First Report of Sesame Mutants Tolerant to Severe Drought Stress during Germination and Early Seedling Growth Stages
Abstract
:1. Introduction
2. Results
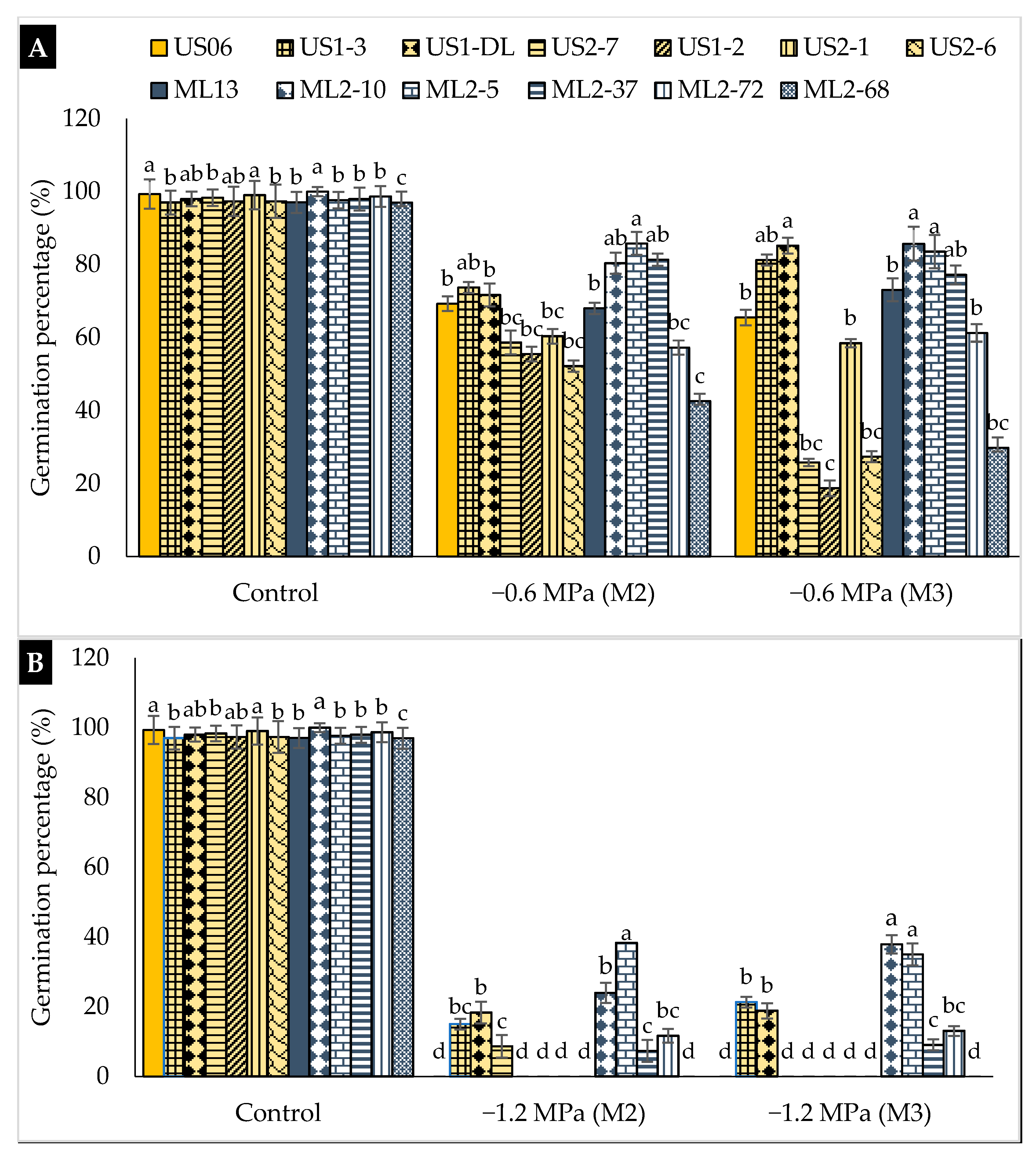
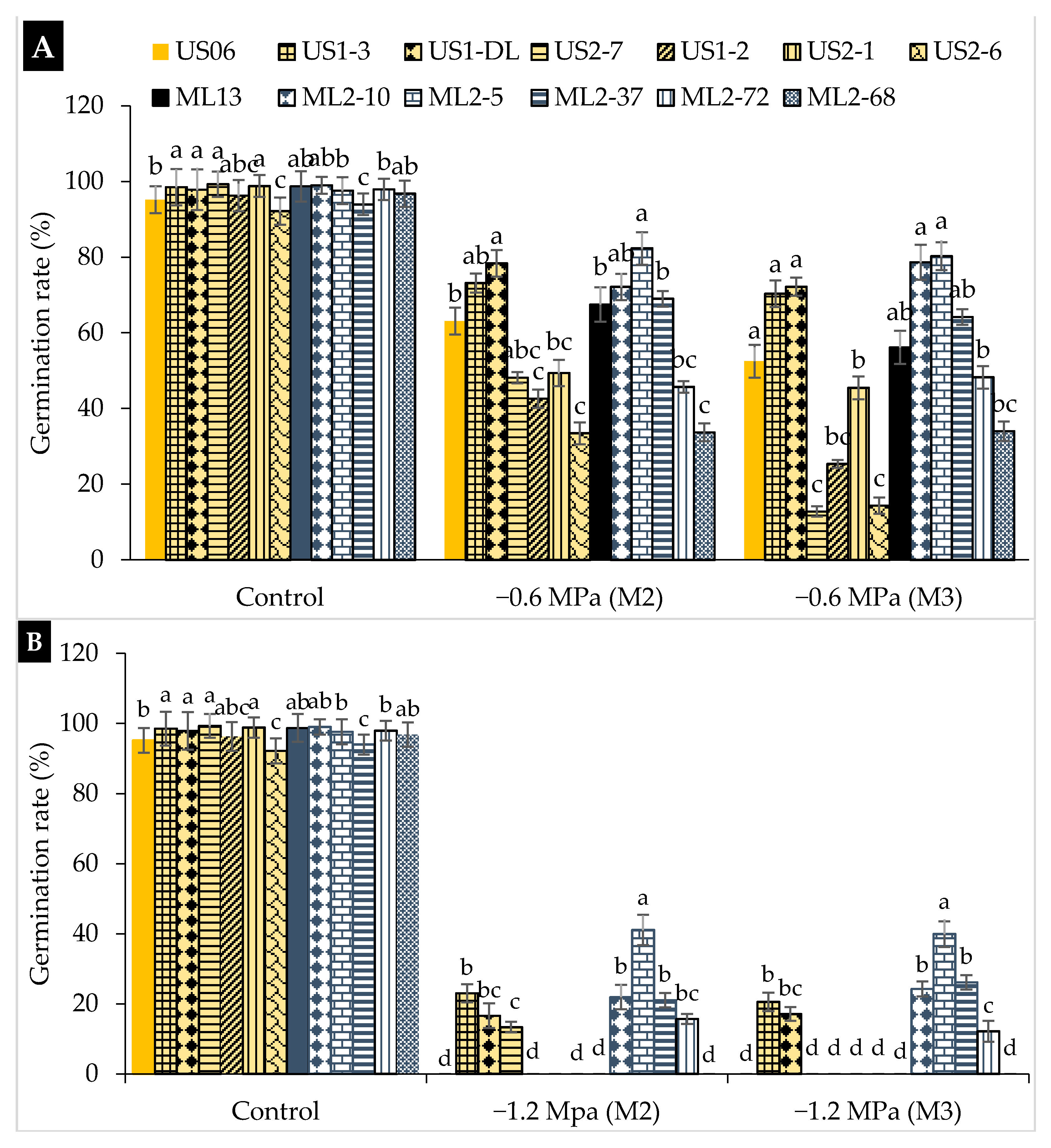
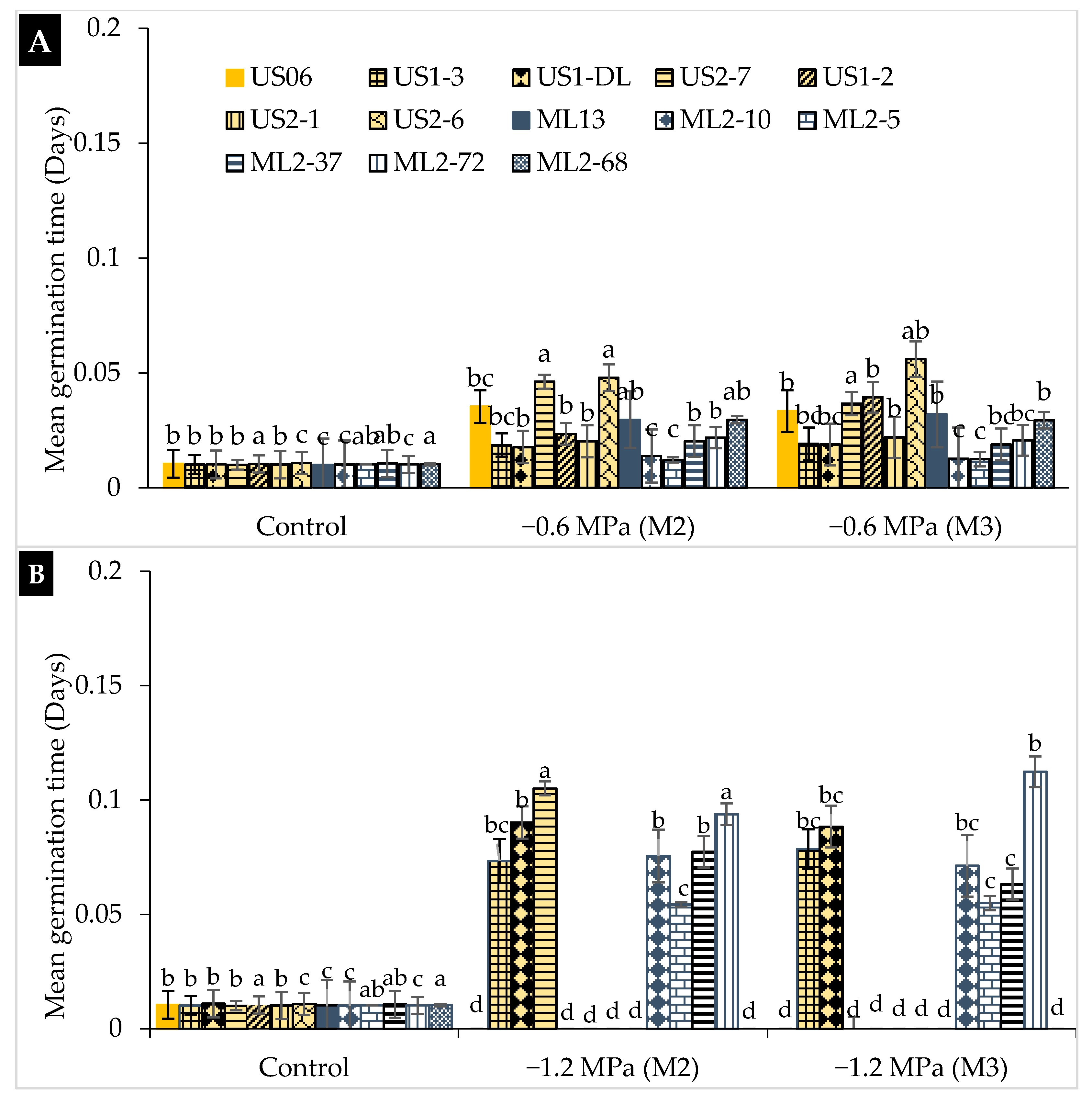
2.1. Drought Stress Effects on Germination
