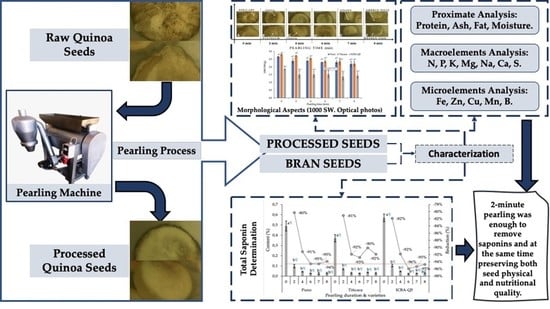
How Does Mechanical Pearling Affect Quinoa Nutrients and Saponin Contents?
Abstract
1. Introduction
2. Results
2.1. ANOVA Results
2.2. Physical Parameters
2.2.1. Pearling Efficiency
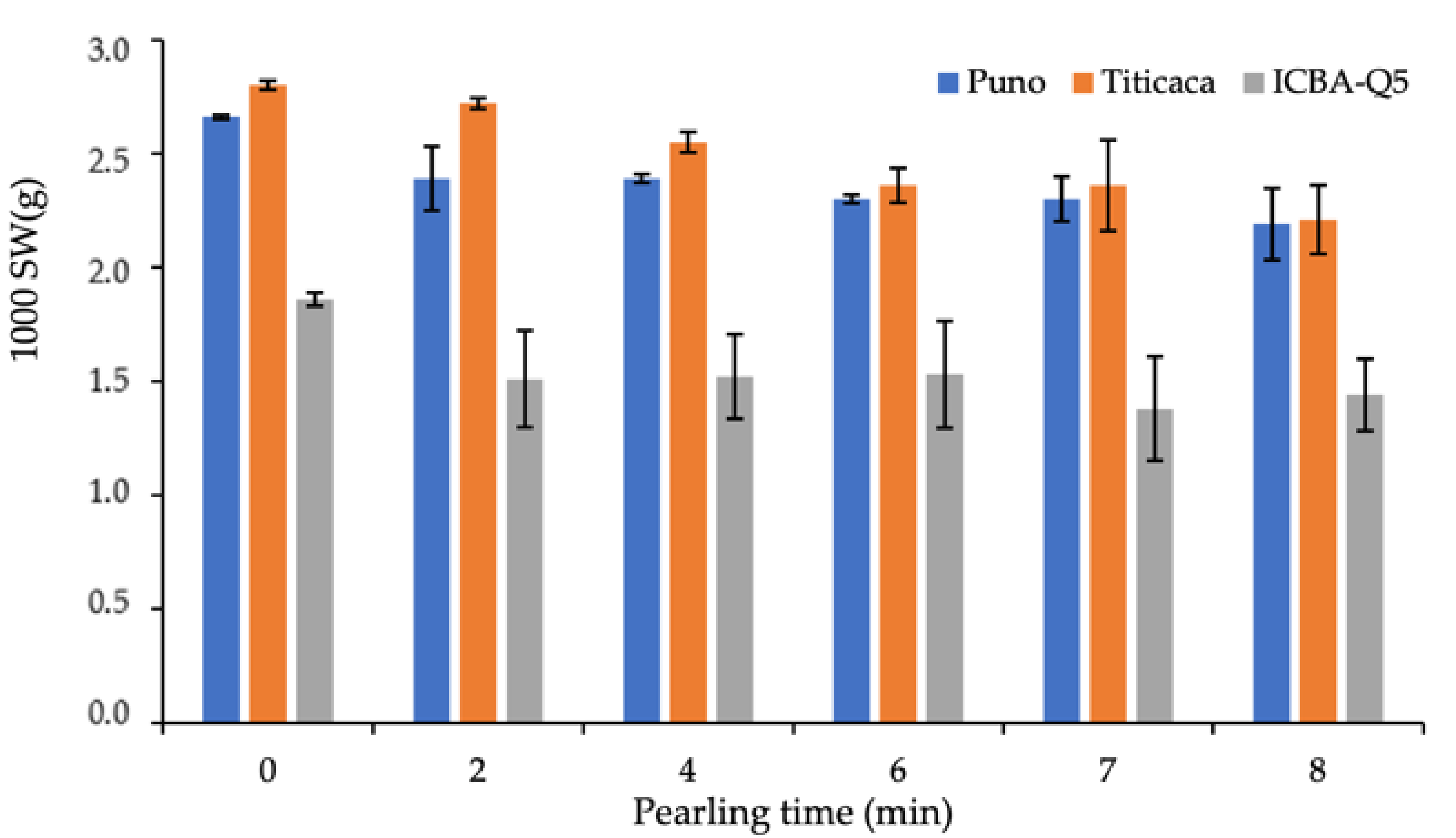
2.2.2. 1000 SW
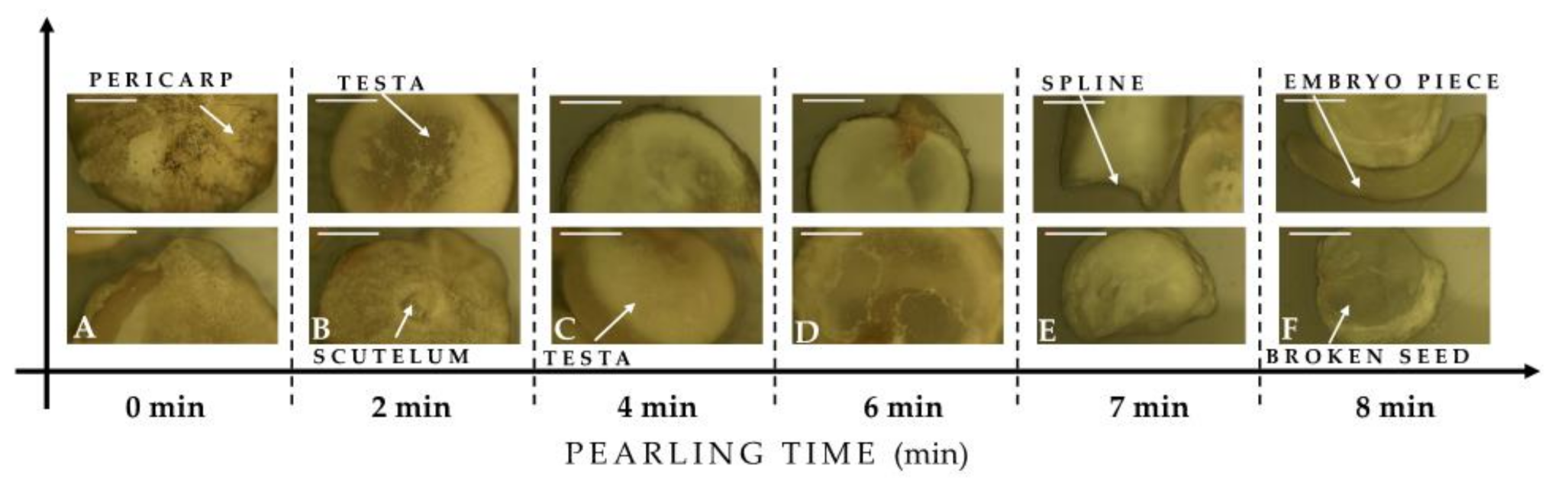
2.2.3. Morphological Aspects
2.3. Chemical Parameters
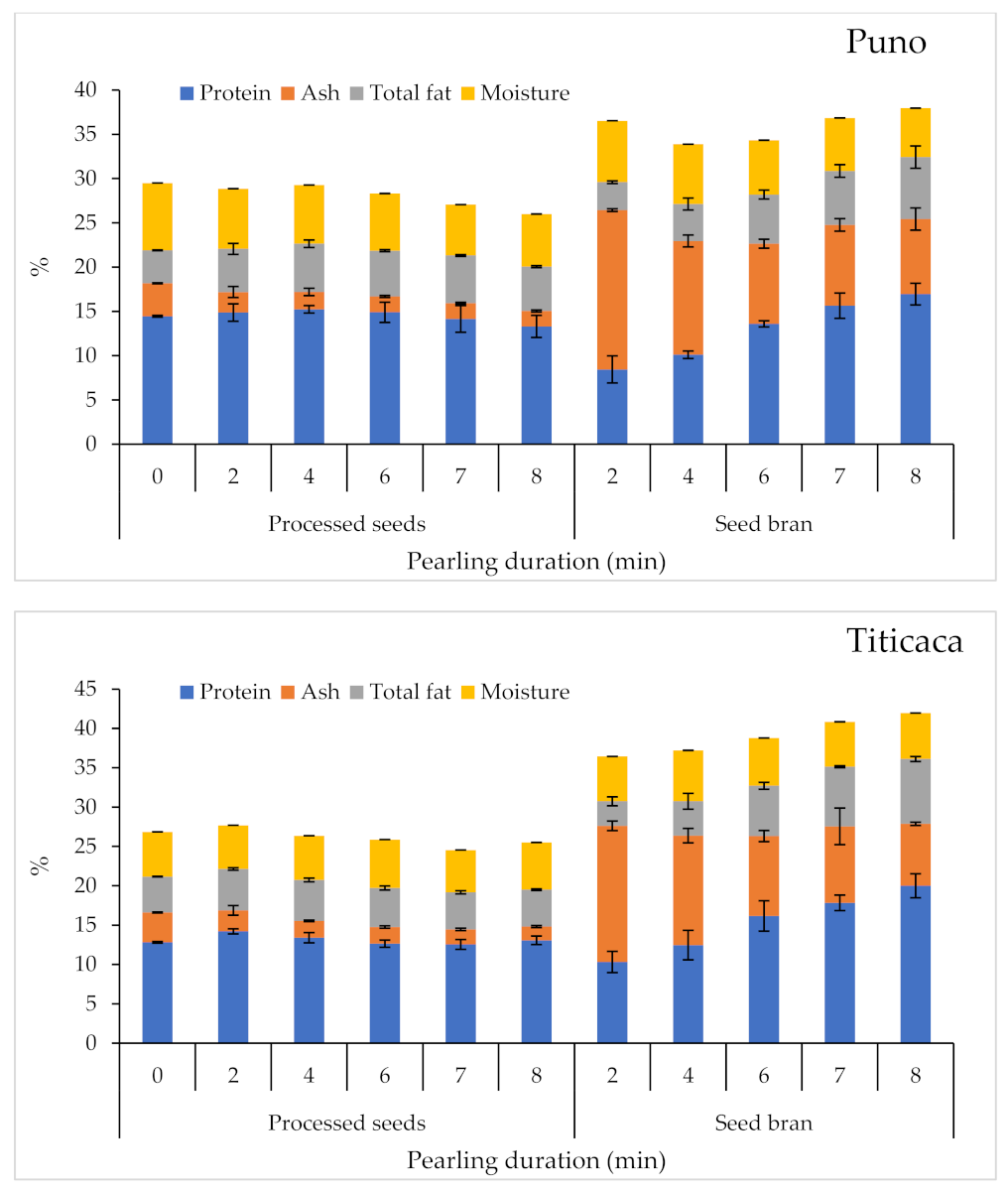
2.3.1. Proximate Analysis
2.3.2. Macronutrient Content
2.3.3. Micronutrient Contents
2.3.4. Saponin Content
2.4. Pearson Correlation
2.5. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Seed Material
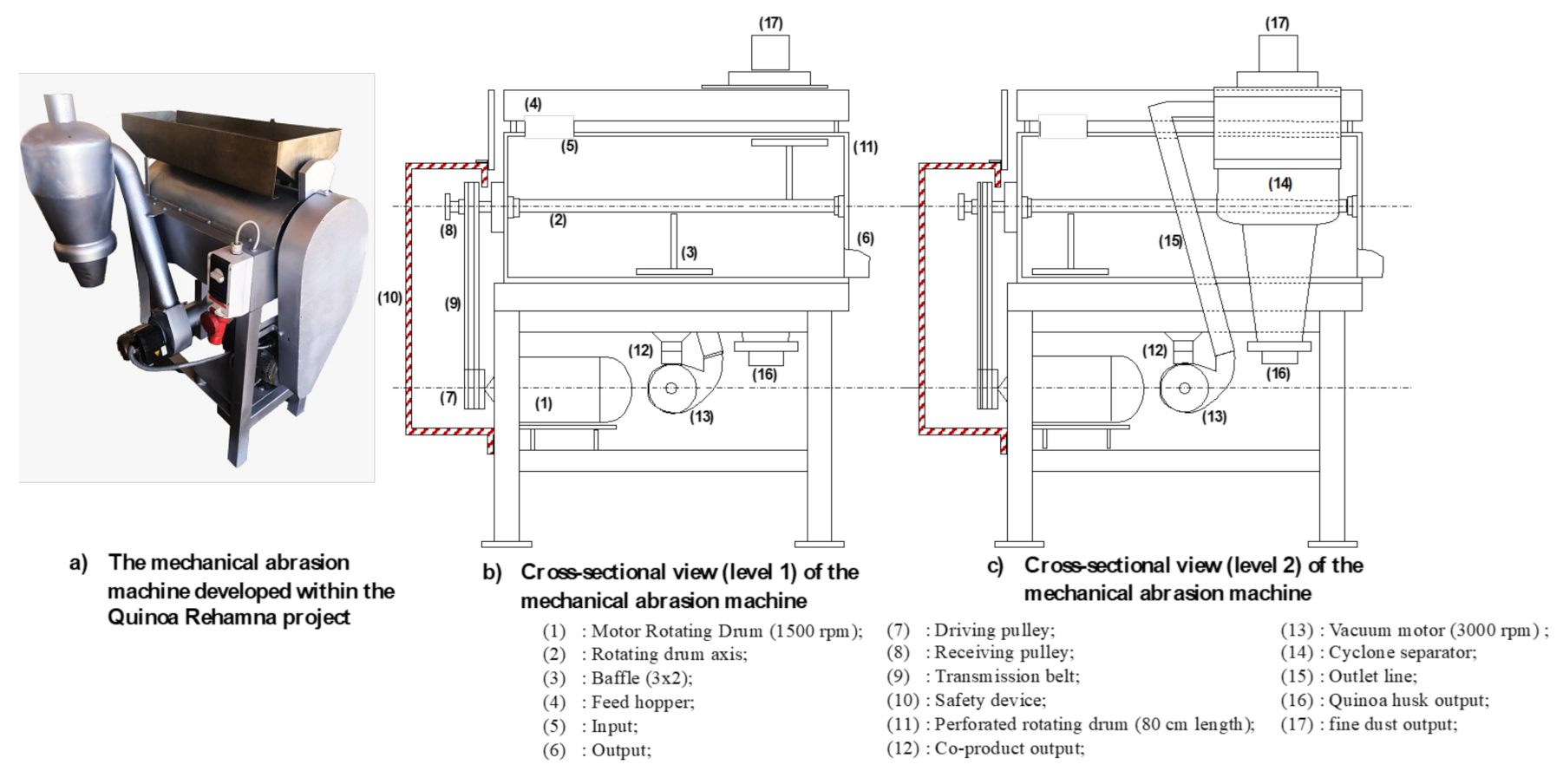
4.2. Quinoa Pearling Machine Design
4.3. Pearling Treatments and Machine Operating Mode
4.4. Physical Analysis
4.5. Chemical Analysis
4.6. Saponin Determination by Liebermann–Buchard Colorimetric Assay
4.6.1. Determining the Wavelength for the Dosage
4.6.2. Dilutions for Range Preparation
4.6.3. Saponin Extraction
4.6.4. Development of the color
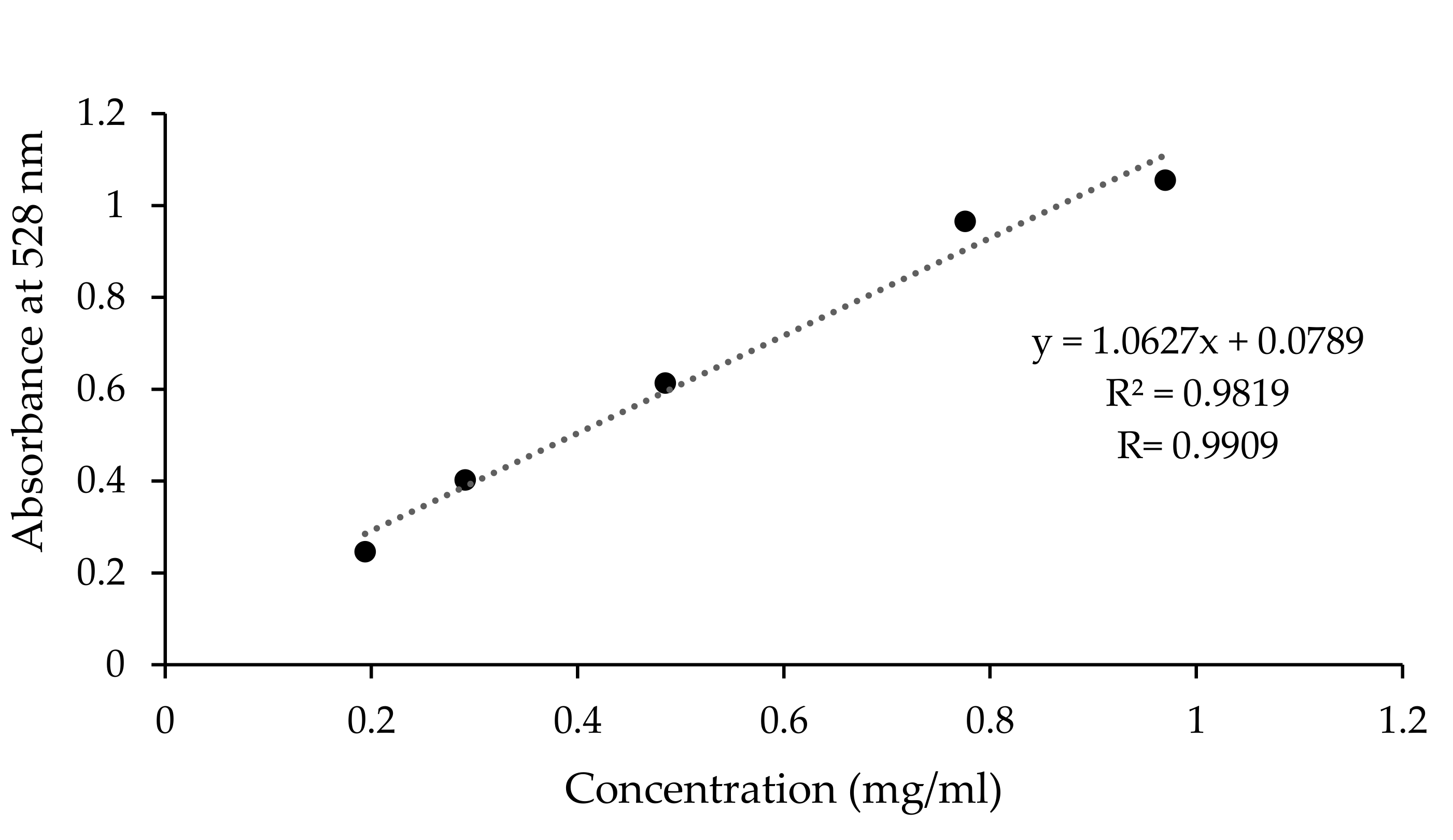
4.6.5. Calibration Curve Preparation
4.7. Statistical Analysis
