853. [Google Scholar] [CrossRef]
- Aphalo, P.J.; Albert, A. Beyond the Visible: A Handbook of Best Practice in Plant UV Photobiology; Helsingin Yliopisto: Helsinki, Finland, 2012. [Google Scholar] [CrossRef]
- Flint, S.D.; Caldwell, M.M. A biological spectral weighting function for ozone depletion research with higher plants. Physiol. Plant. 2003, 117, 137–144. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Rem. Sen. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Li, D.; Tian, M.; Cai, J.; Jiang, D.; Cao, W.; Dai, T. Effects of low nitrogen supply on relationships between photosynthesis and nitrogen status at different leaf position in wheat seedlings. Plant Growth Regul. 2013, 70, 257–263. [Google Scholar] [CrossRef]
- Løvdal, T.; Lillo, C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal. Biochem. 2009, 387, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Dzakovich, M.P.; Ferruzzi, M.G.; Mitchell, C.A. Manipulating Sensory and Phytochemical Profiles of Greenhouse Tomatoes Using Environmentally Relevant Doses of Ultraviolet Radiation. J Agric. Food Chem. 2016, 64, 6801–6808. [Google Scholar] [CrossRef] [PubMed]





| Treatment | Plant Length | WC | Leaf DM | Flowers | Flowers and Fruits a |
|---|---|---|---|---|---|
| Control | 20.6 ± 2.4 | 95.07 ± 2.62 | 0.139 ± 0.061 | 15.0 ± 6.3 | 30.7 ± 6.4 |
| UV-A 1 h | 19.4 ± 2.5 | 93.48 ± 0.95 | 0.204 ± 0.015 * | 11.9 ± 5.2 | 41.6 ± 11.9 * |
| UV-A 4 h | 16.4 ± 3.8 | 91.47 ± 0.95 ** | 0.185 ± 0.023 | 15.8 ± 7.0 | 43.2 ± 10.0 * |
| UV-B 2 min | 16.6 ± 2.3 * | 91.65 ± 0.83 ** | 0.194 ± 0.021 | 10.2 ± 4.8 | 41.0 ± 12.7 |
| UV-B 5 min | 17.7 ± 3.3 | 93.61 ± 1.10 | 0.216 ± 0.028 ** | 12.4 ± 6.7 | 34.7 ± 9.0 |
| Treatment | Chl a | Chl b | Chl a/b | Car | Ant |
|---|---|---|---|---|---|
| Control | 1.44 ± 0.183 | 0.80 ± 0.073 | 1.79 ± 0.077 | 0.45 ± 0.030 | 0.050 ± 0.002 |
| UV-A 1 h | 1.45 ± 0.163 | 0.80 ± 0.088 | 1.81 ± 0.006 | 0.40 ± 0.041 | 0.046 ± 0.001 * |
| UV-A 4 h | 1.40 ± 0.073 | 0.77 ± 0.030 | 1.82 ± 0.042 | 0.39 ± 0.014 | 0.045 ± 0.001 ** |
| UV-B 2 min | 1.69 ± 0.101 | 0.89 ± 0.059 | 1.90 ± 0.012 * | 0.43 ± 0.031 | 0.040 ± 0.001 **** |
| UV-B 5 min | 2.04 ± 0.088 ** | 1.10 ± 0.063 ** | 1.86 ± 0.027 | 0.54 ± 0.033 * | 0.040 ± 0.001 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Correia, C.V.; Correia, C.M.; Moutinho-Pereira, J.; Melo, P.; Dias, M.C.; Santos, C. Physiological, Biochemical and Molecular Assessment of UV-A and UV-B Supplementation in Solanum lycopersicum. Plants 2021, 10, 918. https://doi.org/10.3390/plants10050918
Mariz-Ponte N, Mendes RJ, Sario S, Correia CV, Correia CM, Moutinho-Pereira J, Melo P, Dias MC, Santos C. Physiological, Biochemical and Molecular Assessment of UV-A and UV-B Supplementation in Solanum lycopersicum. Plants. 2021; 10(5):918. https://doi.org/10.3390/plants10050918
Chicago/Turabian StyleMariz-Ponte, Nuno, Rafael J. Mendes, Sara Sario, Cristiana V. Correia, Carlos M. Correia, José Moutinho-Pereira, Paula Melo, Maria Celeste Dias, and Conceição Santos. 2021. "Physiological, Biochemical and Molecular Assessment of UV-A and UV-B Supplementation in Solanum lycopersicum" Plants 10, no. 5: 918. https://doi.org/10.3390/plants10050918
APA StyleMariz-Ponte, N., Mendes, R. J., Sario, S., Correia, C. V., Correia, C. M., Moutinho-Pereira, J., Melo, P., Dias, M. C., & Santos, C. (2021). Physiological, Biochemical and Molecular Assessment of UV-A and UV-B Supplementation in Solanum lycopersicum. Plants, 10(5), 918. https://doi.org/10.3390/plants10050918












