Protocols for Adventitious Regeneration of Amelanchier alnifolia var. cusickii and Lonicera kamtschatica ‘Jugana’
Abstract
1. Introduction
2. Results
2.1. Amelanchier alnifolia var. cusickii Shoot Induction and Multiplication
2.2. Lonicera Kamtschatica ‘Jugana’ Shoot Induction and Multiplication
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Shoot Initiation and Multiplication
4.3. Adventitious Shoot Regeneration
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Byrne, D.H. Trends in fruit breeding. In Fruit Breeding; Badenes, M.R., Byrne, D.H., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, Phenolics, and Antioxidant Capacity in Diverse Small Fruits: Vaccinium, Rubus, andRibes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Hodges, D.; Kalt, W. Health functionality of small fruit. Acta Hortic. 2003, 626, 17–23. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Cuevas-Rodríguez, E.O.; Yousef, G.G.; García-Saucedo, P.A.; López-Medina, J.; Paredes-López, O.; Lila, M.A. Characteriza-tion of anthocyanins and proanthocyanidins in wild and domesticated Mexican blackberries (Rubus spp.). J. Agric. Food Chem. 2010, 58, 7458–7464. [Google Scholar] [CrossRef] [PubMed]
- Ochmian, I.; Kubus, M.; Dobrowolska, A. Description of plants and assessment of chemical properties of three scecies of Amelanchier genus. Dendrobiology 2013, 70, 59–64. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Sochor, J.; Kizek, R. Antioxidant properties od Saskatoon berry (Amelanchier alnifolia Nutt.) fruits. Fruits 2013, 68, 435–444. [Google Scholar] [CrossRef]
- Sedlák, J.; Paprštein, F. Micropropagation of edible honeysuckle. Vědecké Práce Ovocnářské 2013, 23, 157–163. (In Czech) [Google Scholar]
- Malodobry, M.; Bienasz, M.; Dziedzic, E. Evaluation of the yield and some components in the fruit of blue honeysuckle (Lo-nicera caerulea ver. Edulis turcz. Freyn.). Folia Hortic. 2010, 22, 45–50. [Google Scholar] [CrossRef]
- Jurikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (genus Lonicera) and their biological effects. Molecules 2012, 17, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S. Bioreactors and molecular analysis in berry crop micropropagation—A review. Can. J. Plant Sci. 2011, 91, 147–157. [Google Scholar] [CrossRef]
