Combined Selenium and Zinc Biofortification of Bread-Making Wheat under Mediterranean Conditions
Abstract
1. Introduction
2. Results
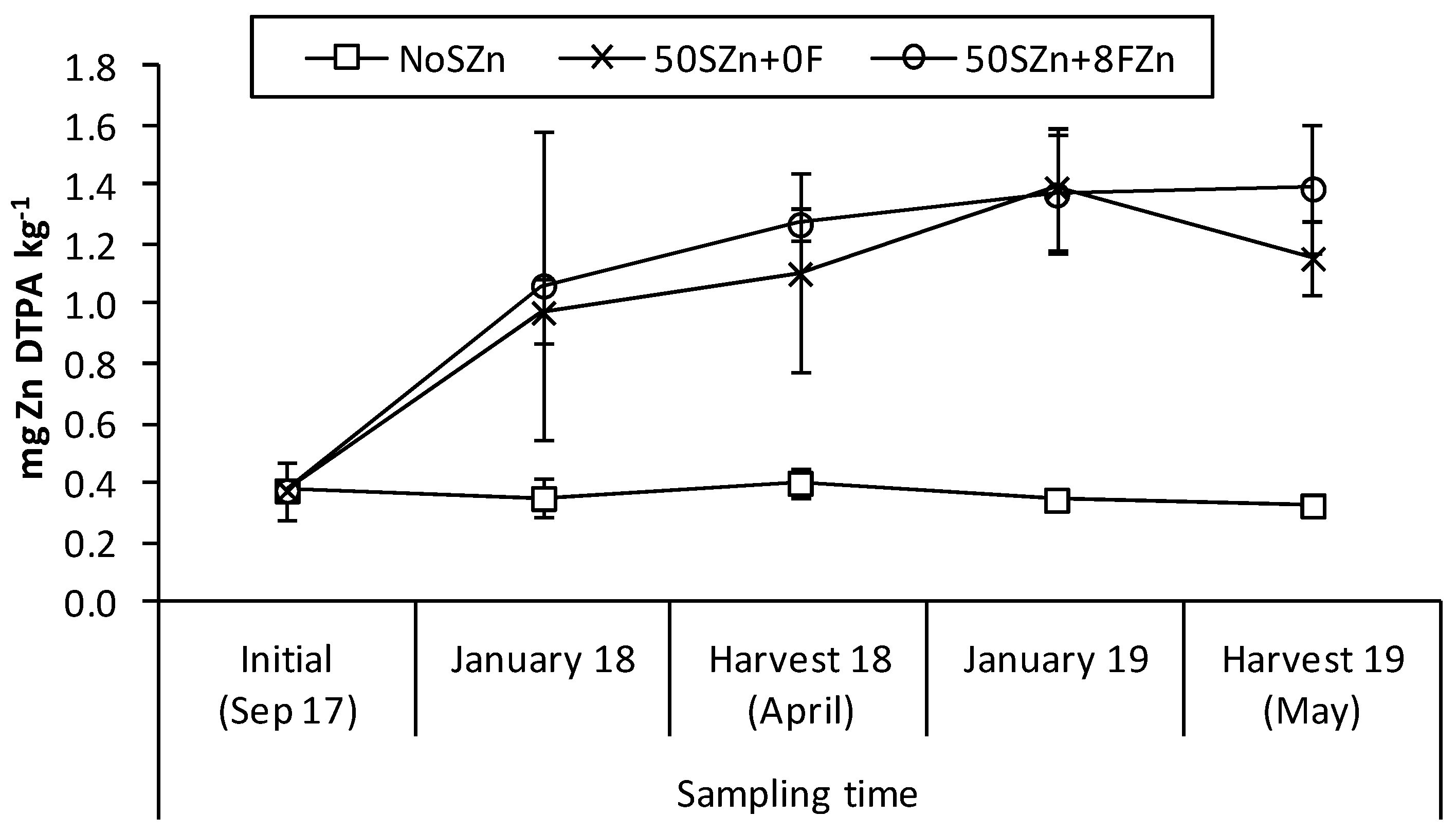
2.1. Evolution of Soil Zn-DTPA in the Soil
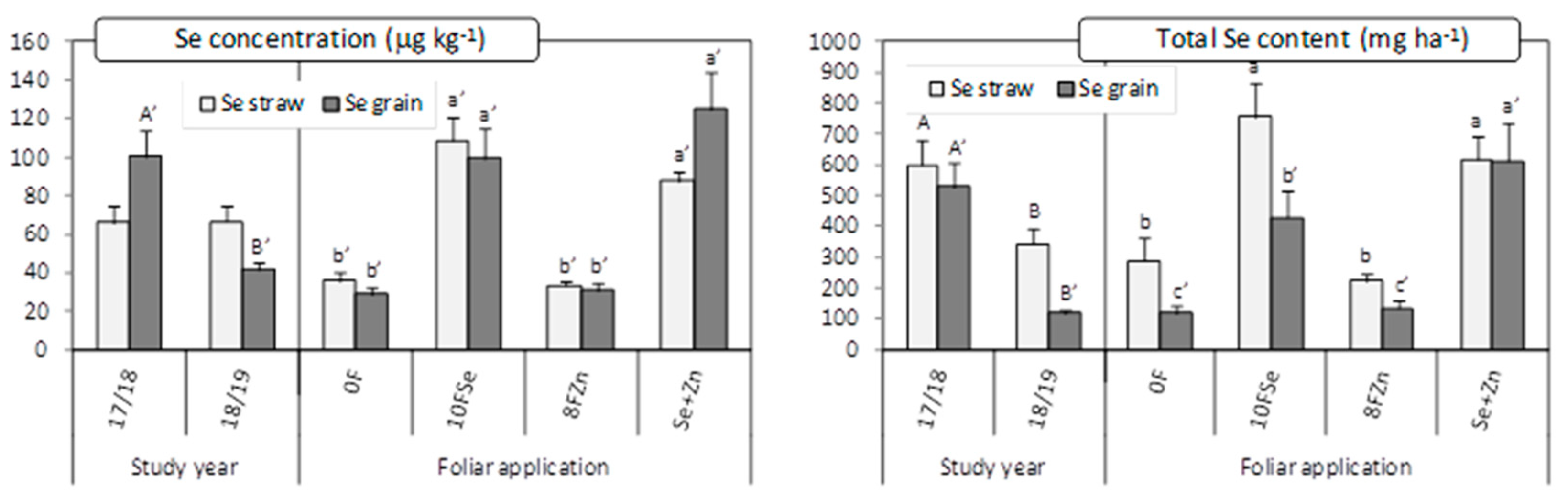
2.2. Zn and Se Concentrations and Contents in Straw and Its Bioavailability
2.3. Zn and Se Concentrations and Contents in Grain and Its Bioavailability
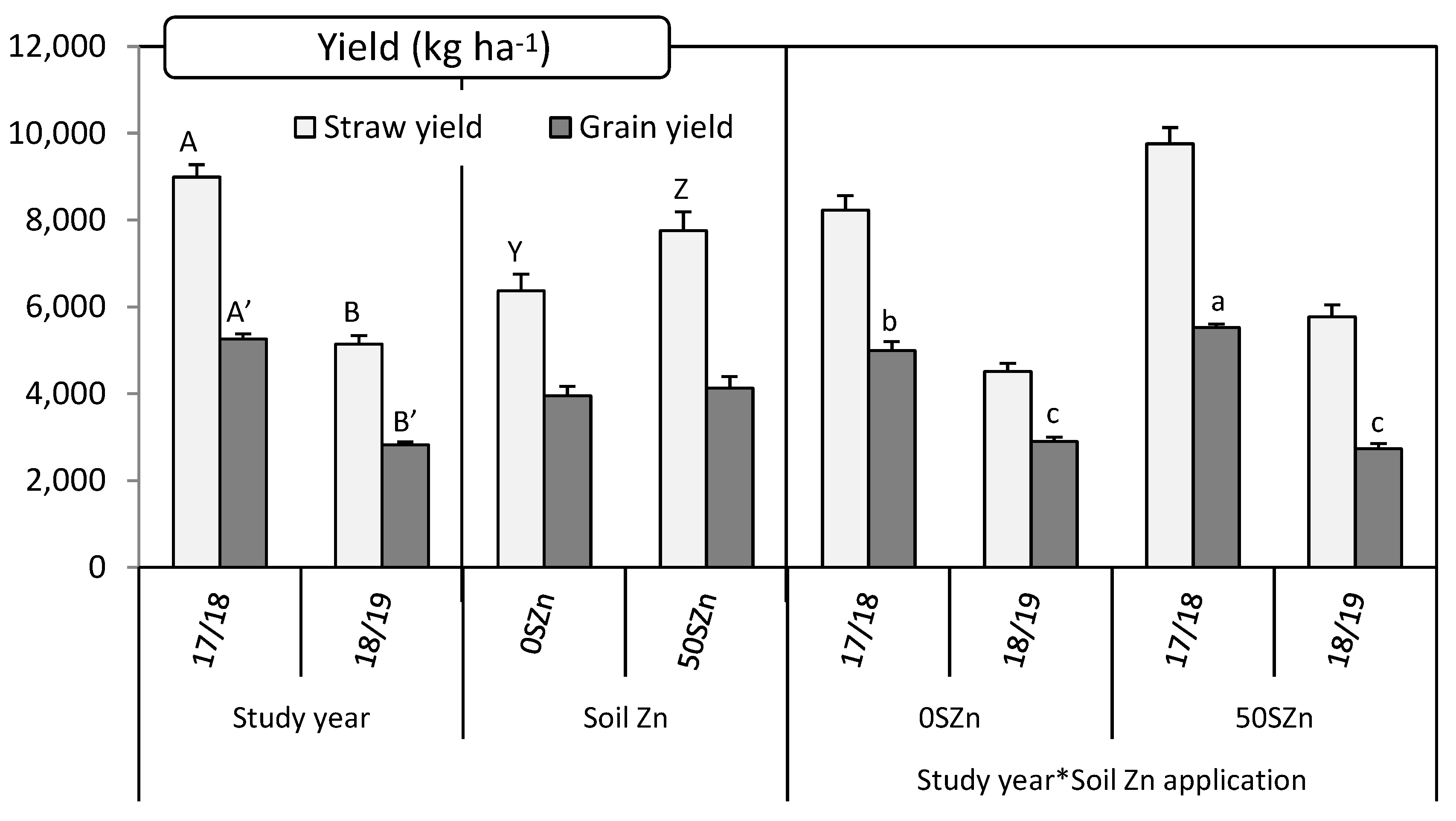
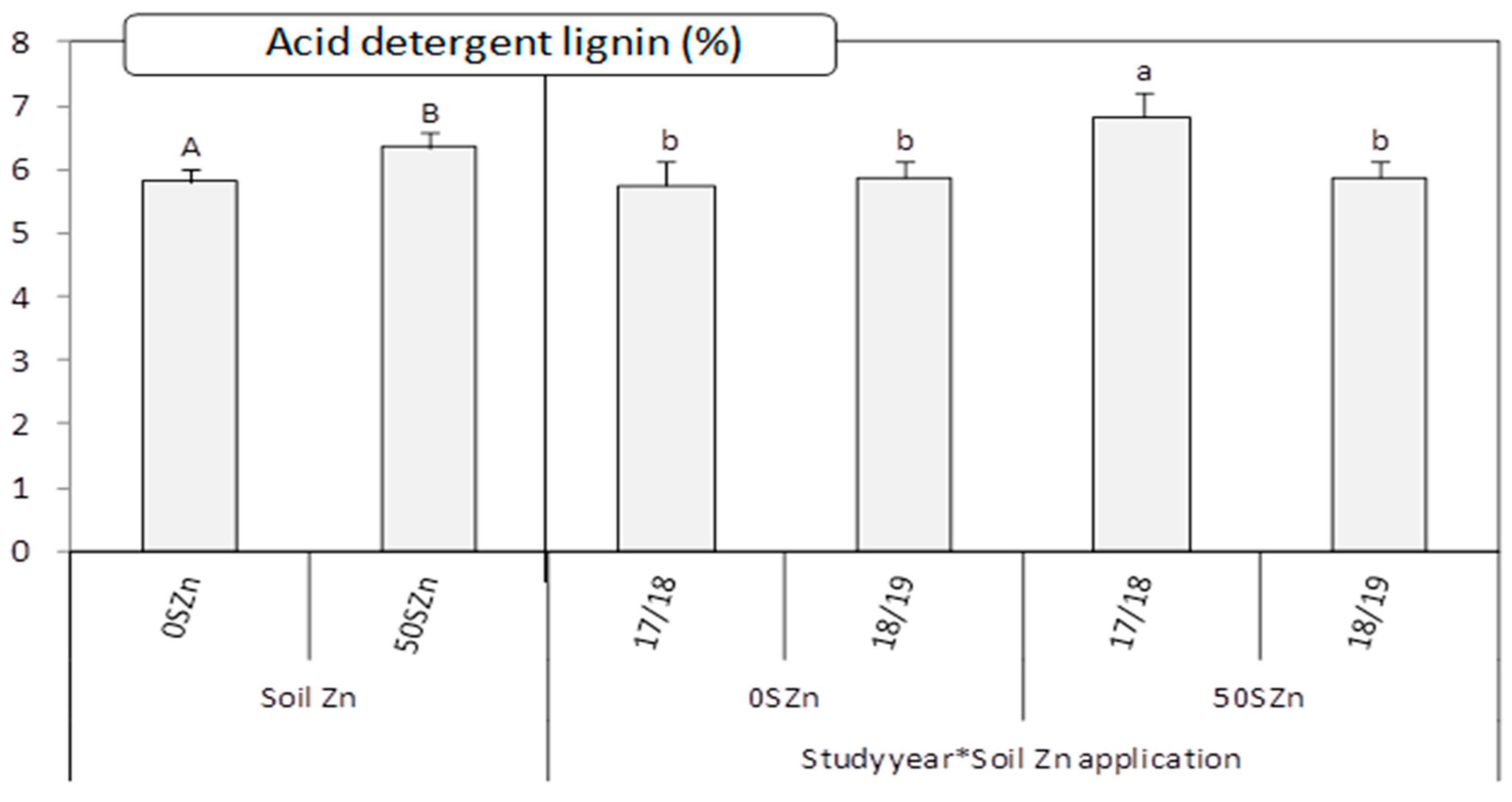
2.4. Effect of Zn and Se Application on Grain and Straw Yield and Straw Nutritive Parameters
3. Discussion
4. Materials and Methods
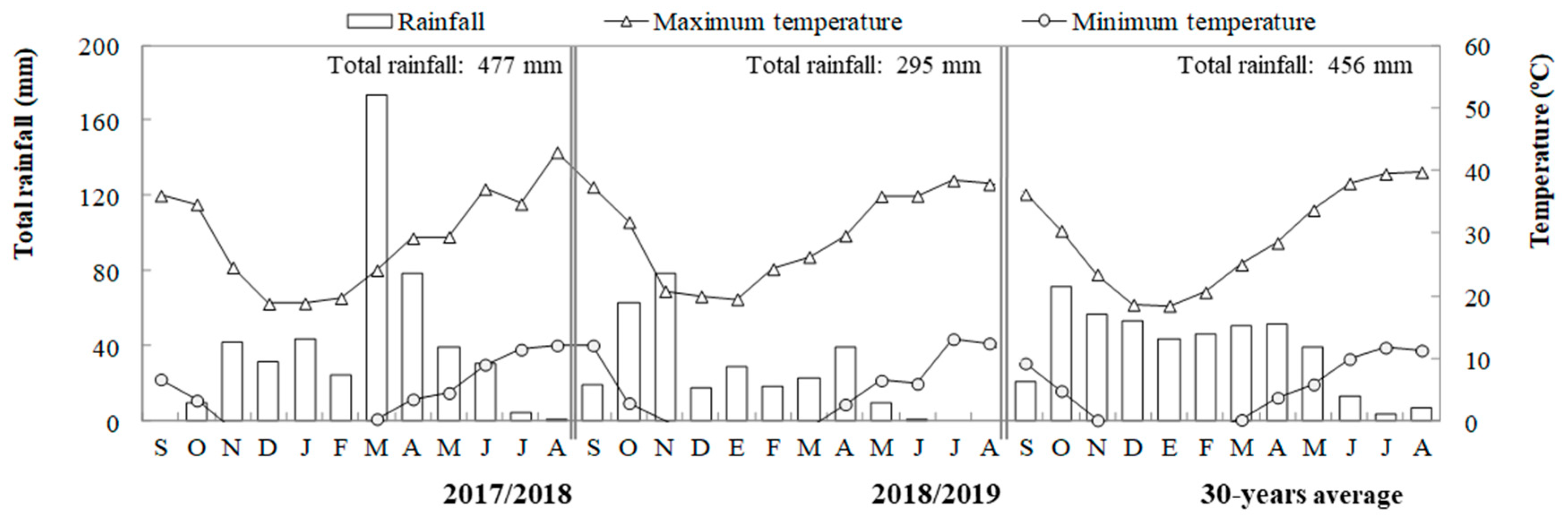
4.1. Site, Experimental Design and Crop Management
4.2. Soil Analysis
4.3. Plant Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Data 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 May 2021).
- Serna Saldívar, S.O. Cereal Grains: Properties, Processing and Nutritional Attributes; CRC Press: Boca Ratón, FL, USA, 2010; p. 747. [Google Scholar]
- Shewry, P.; Halford, N. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef]
- Zúñiga, J.R. Trigo blanco valor nutricional y potencial. Tierra Adentro 2007, 74. Available online: https://biblioteca.inia.cl/handle/123456789/6331 (accessed on 7 May 2021).
- Amarakoon, D.; Thavarajah, D.; McPhee, K.; Thavarajah, P. Iron-, zinc-, and magnesium-rich field peas (Pisum sativum L.) with naturally low phytic acid: A potential food-based solution to global micronutrient malnutrition. J. Food Compos. Anal. 2012, 27, 8–13. [Google Scholar] [CrossRef]