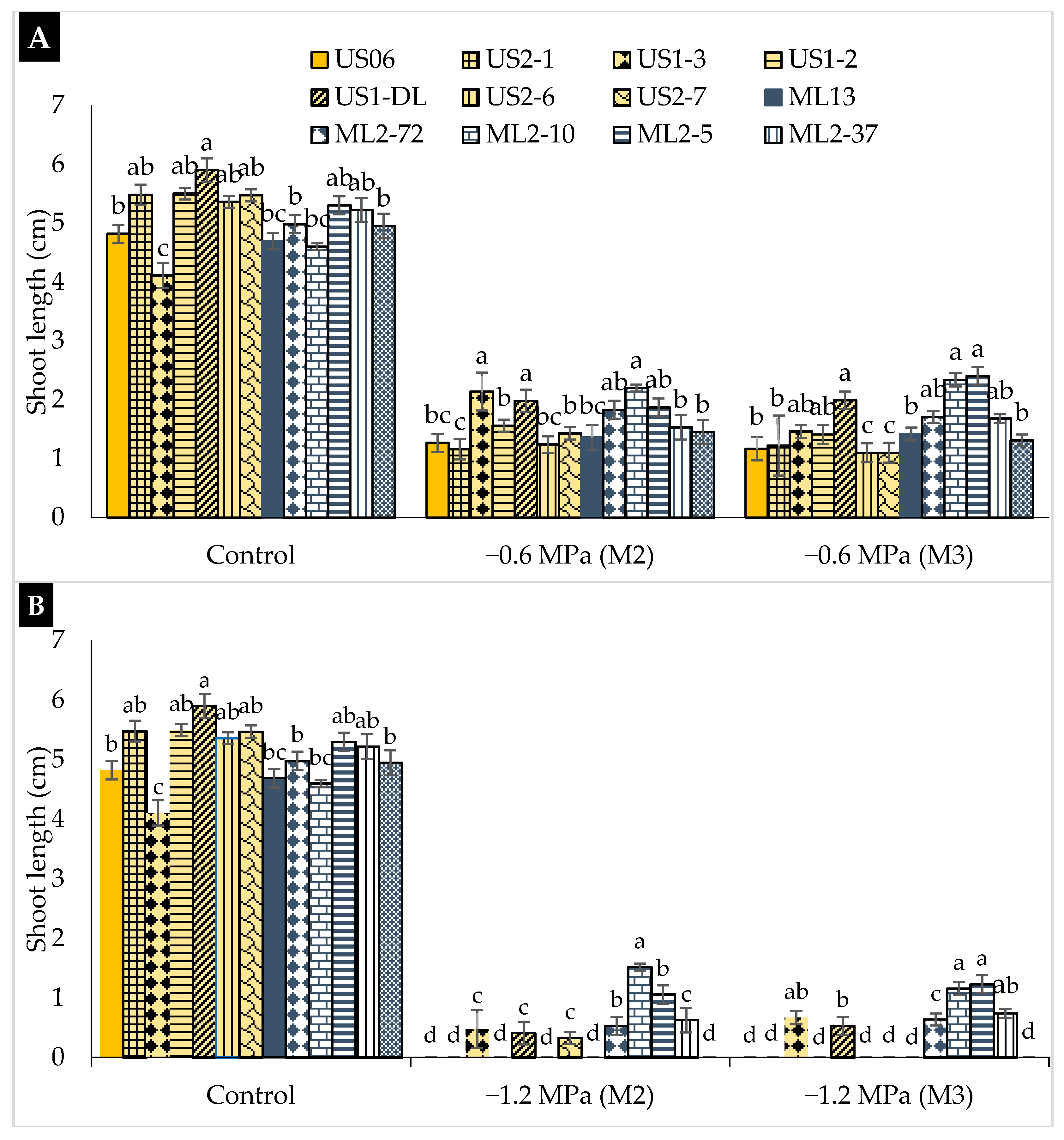
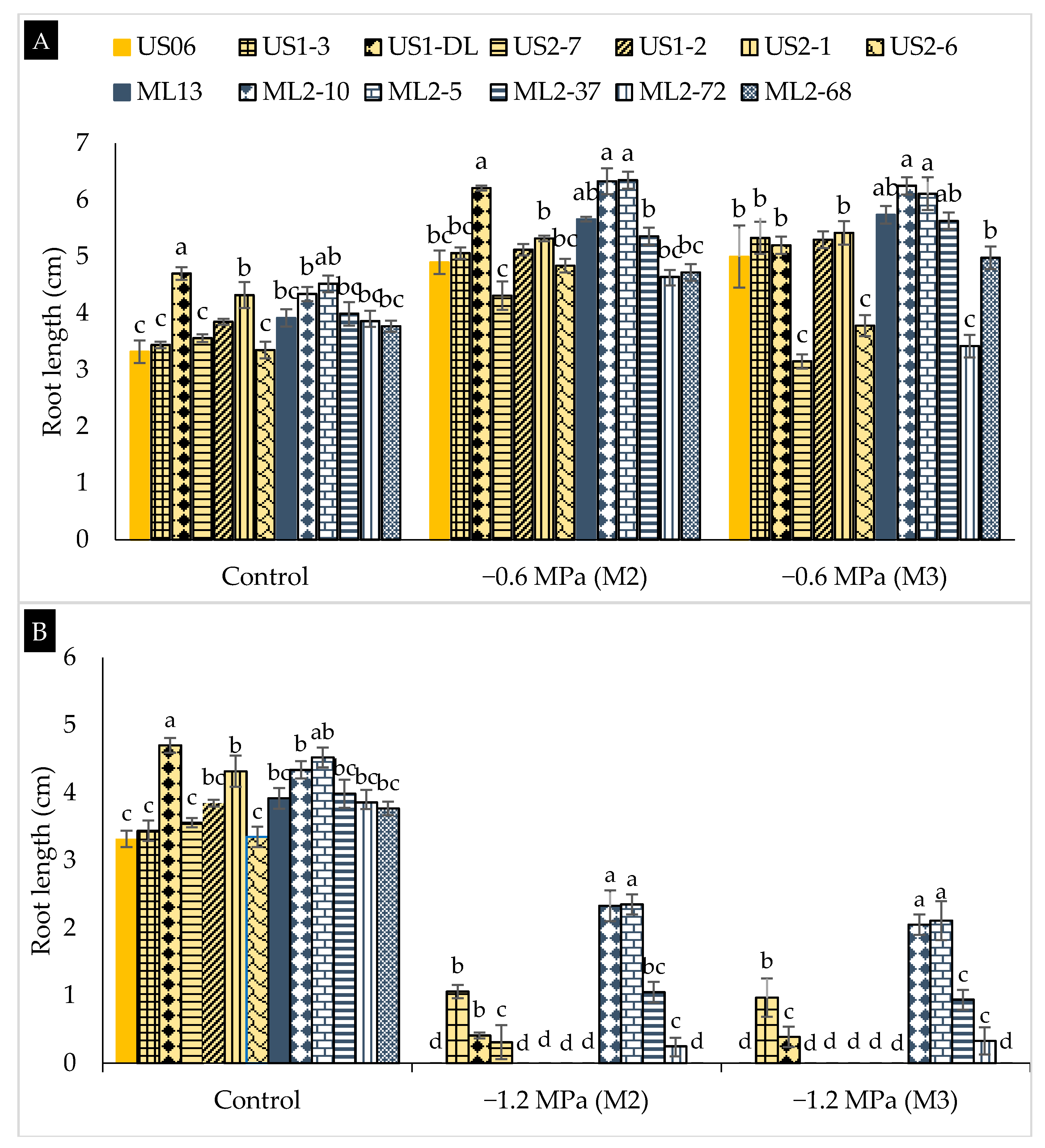
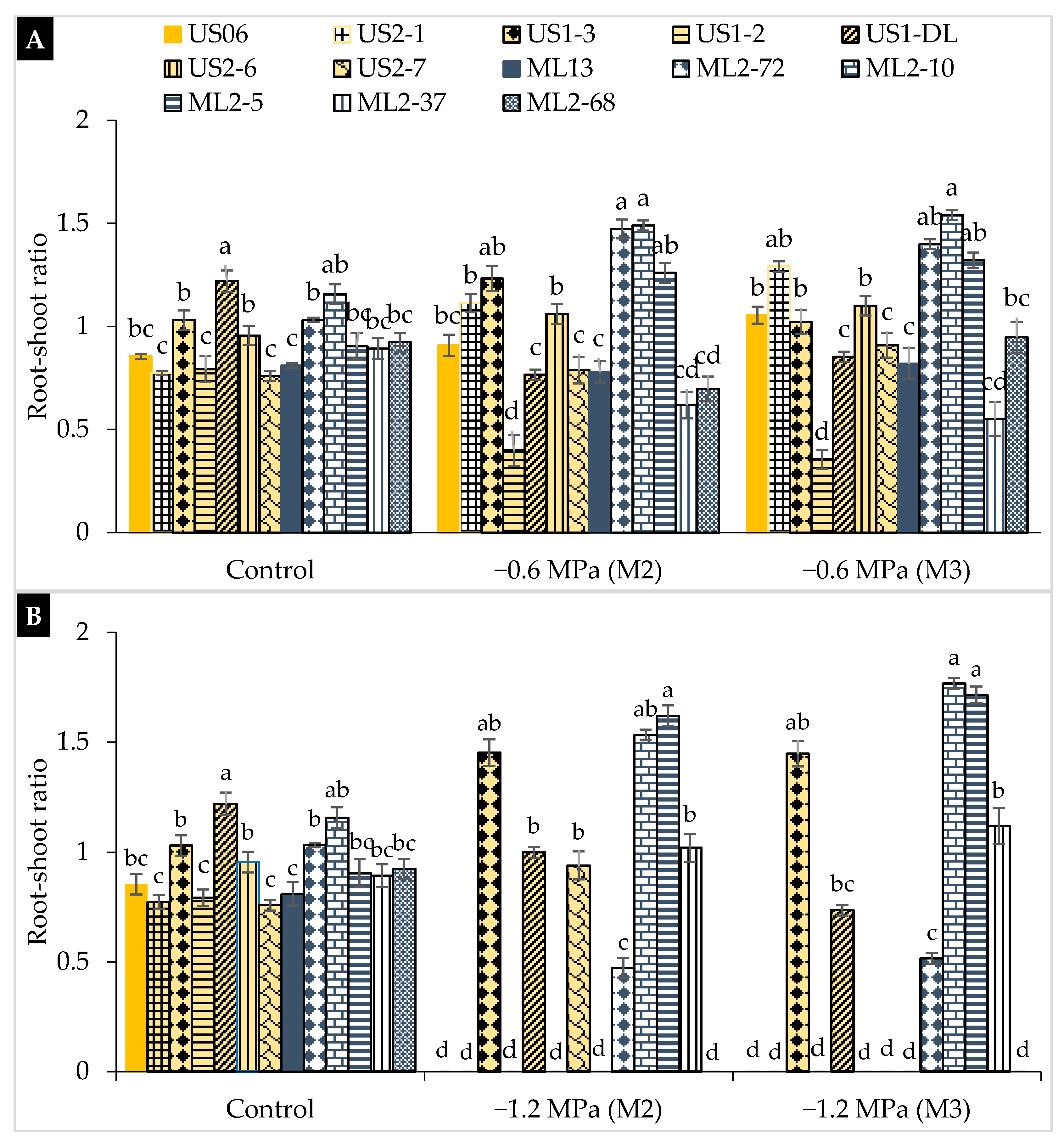
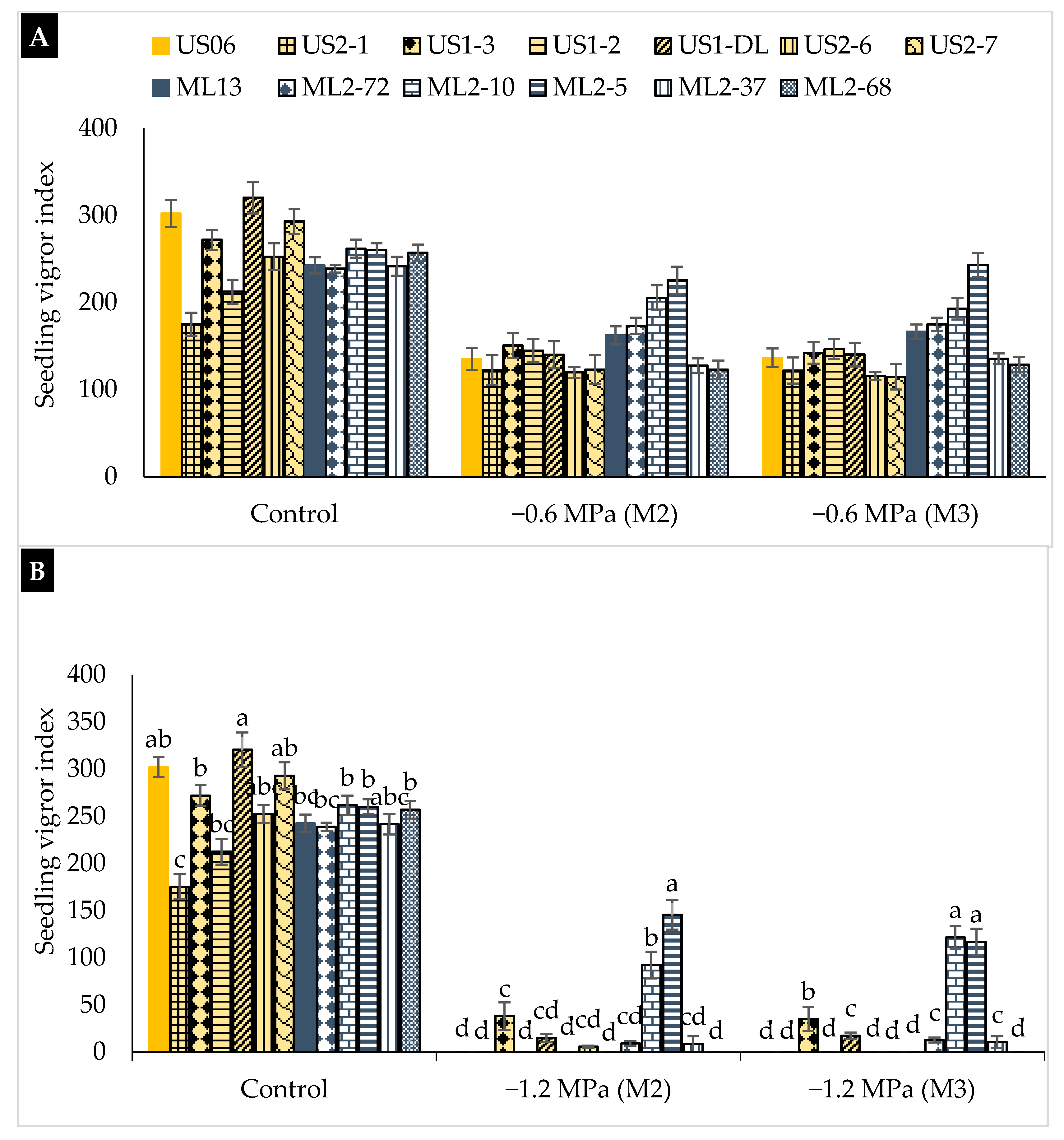
2.2. Drought Stress Effects on Early Seedling
3. Discussion
3.1. Drought Stress Effects on Germination
3.2. Drought Stress Effects on Early Seedling
4. Materials and Methods
4.1. Plant Material
4.2. Seed Treatment under PEG-6000 Solution
4.3. Parameters Calculated/Measured and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moazzami, A.; Kamal-Eldin, A. Sesame Seed Oil. In Gourmet and Health-Promoting Specialty Oils; Elsevier: Amsterdam, The Netherlands, 2009; pp. 267–282. [Google Scholar] [CrossRef]
- Anilakumar, K.R.; Pal, A.; Khanum, F.; Bawa, A.S. Nutritional, Medicinal and Industrial Uses of Sesame (Sesamum indicum L.) Seeds-an Overview. Agric. Conspec. Sci. 2010, 75, 159–168. [Google Scholar]
- Hama, J.R. Comparison of Fatty Acid Profile Changes between Unroasted and Roasted Brown Sesame (Sesamum indicum L.) Seeds Oil. Int. J. Food Prop. 2017, 20, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Arslan, H.; Ekin, Z.; Hatipoglu, H. Performances of sesame genotypes (Sesamum indicum L.) with different seed shell colors in semi-arid climate conditions. Fresen. Environ. Bull. 2018, 27, 8139–8146. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/fr/#data/QC (accessed on 6 March 2021).