- Zatylny, A.M.; Ziehl, W.D.; St-Pierre, R.G. Physiochemical properties of fruit of 16 saskatoon (Amelanchier alnifolia Nutt.) cultivars. Can. J. Plant Sci. 2005, 85, 933–938. [Google Scholar] [CrossRef]
- Holubec, V.K.; Štolc, K.; Paprštein, F. Genetic resources of honeysuckle Lonicera kamtschatica (Sevast.) Pojark., natural condi-tions and variability. In Inovace Pěstování Ovocných Plodín; Výskumný a Šlechtitelský Ústav Ovocnáťský: Holovousy, Czech Republik, 2007; pp. 199–210. [Google Scholar]
- Meiners, J.; Schwab, M.; Szankowski, I. Efficient in vitro regeneration systems for Vaccinium species. Plant Cell Tissue Organ Cult. 2007, 89, 169–176. [Google Scholar] [CrossRef]
- Cappelletti, R.; Sabbadini, S.; Mezzetti, B. The use of TDZ for the efficient in vitro regeneration and organogenesis of straw-berry and blueberry cultivars. Sci. Hortic.-Amst. 2016, 207, 117–124. [Google Scholar] [CrossRef]
- Gahan, P.B.; George, E.F. Adventitious regeneration. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., De Klerk, G.-J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 355–366. [Google Scholar]
- Cambecèdes, J.; Duron, M.; Decourtye, L. Interacting Effects of 2,3,5-Triiodobenzoic Acid, 1-Aminocyclopropane-1-Carboxylic Acid, and Silver Nitrate on Adventitious Bud Formation from Leaf Explants of the Shrubby Honeysuckle, Lonicera nitida Wils. ‘Maigrün’. J. Plant Physiol. 1992, 140, 557–561. [Google Scholar] [CrossRef]
- Georges, D.; Chenieux, J.C.; Ochatt, S.J. Plant regeneration from aged-callus of the woody ornamental species Lonicera japoni-ca cv. “Hall’s Prolific”. Plant Cell Rep. 1993, 13, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Li, Y.; Nie, Q.; Li, J. An efficient procedure for regeneration from leaf-derived calluses of Lonicera macran-thoides ‘Jincuilei’, an important medicinal plant. HortScience 2009, 44, 746–750. [Google Scholar] [CrossRef]