- Lockyer, S.; White, A.; Buttriss, J.L. Biofortified crops for tackling micronutrient deficiencies—What impact are these having in developing countries and could they be of relevance within Europe? Nutr. Bull. 2018, 43, 319–357. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Poblaciones, M.J.; Santamaria, O.; García-White, T.; Rodrigo, S.M. Selenium biofortification in bread-making wheat under Mediterranean conditions: Influence on grain yield and quality parameters. Crop. Pasture Sci. 2014, 65, 362–369. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rodrigo, S.; Santamaria, O. Biofortification of legumes with Selenium in Semiarid Conditions. Selenium 2015, 1, 324–340. [Google Scholar]
- Rodrigo, S.; Santamaria, O.; Perez-Izquierdo, L.; Poblaciones, M.J. Arsenic and selenium levels in rice fields from south-west of Spain: Influence of the years of monoculture. Plant Soil Environ. 2017, 63, 184–188. [Google Scholar]
- Gomez-Coronado, F.; Poblaciones, M.J.; Almeida, A.S.; Cakmak, I. Zinc (Zn) concentration of bread wheat grown under mediterranean conditions as affected by genotype and soil/foliar Zn application. Plant Soil 2016, 401, 331–346. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Beckett, G.J.; Arthur, J.R. Selenium and endocrine systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef]
- Williams, E.; Harrison, M. Selenium: From health to the biological food chain. J. Biotech. Res. 2010, 2, 112–120. [Google Scholar]
- Reid, M.E.; Duffield-Lillico, A.J.; Slate, E.; Natarajan, N.; Turnbull, B.; Jacobs, E.; Combs, G.F., Jr.; Alberts, D.S.; Clark, L.C.; Marshall, J.R. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr. Cancer Int. J. 2008, 60, 155–163. [Google Scholar] [CrossRef]
- Brown, K.H.; Santizo, M.C.; Peerson, J.M.; Begin, F.; Tonin, B. Nutritional quality of complementary feeding regimens and its relationship to dietary diversity and use of processed foods and animal products in low-income guatemalan communities. FASEB J. 2001, 15, A732. [Google Scholar]