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobsen, S.E.; Jensen, C.R.; Liu, F. Improving crop production in the arid Mediterranean climate. F. Crop. Res. 2012, 128, 34–47. [Google Scholar] [CrossRef]
- Choukr-Allah, R.; Rao, N.K.; Hirich, A.; Shahid, M.; Alshankiti, A.; Toderich, K.; Gill, S.; Butt, K.U.R. Quinoa for marginal environments: Toward future food and nutritional security in MENA and central Asia regions. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Benlhabib, O. Les cultures alternatives: Quinoa, amarante et épeautre. Transf. Technol. Agric. 2005, 133, 1–4. [Google Scholar]
- Hirich, A.; Choukr-Allah, R.; Jacobsen, S.E.; El-Youssfi, L.; El-Omari, H. Using deficit irrigation with treated wastewater in the production of quinoa (Chenopodium quinoa Willd.) in Morocco. Rev. Cient. UDO Agric. 2012, 12, 570–583. [Google Scholar]
- Hirich, A.; Jelloul, A.; Choukr-Allah, R.; Jacobsen, S.E. Saline water irrigation of quinoa and chickpea: Seedling rate, stomatal conductance and yield responses. J. Agron. Crop Sci. 2014, 200, 378–389. [Google Scholar] [CrossRef]
- Hirich, A.; Choukr-Allah, R.; Jacobsen, S.E. Deficit irrigation and organic compost improve growth and yield of Quinoa and Pea. J. Agron. Crop Sci. 2014, 200, 390–398. [Google Scholar] [CrossRef]