- Palacios, N.; Christou, P.; Leech, M. Regeneration of Lonicera tatarica plants via adventitious organogenesis from cultured stem explants. Plant Cell Rep. 2002, 20, 808–813. [Google Scholar] [CrossRef]
- Hui, J.X.; Wen, S.C.; Hua, Z.Y.; Ming, L.X. Comparative study on different methods for Lonicera japonica Thunb. microprop-agation and acclimatization. J. Med. Plants Res. 2012, 6, 4389–4393. [Google Scholar]
- Hassanein, A.M.A.; Mahmoud, I.M.A.; Ali, M.I.M.; Ahmed, H.A.M. Essential factors for in vitro regeneration of rose and a protocol for plant regeneration from leaves. Hortic. Sci. 2018, 45, 83–91. [Google Scholar] [CrossRef]
- Matt, A.; Jehle, J.A. In vitro plant regeneration from leaves and internode sections of sweet cherry cultivars (Prunus avium L.). Plant Cell Rep. 2005, 24, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Canli, F.; Tian, L. Regeneration of adventitious shoots from mature stored cotyledons of Japanese plum (Prunus salicina Lind1). Sci. Hortic. 2009, 120, 64–69. [Google Scholar] [CrossRef]
- Feyissa, T.; Zhu, L.H.; Negash, L.; Welander, M. Regeneration and genetic transformation of Hagenia abyssinica (Bruce) JF Gmel. (Rosaceae) with rolB gene. PCTOC 2007, 88, 277–288. [Google Scholar] [CrossRef]
- Quoirin, M.; Lepoivre, P. Improved media for In Vitro culture of Prunus spp. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Vujović, T.; Ružić, D.; Cerović, R. In vitro shoot multiplication as influenced by repeated subculturing of shoots of contem-porary fruit rootstocks. Hortic. Sci. 2012, 39, 101–107. [Google Scholar] [CrossRef]
- Hunková, J.; Libiaková, G.; Fejér, J.; Gajdošová, A. Improved Amelanchier alnifolia Nutt. Ex. M. Roem. shoot proliferation by manipulating iron source. Propag. Ornam. Plants 2017, 17, 103–107. [Google Scholar]
- Linsmaier, E.M.; Skoog, F. Organic Growth Factor Requirements of Tobacco Tissue Cultures. Physiol. Plant. 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Gajdošová, A.; Libiaková, G.; Iliev, I.; Hricová, A. Adventitious shoot induction of Amaranthus cruentus L. in vitro. Propag. Ornam. Plants 2013, 13, 33–39. [Google Scholar]