- Islam, F.; Gill, R.A.; Ali, B.; Farooq, M.A.; Xu, L.; Najeeb, U.; Zhou, W. Chapter 6—Sesame. In Breeding Oilseed Crops for Sustainable Production; Academic Press: Cambridge, MA, USA, 2016; pp. 135–147. [Google Scholar] [CrossRef]
- Hassanzadeh, M.; Asghari, A.; Jamaati-e-Somarin, S.H.; Saeidi, M.; Zabihi-e-Mahmoodabad, R.; Hokmalipour, S. Effects of Water Deficit on Drought Tolerance Indices of Sesame (Sesamum indicum L.) Genotypes in Moghan Region. Res. J. Environ. Sci. 2009, 3, 116–121. [Google Scholar] [CrossRef]
- Boureima, S.; Eyletters, M.; Diouf, M.; Diop, T.A.; Van Damme, P. Sensitivity of Seed Germination and Seedling Radicle Growth to Drought Stress in Sesame, Sesamum indicum L. Res. J. Environ. Sci. 2011, 5, 557. [Google Scholar] [CrossRef]
- Dossa, K.; Diouf, D.; Cissé, N. Genome-Wide Investigation of Hsf Genes in Sesame Reveals Their Segmental Duplication Expansion and Their Active Role in Drought Stress Response. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Dossa, K.; Yehouessi, L.; Likeng-Li-Ngue, B.; Diouf, D.; Liao, B.; Zhang, X.; Cissé, N.; Bell, J. Comprehensive Screening of Some West and Central African Sesame Genotypes for Drought Resistance Probing by Agromorphological, Physiological, Biochemical and Seed Quality Traits. Agronomy 2017, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, H.; Razmjoo, J.; Jafari, A.O. Effect of Drought Stress on Germination and Seedling Growth of Sesame Cultivars (Sesamum indicum L.). Int. J. AgriScience 2012, 2, 423–428. [Google Scholar]
- Dissanayake, I.; Ranwala, S.M.W.; Perera, S.S.N. Germination and Seedling Growth Responses of Sri Lankan Grown Sesame/Thala (Sesamum indicum L.) for Simulated Drought Conditions. J. Natl. Sci. Found. Sri Lanka 2020, 47, 479–490. [Google Scholar] [CrossRef]
- Kermani, S.G.; Saeidi, G.; Sabzalian, M.R.; Gianinetti, A. Drought stress influenced sesamin and sesamolin content and polyphenolic components in sesame (Sesamum indicum L.) populations with contrasting seed coat colors. Food Chem. 2019, 289, 360–368. [Google Scholar] [CrossRef]
- Mundim, F.M.; Pringle, E.G. Whole-plant metabolic allocation under water stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef]
- Najafabadi, M.Y.; Ehsanzadeh, P. Photosynthetic and antioxidative upregulation in drought-stressed sesame (Sesamum indicum L.) subjected to foliar-applied salicylic acid. Photosynthetica 2017, 4, 611–622. [Google Scholar] [CrossRef]
- Sun, J.; Rao, Y.; Yan, T.; Yan, X.; Zhou, H. Effects of drought stress on sesame growth and yield characteristics and comprehensive evaluation of drought tolerance. Chin. J. Oil Crop Sci. 2010, 32, 525–533. [Google Scholar]
- Boureima, S.; Diouf, S.; Amoukou, M.; Van Damme, P. Screening for sources of tolerance to drought in sesame induced mutants: Assessment of indirect selection criteria for seed yield. Int. J. Pure Appl. Biosci. 2016, 4, 45–60. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Oliveira, M.J. Sicklepod (Senna obtusifolia) germination and emergence as affected by environmental factors and seeding depth. Weed Sci. 2006, 54, 903–909. [Google Scholar] [CrossRef]
- Mensah, J.K.; Obadoni, B.O.; Eruotor, P.G.; Onome-Irieguna, F. Simulated Flooding and Drought Effects on Germination, Growth, and Yield Parameters of Sesame (Sesamum indicum L.). Afr. J. Biotechnol. 2006, 5, 1249–1253. [Google Scholar]
- El Harfi, M.; Hanine, H.; Rizki, H.; Latrache, H.; Nabloussi, A. Effect of Drought and Salt Stresses on Germination and Early Seedling Growth of Different Color-Seeds of Sesame (Sesamum indicum). Int. J. Agric. Biol. 2016, 18, 1814–9596. [Google Scholar] [CrossRef]
- Zraibi, L.; Nabloussi, A.; Kajeiou, M.; Elamrani, A.; Khalid, A.; Caid, H.S. Comparative Germination and Seedling Growth Response to Drought and Salt Stresses in a Set of Safflower (Carthamus tinctorius) Varieties. Seed Technol. 2011, 33, 40–52. [Google Scholar]
- Office Régional de Mise en Valeur Agricole, ORMVA-Tadla. Available online: https://www.ormva-tadla.ma/ormvat/office (accessed on 5 March 2021).