- Roth, D.E.; Richard, S.A.; Black, R.E. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: Meta-analysis and meta-regression of randomized trials. Int. J. Epidemiol. 2010, 39, 795–808. [Google Scholar] [CrossRef]
- Mufarrege, D.J.; Aguilar, D.E. Suplementación con zinc de los bovinos para carne en la provincia de Corrientes (Argentina). EEA INTA Mercedes Corrientes Not. Coment. 2001, 348, 1–4. [Google Scholar]
- Rosa, D.E.; Fazzio, L.E.; Picco, S.J.; Furnus, C.C.; Mattioli, G.A. Metabolismo y deficiencia de zinc en bovinos. Laboratorio de nutrición mineral y fisiología reproductiva. Analecta Vet. 2008, 28, 34–44. [Google Scholar]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Steidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Read, S.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Taylor, E.; Bennett, K.; Saad, R.; Rayman, M. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Ma, J.; Mu, Y.; Wang, Y.; Yang, S.; Wu, Y.; Wu, F.; Zhou, Y. Selenium (Se) plays a key role in the biological effects of some viruses: Implications for COVID-19. Environ. Res. 2021, 196, 110984. [Google Scholar] [CrossRef]
- Hoang, B.X.; Hoang, H.Q.; Han, B. Zinc Iodide in combination with Dimethyl Sulfoxide for treatment of SARS-CoV-2 and other viral infections. Med. Hypotheses 2020, 143, 109866. [Google Scholar] [CrossRef]
- Sethuram, R.; Bai, D.; Abu-Soud, H.M. Potential role of zinc in the COVID-19 disease process and its probable impact on reproduction. Reprod. Sci. 2021, 7, 1–6. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.; Santamaría, O.; López-Bellido, F.J.; Poblaciones, M.J. Agronomic selenium biofortification of two-rowed barley under mediterranean conditions. Plant Soil Environ. 2013, 59, 115–120. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Poblaciones, M.J. Selenium application timing: Influence in wheat grain and flour selenium accumulation under Mediterranean conditions. J. Agric. Sci. 2014, 6, 23–30. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef] [PubMed]
- Poblaciones, M.J.; Rengel, Z. Combined foliar selenium and zinc biofortification in field pea (Pisum sativum L.): Accumulation and bioavailability in raw and cooked grains. Crop. Pasture Sci. 2017, 68, 265–271. [Google Scholar] [CrossRef]
- Reynolds-Marzal, M.D.; Rivera-Martín, A.M.; Rodrigo, S.M.; Santamaria, O.; Poblaciones, M.J. Biofortification of Forage Peas with Combined Application of Selenium and Zinc under Mediterranean Conditions. J. Soil Sci. Plant Nutr. 2021, 21, 286–300. [Google Scholar] [CrossRef]
- National Research Council (NRC). Reference Intakes for Vitamin A, vitamin K, As, B, Cr, Cu, I, Fe, Mn, Mo, Ni, Si and Zn; Institute of Medicine/Food and Nutrition Board, National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Elmadfa, I.; Meyer, A.; Nowak, V.; Hasenegger, V.; Putz, P.; Verstraeten, R.; Remaut-DeWinter, A.M.; Kolsteren, P.; Dostálová, J.; Dlouhý, P.; et al. The European nutrition and health report 2009. Forum Nutr. 2009, 62, 1–405. [Google Scholar]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; CABI: Cambridge, MA, USA, 2010. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic: London, UK, 1995. [Google Scholar]
- Longnecker, N.E.; Robson, A.D. Distribution and Transport of Zinc in Plants. In Zinc in Soils and Plants; Robson, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 79–91. [Google Scholar]