- Van Le, B.; My, N.D.; Jeanneau, M.; Sadik, S.; Tu, S.; Vidal, J.; Vân, K.T.T. Rapid plant regeneration of a C 4 dicot species: Amaranthus edulis. Plant Sci. 1998, 132, 45–54. [Google Scholar] [CrossRef]
- Fira, A.; Clapa, D.; Cristea, V.; Plopa, C. In vitro propagation of Lonicera kamtschatica. Agric. Sci. Pract. 2014, 1–2, 90–99. [Google Scholar]
- Hunková, J.; Libiaková, G.; Fejér, J.; Vujovic, T.; Gajdosová, A. Testing of different iron sources and concentrations on shoot multiplication of blackberry (Rubus fruticosus L.). Genetika 2018, 50, 351–356. [Google Scholar] [CrossRef]
- Hunková, A.; Libiaková, G.; Gajdošová, A. Less-known small fruit species and their propagation using in vitro techniques. In Proceedings of the Recent advances in neglected and under-utilized species research, Texcoco, Mexico, 25–27 November 2015; pp. 21–24. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Dziedzic, E. Propagation of blue honeysuckle (Lonicera caerulea var. Kamtschatica pojark) in in vitro culture. J. Basic Appl. Sci. 2008, 16, 93–100. [Google Scholar]
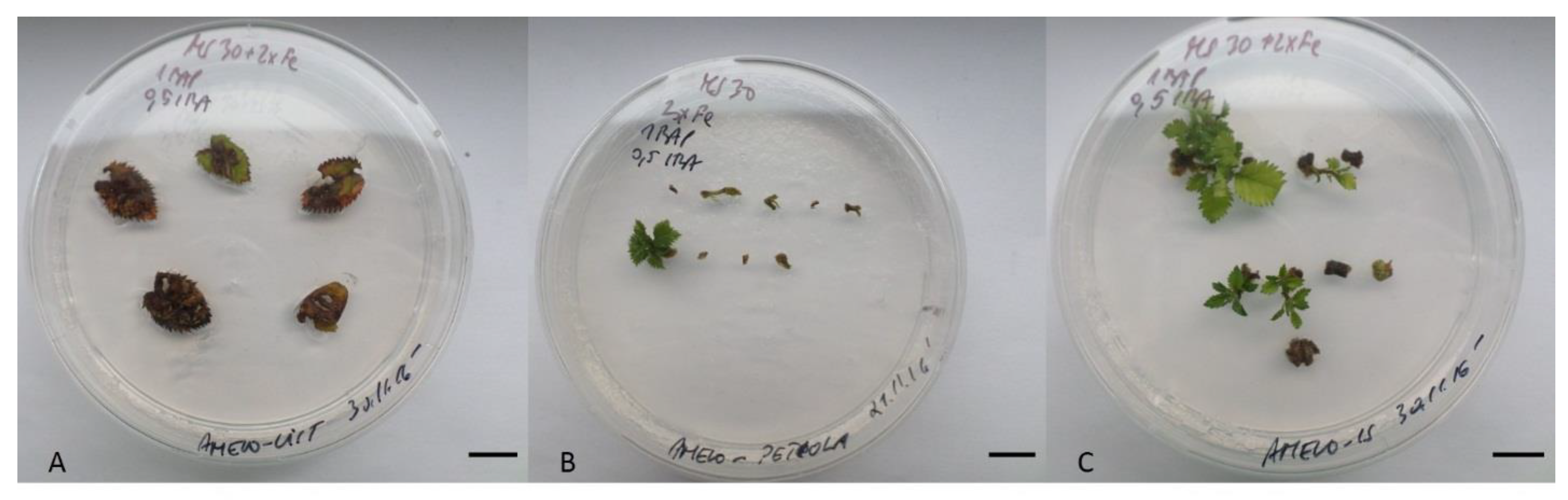
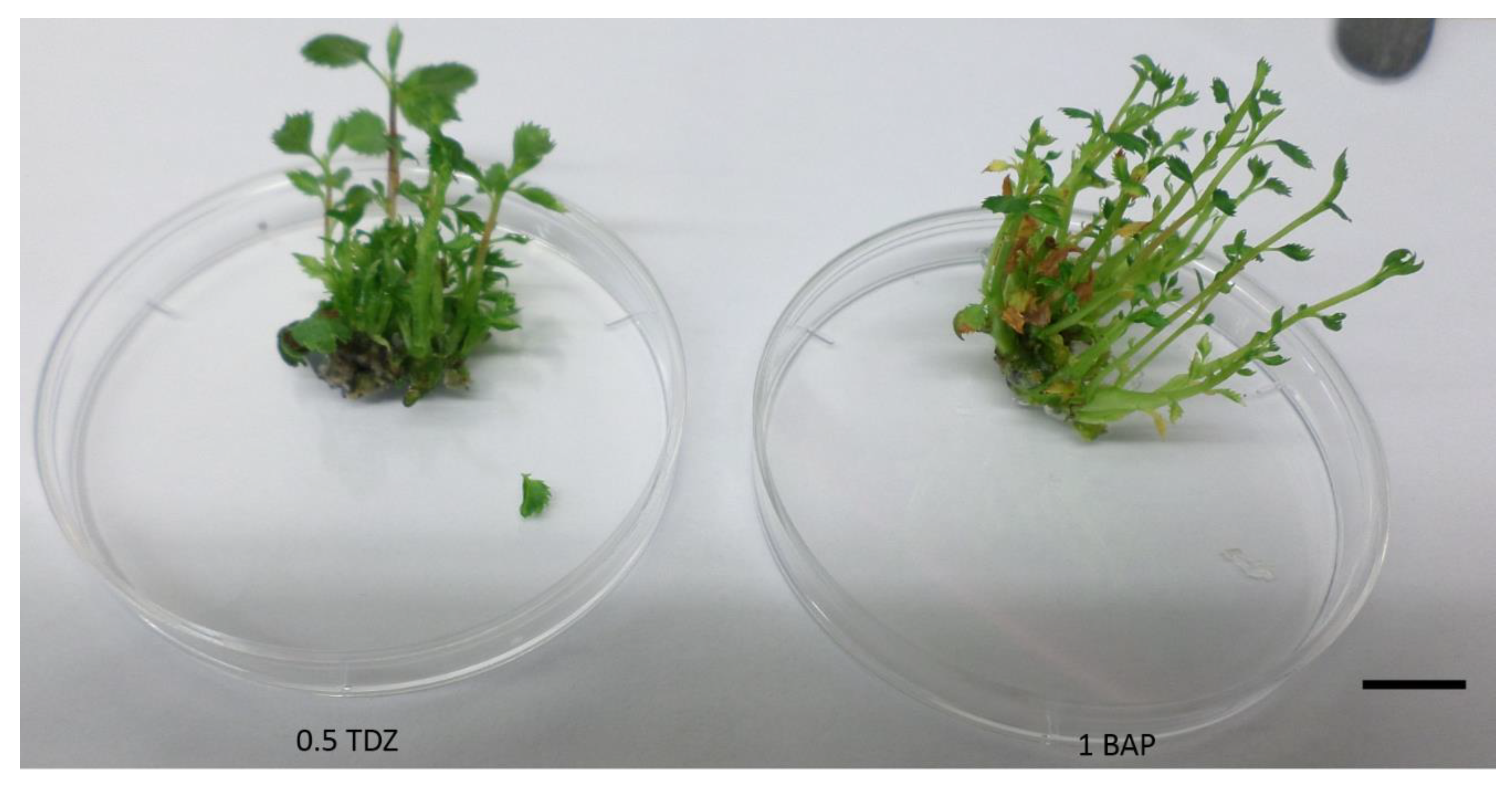
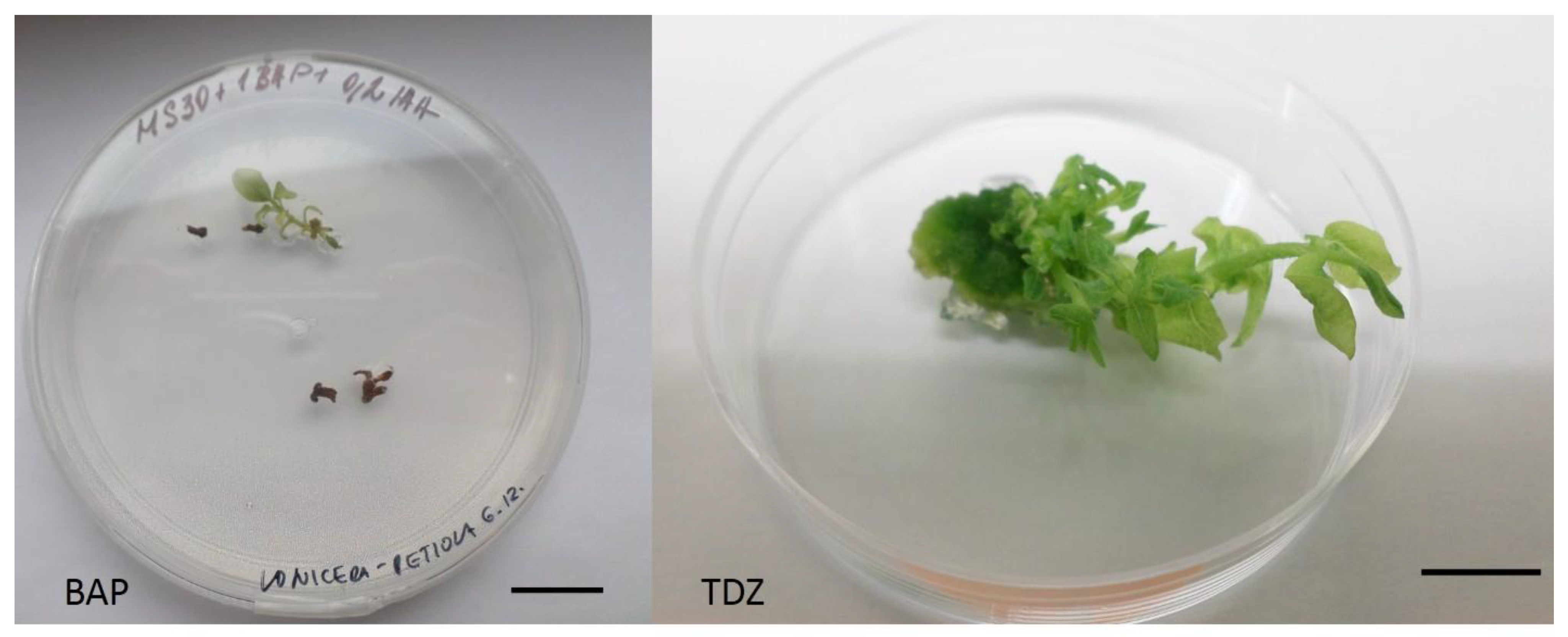

| PGR Combinations (mg/L) | Shoot Induction | Regenerated Shoots | ||||
|---|---|---|---|---|---|---|
| L | P | IS | L | P | IS | |
| 1 BAP + 0.5 IBA | 0.000 e | 0.040 de | 0.160 cd | 0.000 c | 0.280 c | 0.240 c |
| 2 BAP + 0.5 IBA | 0.000 e | 0.040 de | 0.040 de | 0.000 c | 0.040 c | 0.640 bc |
| 3 BAP + 0.5 IBA | 0.000 e | 0.000 e | 0.320 b | 0.000 c | 0.000 c | 1.520 ab |
| 1 TDZ + 0.5 IBA | 0.040 de | 0.000 e | 0.240 bc | 0.040 c | 0.000 c | 0.360 c |
| 2 TDZ + 0.5 IBA | 0.000 e | 0.000 e | 0.680 a | 0.000 c | 0.000 c | 2.120 a |
| 3 TDZ + 0.5 IBA | 0.000 e | 0.000 e | 0.320 b | 0.000 c | 0.000 c | 2.240 a |
| Effect | Callus Formation | Shoot Induction | Number of Shoots | ||||
|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | P | |
| explant | 2 | 4.482 | 0.012 * | 61.778 | 0.000 * | 23.249 | 0.000 * |
| hormone | 5 | 6.304 | 0.000 * | 5.203 | 0.000 * | 1.961 | 0.083 |
| explant * hormone | 10 | 1.829 | 0.054 | 6.598 | 0.000 * | 2.521 | 0.005 * |
| Error | 432 | ||||||