- El Harfi, M.; Jbilou, M.; Hanine, H.; Rizki, H.; Fechtali, M.; Nabloussi, A. Genetic Diversity Assessment of Moroccan Sesame (Sesamum indicum L.) Populations Using Agro-Morphological Traits. J. Agric. Sci. Technol. A 2018, 8, 296–305. [Google Scholar]
- El Harfi, M.; Charafi, J.; Houmanat, K.; Hanine, H.; Nabloussi, A. Assessment of Genetic Diversity in Moroccan Sesame (Sesamum indicum) Using ISSR Molecular Markers. OCL 2021, 28, 3. [Google Scholar] [CrossRef]
- Kouighat, M.; Channaoui, S.; Labhilili, M.; El Fechtali, M.; Nabloussi, A. Novel Genetic Variability in Sesame Induced via Ethyl Methane Sulfonate. J. Crop. Improv. 2020, 1–12. [Google Scholar] [CrossRef]
- Yigit, N.; Sevik, H.; Cetin, M.; Kaya, N. Determination of the effect of drought stress on the seed germination in some plant species. Water Stress Plants 2016, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Ávila, M.R.; de Braccini, A.L.; Scapim, C.A.; Fagliari, J.R.; dos Santos, J.L. Influência Do Estresse Hídrico Simulado Com Manitol Na Germinação de Sementes e Crescimento de Plântulas de Canola. Rev. Bras. Sementes 2007, 29, 98–106. [Google Scholar] [CrossRef]
- Mohammadi, G.R.; Amiri, F. The Effect of Priming on Seed Performance of Canola (Brassica napus L.) under Drought Stress. Am. Eurasian J. Agric. Environ. Sci. 2010, 9, 202–207. [Google Scholar]
- Channaoui, S.; El Idrissi, I.S.; Mazouz, H.; Nabloussi, A. Reaction of Some Rapeseed (Brassica napus L.) Genotypes to Different Drought Stress Levels during Germination and Seedling Growth Stages. OCL 2019, 26, 23. [Google Scholar] [CrossRef] [Green Version]
- Luan, Z.; Xiao, M.; Zhou, D.; Zhang, H.; Tian, Y.; Wu, Y.; Guan, B.; Song, Y. Effects of Salinity, Temperature, and Polyethylene Glycol on the Seed Germination of Sunflower (Helianthus annuus L.). Sci. World J. 2014, 9. [Google Scholar] [CrossRef]
- Botía, P.; Carvajal, M.; Cerdá, A.; Martínez, V. Response of Eight Cucumis melo Cultivars to Salinity during Germination and Early Vegetative Growth. Agronomie 1998, 18, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Almas, D.E.; Bagherikia, S.; Mashaki, K.M. Effects of Salt and Water Stresses on Germination and Seedling Growth of Artemisia vulgaris L. Int. J. Agric. Crop. Sci. 2013, 6, 762. [Google Scholar]
- Gill, P.K.; Sharma, A.D.; Singh, P.; Bhullar, S.S. Changes in Germination, Growth and Soluble Sugar Contents of Sorghum bicolor (L.) Moench Seeds under Various Abiotic Stresses. Plant Growth Regul. 2003, 40, 157–162. [Google Scholar] [CrossRef]
- McDonald, M.B. Physiology of Seed Germination; The Ohio State University: Columbus, OH, USA, 2007. [Google Scholar]
- Haouari, C.C.; Nasraoui, A.H.; Carrayol, E.; Gouia, H. Variations In Amylase and-Glycosidase Activities in Two Genotypes of Wheat under NaCl Salinity Stress. Afr. J. Agric. Res. 2013, 8, 2038–2043. [Google Scholar]
- Ahmad, S.; Ahmad, R.; Ashraf, M.Y.; Ashraf, M.; Waraich, E.A. Sunflower (Helianthus annuus L.) Response to Drought Stress at Germination and Seedling Growth Stages. Pak. J. Bot. 2009, 41, 647–654. [Google Scholar]
- Gordin, C.R.B.; Scalon, S.D.P.Q.; Masetto, T.E. Disponibilidade Hídrica Do Substrato e Teor de Água Da Semente Na Germinação de Niger. Pesqui. Agropecu. Trop. 2015, 45, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Bakhshandeh, E.; Jamali, M.; Afshoon, E.; Gholamhossieni, M. Using Hydrothermal Time Concept to Describe Sesame (Sesamum indicum L.) Seed Germination Response to Temperature and Water Potential. Acta Physiol. Plant 2017, 39, 1–9. [Google Scholar] [CrossRef]
- Santarém, E.R.; Almeida-Cortez, J.S.; da Silveira, T.; Ferreira, A.G. Efeito Do Estresse Hídrico Na Germinação e Crescimento Inicial de Três Espécies de Leguminosas. Acta Bot. Bras. 1996, 10, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Okçu, G.; Kaya, M.D.; Atak, M. Effects of Salt and Drought Stresses on Germination and Seedling Growth of Pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]
- Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiol 2014, 166, 1943–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bor, M.; Seckin, B.; Ozgur, R.; Yılmaz, O.; Ozdemir, F.; Turkan, I. Comparative Effects of Drought, Salt, Heavy Metal and Heat Stresses on Gamma-Aminobutryric Acid Levels of Sesame (Sesamum indicum L.). Acta Physiol. Plant 2009, 31, 655–659. [Google Scholar] [CrossRef]