- Filali, K. Caractérisation et évaluation du rendement de lignées de quinoa (Chenopodium quinoa) dans la région de Rhamna. Amélioration Génétique des Productions Agricoles. Master’s Thesis, Institute Agronomique et Vétérinaire Hassan II, Rabat, Morocco, 2011; p. 109. [Google Scholar]
- Rafik, S.; Chaoui, M.; Assabban, Y.; Jazi, S.; Choukr-Allah, R.; El Gharouss, M.; Hirich, A. Quinoa value chain, adoption, and market assessment in Morocco. Environ. Sci. Pollut. Res. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rafik, S.; Rahmani, M.; Choukr-Allah, R.; El Gharous, M.; Calle, J.P.R.; Filali, K.; Hirich, A. Techno-economic assessment of quinoa production and transformation in Morocco. Environ. Sci. Pollut. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Hirich, A.; Rafik, S.; Rahmani, M.; Fetouab, A.; Azaykou, F.; Filali, K.; Ahmadzai, H.; Jnaoui, Y.; Soulaimani, A.; Moussafir, M.; et al. Development of Quinoa Value Chain to Improve Food and Nutritional Security in Rural Communities in Rehamna, Morocco: Lessons Learned and Perspectives. Plants 2021, 10, 301. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Khakimov, B.; Engelsen, S.B.; Bak, S.; Biondi, S.; Jacobsen, S.E. Quinoa seed coats as an expanding and sustainable source of bioactive compounds: An investigation of genotypic diversity in saponin profiles. Ind. Crops Prod. 2017, 104, 156–163. [Google Scholar] [CrossRef]
- Abugoch James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58. [Google Scholar] [CrossRef]
- Lindeboom, N. Studies on the characterization, biosynthesis and isolation of starch and protein from quinoa (Chenopodium quinua Willd.). Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2005; p. 170. [Google Scholar]
- Jancurová, M.; Minarovičová, L.; Dandár, A. Quinoa—A review. Czech J. Food Sci. 2009, 27, 71–79. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa—An Indian perspective. Ind. Crops Prod. 2016, 23, 73–87. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhu, F. Physicochemical properties of quinoa starch. Carbohydr. Polym. 2016, 137, 328–338. [Google Scholar] [CrossRef]