| PGR Combinations (mg/L) | Callus Formation | Shoot Induction | Regenerated Shoots |
|---|---|---|---|
| 1 BAP + 0.2 IAA | 0.000 b | 0.040 ab | 0.040 bc |
| 2 BAP + 0.2 IAA | 0.000 b | 0.000 b | 0.000 c |
| 3 BAP + 0.2 IAA | 0.000 b | 0.000 b | 0.000 c |
| 0.5 BAP + 1.5 KIN + 0.2 IAA | 0.000 b | 0.027 ab | 0.027 bc |
| 1 TDZ + 0.2 IAA | 0.133 a | 0.080 a | 0.360 a |
| 2 TDZ + 0.2 IAA | 0.200 a | 0.053 ab | 0.227 ab |
| 3 TDZ + 0.2 IAA | 0.173 a | 0.013 b | 0.027 bc |
| Effect | Callus Formation | Shoot Induction | Number of Shoots | ||||
|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | |
| explant | 2 | 1.002 | 0.368 | 2.867 | 0.058 | 2.863 | 0.058 |
| hormone | 6 | 5.518 | 0.000 * | 2.289 | 0.034 * | 3.501 | 0.002 * |
| explant * hormone | 12 | 0.562 | 0.873 | 1.622 | 0.082 | 1.692 | 0.0653 |
| Error | 504 | ||||||
| Species | PGR (in mg/L) | |
|---|---|---|
| Shoot Induction | Shoot Multiplication | |
| A. alnifolia var. cusickii | 1–3 BAP + 0.5 IBA | 1 BAP + 0.5 IBA |
| 1–3 TDZ + 0.5 IBA | 0.5 TDZ + 0.5 IBA | |
| L. kamtschatica ‘Jugana’ | 1–3 BAP + 0.2 IAA | 1 TDZ + 0.2 IAA |
| 1–3 TDZ + 0.2 IAA | ||
| 0.5 BAP + 1.5 KIN + 0.2 IAA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunková, J.; Szabóová, M.; Gajdošová, A. Protocols for Adventitious Regeneration of Amelanchier alnifolia var. cusickii and Lonicera kamtschatica ‘Jugana’. Plants 2021, 10, 1155. https://doi.org/10.3390/plants10061155
Hunková J, Szabóová M, Gajdošová A. Protocols for Adventitious Regeneration of Amelanchier alnifolia var. cusickii and Lonicera kamtschatica ‘Jugana’. Plants. 2021; 10(6):1155. https://doi.org/10.3390/plants10061155
Chicago/Turabian StyleHunková, Júlia, Monika Szabóová, and Alena Gajdošová. 2021. "Protocols for Adventitious Regeneration of Amelanchier alnifolia var. cusickii and Lonicera kamtschatica ‘Jugana’" Plants 10, no. 6: 1155. https://doi.org/10.3390/plants10061155
APA StyleHunková, J., Szabóová, M., & Gajdošová, A. (2021). Protocols for Adventitious Regeneration of Amelanchier alnifolia var. cusickii and Lonicera kamtschatica ‘Jugana’. Plants, 10(6), 1155. https://doi.org/10.3390/plants10061155