- Ghaderzadeh, S.; Mirzaei Aghjeh-Gheshlagh, F.; Nikbin, S.; Navidshad, B. A Review on properties of selenium in animal nutrition. Iran. J. Appl. Anim. Sci. 2016, 6, 753–761. [Google Scholar]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; de Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.A.; Muhammad Sabir Tariq, R.; Ayub, M.A.; Hossain, A.; Hassan, M.M.; Brestic, M.; Sohidul Islam, M.; et al. Selenium Alleviates the Adverse Effect of Drought in Oilseed Crops Camelina (Camelina sativa L.) and Canola (Brassica napus L.). Molecules 2021, 26, 1699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Mohtashami, R.; Movahhedi, M.D.; Balouchi, H.; Faraji, H. Improving yield, oil content and water productivity of dryland canola by supplementary irrigation and selenium spraying. Agric. Water Manag. 2020, 232, 106046. [Google Scholar] [CrossRef]
- Manojlović, M.S.; Lončarić, Z.; Cabilovski, R.R.; Popović, B.; Karalić, K.; Ivezić, V.; Ademi, A.; Singh, B.R. Biofortification of wheat cultivars with selenium. Soil Plant Sci. 2019, 69, 715–724. [Google Scholar] [CrossRef]
- Johnson, L. Trends and annual fluctuations in selenium concentrations in wheat grain. Plant Soil 1991, 138, 67–73. [Google Scholar] [CrossRef]
- Moraghan, J.T.; Mascagni, H.J., Jr. Environmental and soil factors affecting micronutrient deficiencies and toxicities. In Micronutrients in Agriculture; Mordvedt, J.J., Cox, F.R., Shumann, L.M., Welch, R.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1991; pp. 371–425. [Google Scholar]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biol. Technol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Sevanto, S. Phloem transport and drought. J. Exp. Bot. 2014, 65, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Sotiropoulos, T.; Brown, P. Foliar Fertilization: Scientific Principles and Field Practices; International Fertilizer Industry Association (IFA): Paris, France, 2013.