- Comai, S.; Bertazzo, A.; Bailoni, L.; Zancato, M.; Costa, C.V.L.; Allegri, G. The content of proteic and nonproteic (free and protein-bound) tryptophan in quinoa and cereal flours. Food Chem. 2007, 100, 1350–1355. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Matilla, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Gohara, A.K.; Souza, A.H.P.; de Souza, N.E.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Evaluation of nutritional compounds in new amaranth and quinoa cultivars. Food Sci. Technol. 2013, 33, 339–344. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Sayas-Barberá, M.E.; Navarro-Rodríguez de Vera, C.; Lucas-González, R.; Roldán-Verdú, A.; Botella-Martínez, C.; Pérez-Alvarez, J.A. Chia, Quinoa, and Their Coproducts as Potential Antioxidants for the Meat Industry. Plants 2020, 9, 1359. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- De Costa, F.; Yendo, A.C.A.; Fleck, J.D.; Gosmann, G.; Fett-Neto, A.G. Immunoadjuvant and Anti-Inflammatory Plant Saponins: Characteristics and Biotechnological Approaches Towards Sustainable Production. Mini-Rev Med. Chem. 2011, 11, 857–880. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Abdel, H.; Salman, K.; Sulaiman, R. Extraction and quanti fi cation of saponins: A review. Food Res Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Norma Boliviana. NB NA 0038 Granos Andinos—Pseudo Cereales—Quinua en grano Clasificación y Requisitos. 