- Saxena, D.; Flores, S.; Stotzky, G. Bt Toxin is Released in Root Exudates from 12 Transgenic Corn Hybrids Representing Three Transformation Events. Soil Biol. Biochem. 2002, 34, 133–137. [Google Scholar] [CrossRef]
- Sikder, S.; Hasan, M.A.; Hossain, M.S. Germination Characteristics and Mobilization of Seed Reserves in Maize Varieties as Influenced by Temperature Regimes. J. Agric. Rural. Dev. 2009, 7, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Mir, R.R.; Zaman-Allah, M.; Sreenivasulu, N.; Trethowan, R.; Varshney, R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012, 125, 625–645. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.G.M.D.; Sajjad, M.; Li, M.; Azmat, M.A.; Rizwan, M.; Maqsood, R.H.; Khan, S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability 2019, 11, 2584. [Google Scholar] [CrossRef] [Green Version]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of Drought Tolerance in Wheat Genotypes at Early Stages of Plant Growth. J. Agron. Crop. Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Hyles, J.; Joaquim, P.; Azanza, F.; Bonnett, D.; Ellis, M.E.; Moore, C.; Richards, R.A. A QTL on Chromosome 6A in Bread Wheat (Triticum aestivum) is Associated with Longer Coleoptiles, Greater Seedling Vigour and Final Plant Height. Theor. Appl. Genet. 2007, 115, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Koskosidis, A.; Khah, E.; Mavromatis, A.; Pavli, O.; Vlachostergios, D.N. Effect of PEG-Induced Drought Stress on Germination of Ten Chickpea (Cicer arietinum L.) Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- ISTA. International Seed Testing Association. Seed Sci. Technol. 1999, 27, 1–333. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, H.; Khazaei, F.; Yari, L.; Sheidaei, S. Effect of seed osmopriming on seed germination behavior and vigor of soybean (Glycine max L.). ARPN J. Agric. Biol. Sci. 2011, 6, 39–43. [Google Scholar]
| Source of Variation | Degree of Freedom | Germination Percentage | Germination Rate | Mean Germination Time | Seedling Vigor Index | Root Length | Shoot Length | Root/Shoot Ratio |
|---|---|---|---|---|---|---|---|---|
| Genotype (G) | 12 | 418.43 *** | 290.89 *** | 0.070 *** | 23,273.49 *** | 3.12 *** | 2.91 *** | 18,970.66 *** |
| Drought (D) | 2 | 147,508.51 *** | 14,839.06 *** | 0.0210 *** | 919,444.07 *** | 64.28 *** | 354.17 ** | 3394.67 *** |
| Generation (M) | 1 | 51.92 * | 209.72 | 0.0117 | 490,553.31 ** | 0.02 | 3.12 | 413.98 * |
| G × D | 24 | 139.17 *** | 247.95 ** | 0.0060 *** | 13,028.48 *** | 1.29 *** | 1.88 *** | 9812.85 *** |
| M × G | 12 | 403.02 * | 221.93 | 0.067 | 25,990.86 | 3.05 | 2.63 * | 19,039.58 |
| Genotypes | Characteristics |
|---|---|
| “US06” | Parent (wild-type), white seeds, and one capsule/axil |
| “US2-7” | Mutant, white seeds, and high 1000-seed weight |
| “US1-DL” | Mutant, white seeds, and late maturity |
| “US1-3” | Mutant, white seeds, and early flowering |
| “US2-1” | Mutant, white seeds, and three capsules/axil |
| “US1-2” | Mutant, very large leaf, and high seed yield |
| “US2-6” | Mutant, pale black seeds, early flowering, and maturity |
| “ML13” | Parent (wild-type), beige seeds, and one capsule/axil |
| “ML2-5” | Mutant, brown seeds, and large capsule |
| “ML2-10” | Mutant, brown seeds, and high plant branching |
| “ML2-72” | Mutant, light brown seeds, and high 1000-seed weight |
| “ML2-37” | Mutant, beige seeds, and thick leaf |
| “ML2-68” | Mutant, grey seeds, and three capsules/axil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouighat, M.; Hanine, H.; El Fechtali, M.; Nabloussi, A. First Report of Sesame Mutants Tolerant to Severe Drought Stress during Germination and Early Seedling Growth Stages. Plants 2021, 10, 1166. https://doi.org/10.3390/plants10061166
Kouighat M, Hanine H, El Fechtali M, Nabloussi A. First Report of Sesame Mutants Tolerant to Severe Drought Stress during Germination and Early Seedling Growth Stages. Plants. 2021; 10(6):1166. https://doi.org/10.3390/plants10061166
Chicago/Turabian StyleKouighat, Mohamed, Hafida Hanine, Mohamed El Fechtali, and Abdelghani Nabloussi. 2021. "First Report of Sesame Mutants Tolerant to Severe Drought Stress during Germination and Early Seedling Growth Stages" Plants 10, no. 6: 1166. https://doi.org/10.3390/plants10061166
APA StyleKouighat, M., Hanine, H., El Fechtali, M., & Nabloussi, A. (2021). First Report of Sesame Mutants Tolerant to Severe Drought Stress during Germination and Early Seedling Growth Stages. Plants, 10(6), 1166. https://doi.org/10.3390/plants10061166