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid enhancement of bioavailable micronutrients in food grains. J. Food. Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Ellis, R. Usefulness of the dietary phytic acid/zinc molar ratio as an index of zinc bioavailability to rats and humans. Biol. Trace Elem. Res. 1989, 19, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Saboor, A.; Ali, M.A.; Ahmed, N.; Skalicky, M.; Danish, S.; Fahad, S.; Hassan, F.; Hassan, M.M.; Brestic, M.; El Sabagh, A.; et al. Biofertilizer-Based Zinc Application Enhances Maize Growth, Gas Exchange Attributes, and Yield in Zinc-Deficient Soil. Agriculture 2021, 11, 310. [Google Scholar] [CrossRef]
- Zinzala, V.N.; Narwade, A.V.; Karmakar, N.; Patel, P.B. Influence of Zinc Applications on Photosynthesis, Transpiration and Stomatal Conductance in Kharif Rice (Oryza sativa L.) Genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 150–168. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zou, C.Q.; Mirza, Z.; Li, H.; Zhang, Z.Z.; Li, D.P.; Xu, C.L.; Zhou, X.B.; Shi, X.J.; Xie, D.T.; et al. Cost of agronomic biofortification of wheat with zinc in China. Agron. Sustain. Dev. 2016, 36, 44–50. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining organic carbon in soils: Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1934, 63, 251–263. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen total. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Lindsay, W.L.; Norwell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Zhao, F.J.; McGrath, S.P. Extractable sulphate and organic sulphur in soils and their availability to plants. Plant Soil 1994, 164, 243–250. [Google Scholar] [CrossRef]
- AOCS. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Adams, M.L.; Lombi, E.; Zhao, F.J.; McGrath, S.P. Evidence of low selenium concentration in UK bread-making wheat grain. J. Sci. Food Agric. 2002, 82, 1160–1165. [Google Scholar] [CrossRef]
- Haug, W.; Lantzsch, H.J. Sensitive method for the rapid determination of phtytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]

| Part | Year (Y) | Zn Soil Applic. (S) | Foliar Applic. (F) | Y*S | Y*F | S*F | Y*S*F | |
|---|---|---|---|---|---|---|---|---|
| DF | 1 | 1 | 3 | 1 | 3 | 3 | 3 | |
| Zn (mg kg−1) | Straw 1 | 35.92 * | 0.23 | 185.9 *** | 1.25 | 13.70 *** | 0.26 | 1.33 |
| Grain | 1.36 | 7.30 * | 26.71 *** | 0.00 | 3.75 * | 0.10 | 0.49 | |
| Se (μg kg−1) | Straw 1 | 0.07 | 0.06 | 27.78 *** | 0.04 | 0.22 | 0.34 | 0.37 |
| Grain 2 | 75.01 ** | 0.33 | 55.93 *** | 0.17 | 2.36 | 0.05 | 0.70 | |
| TZn (g kg−1) | Straw 1 | 5.39 | 6.31 | 100.6 *** | 1.48 | 0.69 | 0.53 | 1.09 |
| Grain | 101.2 ** | 24.01 ** | 25.92 *** | 15.83 ** | 13.78 *** | 0.50 | 0.26 | |
| TSe (mg kg−1) | Straw 1,2 | 29.45 * | 1.04 | 47.86 *** | 0.03 | 0.91 | 0.81 | 0.85 |
| Grain 2 | 365.4 *** | 0.50 | 53.62 *** | 1.23 | 2.34 | 0.07 | 0.92 | |
| Phytic acid (g kg−1) | Straw 1 | 1.04 | 0.30 | 0.24 | 0.11 | 2.18 | 1.26 | 1.47 |