2007. Available online: http://www.conal.gob.ar/CONASE/actas/ActaCONASE_2013_04Abr18_AnexoI.pdf (accessed on 18 April 2021).
- Koziol, M.J. Afrosimetric estimation of threshold saponin concentration for bitterness in quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 1991, 54, 211–219. [Google Scholar] [CrossRef]
- Gargiulo, L.; Grimberg, Å.; Repo-Carrasco-Valencia, R.; Carlsson, A.S.; Mele, G. Morpho-densitometric traits for quinoa (Chenopodium quinoa Willd.) seed phenotyping by two X-ray micro-CT scanning approaches. J. Cereal Sci. 2019, 90. [Google Scholar] [CrossRef]
- Ando, H.; Chen, Y.-C.; Tang, H.; Shimizu, M.; Watanabe, K.; Mitsunaga, T. Food Components in Fractions of Quinoa Seed. Food Sci. Technol. Res. 2002, 8, 80–84. [Google Scholar] [CrossRef]
- D’Amico, S.; Jungkunz, S.; Balasz, G.; Foeste, M.; Jekle, M.; Tömösköszi, S.; Schoenlechner, R. Abrasive milling of quinoa: Study on the distribution of selected nutrients and proteins within the quinoa seed kernel. J. Cereal Sci. 2019, 86, 132–138. [Google Scholar] [CrossRef]
- Czekus, B.; Pećinar, I.; Petrović, I.; Paunović, N.; Savić, S.; Jovanović, Z.; Stikić, R. Raman and Fourier transform infrared spectroscopy application to the Puno and Titicaca cvs. of quinoa seed microstructure and perisperm characterization. J. Cereal Sci. 2019, 87, 25–30. [Google Scholar] [CrossRef]
- Nickel, J.; Spanier, L.P.; Botelho, F.T.; Gularte, M.A.; Helbig, E. Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd grains. Food Chem. 2016, 209, 139–143. [Google Scholar] [CrossRef]
- Ridout, C.L.; Price, K.R.; Dupont, M.S.; Parkerc, M.L.; Fenwick, G.R. Quinoa Saponins -Analysis and Preliminary Investigations into the Effects of Reduction by Processing. J. Sci. Food Agric. 1991, 54, 165–176. [Google Scholar] [CrossRef]
- Bazile, D.; Bertero, H.D.; Nieto, C. State of the Art Report on Quinoa Around the World in 2013; United Nations Organization of Food and Agriculture: Rome, Italy, 2015. [Google Scholar]
- CODEX Standard for Quinoa-CXS 333–2019. 2019, CODEX Aliment.-Int. Food Stand. United Nations Organization of Food and Agriculture (FAO), Rome, Italy. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B333-2019%252FCXS_333e.pdf (accessed on 18 April 2021).