| Grain | 0.04 | 3.09 | 0.33 | 0.93 | 0.45 | 0.20 | 0.14 | |
| Ph/Zn | Straw 1 | 49.29 * | 1.62 | 96.10 *** | 0.46 | 6.61 ** | 0.90 | 1.09 |
| Grain | 0.03 | 5.09 | 39.30 *** | 0.02 | 4.29 * | 0.68 | 0.61 | |
| Ph/Se | Straw 1 | 0.05 | 0.04 | 57.46 *** | 0.02 | 0.85 | 0.10 | 0.30 |
| Grain 2 | 61.83 ** | 0.24 | 54.70 *** | 0.13 | 1.68 | 0.06 | 0.79 | |
| Yield (kg ha−1) | Straw | 195.4 *** | 28.55 ** | 0.64 | 0.27 | 1.58 | 1.70 | 0.78 |
| Grain | 394.7 *** | 2.23 | 1.70 | 8.01 ** | 2.19 | 1.42 | 0.55 | |
| 1000 gw (g) | Grain | 60.75 ** | 0.16 | 2.72 | 0.30 | 1.24 | 0.08 | 1.67 |
| Hect. weight (kg hL−1) | Grain | 31.38 * | 0.29 | 2.34 | 3.16 | 0.91 | 0.82 | 0.78 |
| NDF (%) | Straw | 1766 *** | 1.46 | 0.88 | 0.18 | 0.42 | 0.60 | 0.12 |
| ADF (%) | Straw | 937.1 *** | 2.19 | 0.23 | 0.69 | 0.24 | 0.63 | 0.09 |
| ADL (%) | Straw | 2.14 | 8.13 * | 1.22 | 7.65* | 1.52 | 0.88 | 1.53 |
| Ashes (%) | Straw 2 | 102.1 ** | 0.07 | 0.32 | 3.35 | 0.38 | 1.74 | 1.09 |
| Mg (mg kg−1) | Straw 1 | 16.92 | 14.71* | 0.56 | 3.77 | 0.48 | 0.87 | 0.79 |
| Grain | 0.35 | 0.02 | 1.03 | 1.98 | 1.58 | 0.58 | 1.35 | |
| Ca (mg kg−1) | Straw 1 | 8.25 | 85.14 *** | 0.44 | 71.44 ** | 0.37 | 1.05 | 1.58 |
| Grain | 2.73 | 0.49 | 1.17 | 1.94 | 2.10 | 1.07 | 2.33 | |
| Fe (mg kg−1) | Straw 1 | 101.8 ** | 1.18 | 0.92 | 0.17 | 1.15 | 2.41 | 0.41 |
| Grain | 14.56 * | 0.40 | 0.69 | 5.87 | 3.85 * | 0.89 | 0.49 | |
| Ph/Mg | Straw 1 | 18.60 * | 10.54 * | 0.96 | 1.41 | 1.09 | 0.51 | 0.67 |
| Grain | 0.00 | 0.18 | 1.31 | 3.00 | 1.82 | 1.45 | 1.57 | |
| Ph/Ca | Straw 1 | 9.94 | 18.00 * | 0.93 | 15.13 * | 1.53 | 0.95 | 1.80 |
| Grain | 0.14 | 1.10 | 1.57 | 2.33 | 2.08 | 1.52 | 2.55 | |
| Ph/Fe | Straw 1 | 403.9 ** | 2.34 | 1.86 | 0.12 | 1.71 | 2.45 | 0.30 |
| Grain | 25.57 * | 0.31 | 0.54 | 9.97* | 4.21* | 0.90 | 0.51 |
| Factor | Treatment | Study Year | ||||
|---|---|---|---|---|---|---|
| 2017/2018 | 2018/2019 | Average | ||||
| Phytate:Zn | Straw | Foliar application | 0F | 52.9 ± 10.1 bc | 84.5 ± 8.4 a | 68.7 ± 7.8 Z |
| 10FSe | 45.0 ± 5.5 c | 66.5 ± 4.7 b | 55.8 ± 4.7 Y | |||
| 8FZn | 7.2 ± 0.5 d | 4.4 ± 0.3 d | 5.8 ± 0.5 X | |||
| 8FZn + 10FSe | 7.1 ± 0.5 d | 4.9 ± 0.3 d | 6.0 ± 0.4 X | |||
| Average | 28.0 ± 5.1 B | 40.1 ± 8.0 A | ||||
| Grain | Foliar application | 0F | 17.3 ± 1.0 a | 16.4 ± 0.8 ab | 16.9 ± 0.6 Z | |
| 10FSe | 17.1 ± 0.8 ab | 15.5 ± 0.4 b | 16.3 ± 0.5 Z | |||
| 8FZn | 11.7 ± 0.3 d | 12.9 ± 0.5 cd | 12.3 ± 0.3 Y | |||
| 8FZn + 10FSe | 12.0 ± 0.7 d | 13.6 ± 0.4 c | 12.8 ± 0.4 Y | |||
| Average | 14.5 ± 0.6 | 14.6 ± 0.4 | ||||
| Phytate:Se | Straw | Foliar application | 0F | 19.9 ± 2.9 | 22.5 ± 1.8 | 21.2 ± 1.6 Z |
| 10FSe | 8.1 ± 1.8 | 6.2 ± 0.5 | 7.2 ± 0.9 Y | |||
| 8FZn | 20.9 ± 1.3 | 21.8 ± 3.1 | 21.4 ± 1.5 Z | |||
| 8FZn + 10FSe | 8.2 ± 0.7 | 7.7 ± 0.5 | 8.0 ± 0.4 Y | |||
| Average | 14.3 ± 1.5 | 14.5 ± 1.8 | ||||
| Grain | Foliar application | 0F | 24. 0 ± 3.1 | 35.0 ± 5.7 | 29.5 ± 3.4 Z | |
| 10FSe | 5.5 ± 0.8 | 14.0 ± 1.3 | 9.8 ± 1.4 Y | |||
| 8FZn | 20.4 ± 4.0 | 37.3 ± 5.5 | 28.9 ± 3.9 Z | |||
| 8FZn + 10FSe | 4.5 ± 0.8 | 11.0 ± 1.2 | 7.7 ± 1.1 Y | |||
| Average | 13.6 ± 2.0 B | 24.3 ± 2.8 A | ||||
| Factor | Treatment | Study Year | ||||
|---|---|---|---|---|---|---|
| 2017/2018 | 2018/2019 | Average | ||||
| Straw | Mg (g kg−1) | Soil Zn application | 0SZn | 1.05 ± 0.05 | 0.77 ± 0.03 | 0.91 ± 0.04 Z |
| 50SZn | 0.85 ± 0.03 | 0.71 ± 0.02 | 0.78 ± 0.02 Y | |||
| Average | 0.95 ± 0.03 | 0.74 ± 0.02 | ||||
| Ph/Mg | Soil Zn application | 0SZn | 0.20 ± 0.01 | 0.27 ± 0.01 | 0.24 ± 0.01 Y | |
| 50SZn | 0.25 ± 0.01 | 0.30 ± 0.01 | 0.27 ± 0.01 Z | |||
| Average | 0.22 ± 0.01 B | 0.28 ± 0.01 A | ||||
| Ca (g kg−1) | Soil Zn application | 0SZn | 3.73 ± 0.15 z | 2.67 ± 0.06 x | 3.20 ± 0.14 Z | |
| 50SZn | 3.05 ± 0.13 y | 2.64 ± 0.08 x | 2.84 ± 0.09 Y | |||
| Average | 3.39 ± 0.12 | 2.65 ± 0.05 | ||||
| Ph/Ca | Soil Zn application | 0SZn | 0.09 ± 0.00 x | 0.13 ± 0.00 z | 0.11 ± 0.00 Y | |
| 50SZn | 0.12 ± 0.00 y | 0.13 ± 0.00 z | 0.12 ± 0.00 Z | |||
| Average | 0.10 ± 0.00 | 0.13 ± 0.00 | ||||
| Grain | Fe (mg kg−1) | Foliar application | 0F | 36.3 ± 0.9 bc | 35.0 ± 2.6 bcd | 35.6 ± 1.3 |
| 10FSe | 39.3 ± 1.9 ab | 34.7 ± 2.9 bcd | 37.0 ± 1.7 | |||
| 8FZn | 37.8 ± 1.8 ab | 32.0 ± 0.6 cd | 34.9 ± 1.2 | |||
| 8FZn + 10FSe | 42.3 ± 2.9 a | 30.6 ± 0.9 d | 36.4 ± 2.1 | |||
| Average | 38.9 ± 1.0 A | 33.1 ± 1.0 B | ||||
| Ph/Fe | Soil Zn application | 0SZn | 1.24 ± 0.02 x | 1.65 ± 0.02 z | 1.44 ± 0.04 | |
| 50SZn | 1.38 ± 0.05 y | 1.44 ± 0.05 y | 1.41 ± 0.04 | |||
| Foliar application | 0F | 1.38 ± 0.04 cd | 1.47 ± 0.09 bc | 1.43 ± 0.05 | ||
| 10FSe | 1.29 ± 0.06 de | 1.49 ± 0.09 abc | 1.39 ± 0.06 | |||
| 8FZn | 1.34 ± 0.07 cde | 1.56 ± 0.03 ab | 1.45 ± 0.05 | |||
| 8FZn + 10FSe | 1.21 ± 0.08 e | 1.64 ± 0.05 a | 1.43 ± 0.07 | |||
| Average | 1.31 ± 0.03 B | 1.54 ± 0.03 A | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds-Marzal, D.; Rivera-Martin, A.; Santamaria, O.; Poblaciones, M.J. Combined Selenium and Zinc Biofortification of Bread-Making Wheat under Mediterranean Conditions. Plants 2021, 10, 1209. https://doi.org/10.3390/plants10061209
Reynolds-Marzal D, Rivera-Martin A, Santamaria O, Poblaciones MJ. Combined Selenium and Zinc Biofortification of Bread-Making Wheat under Mediterranean Conditions. Plants. 2021; 10(6):1209. https://doi.org/10.3390/plants10061209
Chicago/Turabian StyleReynolds-Marzal, Dolores, Angelica Rivera-Martin, Oscar Santamaria, and Maria J. Poblaciones. 2021. "Combined Selenium and Zinc Biofortification of Bread-Making Wheat under Mediterranean Conditions" Plants 10, no. 6: 1209. https://doi.org/10.3390/plants10061209
APA StyleReynolds-Marzal, D., Rivera-Martin, A., Santamaria, O., & Poblaciones, M. J. (2021). Combined Selenium and Zinc Biofortification of Bread-Making Wheat under Mediterranean Conditions. Plants, 10(6), 1209. https://doi.org/10.3390/plants10061209