- Mhada, M.; Metougui, M.L.; El Hazzam, K.; El Kacimi, K.; Yasri, A. Variations of Saponins, Minerals and Total Phenolic Compounds due to Processing and Cooking of Quinoa (Chenopodium quinoa Willd.) Seeds. Foods 2020, 9, 660. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef]
- Riccardi, M.; Mele, G.; Pulvento, C.; Lavini, A.; D’Andria, R.; Jacobsen, S.E. Non-destructive evaluation of chlorophyll content in quinoa and amaranth leaves by simple and multiple regression analysis of RGB image components. Photosynth. Res. 2014, 120, 263–272. [Google Scholar] [CrossRef]
- Valcárcel-Yamani, B.; Lannes, S.C.D.S. Applications of Quinoa (Chenopodium Quinoa Willd.) and Amaranth (Amaranthus spp.) and Their Influence in the Nutritional Value of Cereal Based Foods. Food Public Heal. 2012, 2, 265–275. [Google Scholar] [CrossRef]
- Koziol, M.J. Chemical Composition and Nutritional Evaluation of Quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Wu, L.G.; Wang, A.; Shen, R.; Qu, L. The effect of slight milling on nutritional composition and morphology of quinoa (Chenopodium quinoa) grain. Int. J. Food Eng. 2020, 16. [Google Scholar] [CrossRef]
- Prado, F.E.; Fernández-Turiel, J.L.; Tsarouchi, M.; Psaras, G.K.; González, J.A. Variation of seed mineral concentrations in seven quinoa cultivars grown in two agroecological sites. Cereal Chem. 2014, 91, 453–459. [Google Scholar] [CrossRef]
- Nascimento, A.C.; Mota, C.; Coelho, I.; Gueifão, S.; Santos, M.; Matos, A.S.; Gimenez, A.; Lobo, M.; Samman, N.; Castanheira, I. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: Proximates, minerals and trace elements. Food Chem. 2014, 148, 420–426. [Google Scholar] [CrossRef]
- Prego, I.; Maldonado, S.; Otegui, M. Seed structure and localization of reserves in Chenopodium quinoa. Ann. Bot. 1998, 82, 481–488. [Google Scholar] [CrossRef]
- Konishi, Y.; Hirano, S.; Tsuboi, H.; Wada, M. Distribution of minerals in quinoa (Chenopodium quinoa Willd.) seeds. Biosci. Biotechnol. Biochem. 2004, 68, 231–234. [Google Scholar] [CrossRef]
- Balwinder, S.; Amritpal, K. Control of insect pests in crop plants and stored food grains using plant saponins: A review. LWT 2018, 87, 93–101. [Google Scholar] [CrossRef]
- Zegarra Vilchez, G.H. Actividad Deterrente y Acaricida de Principios Activos de Quinuas Amargas, Aceites Esenciales y Tarwi. Bachelor’s Thesis, Facultad de Ciencias e Ingeniería, Pontificia Universidad Católica del Peru, Lima, Peru, 2011. [Google Scholar]
- Guzmán, B.; Tenorio, R.; Cruz, D.; Espinal, C.; Alvarado, J.; y Mollinedo, P. Saponins from Chenopodium quinoa Willd. and Chenopodium pallidicaule Aellen as biocontrollers of Phytopathogen fungi and Hemolysis agents. Revista Boliviana de química 2015, 32, 8–14. [Google Scholar]
- Karimian, J.; Abedi, S.; Shirinbakhshmasoleh, M.; Moodi, F.; Moodi, V.; Ghavami, A. The effects of quinoa seed supplementation on cardiovascular risk factors: A systematic review and meta-analysis of controlled clinical trials. Phytother. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Güçlü-Üstündag, O.; Mazzaa, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Tippel, J.; Gies, K.; Harbaum-Piayda, B.; Steffen-Heins, A.; Drusch, S. Composition of Quillaja saponin extract affects lipid oxidation in oil-in-water emulsions. Food Chem. 2016, 221, 386–394. [Google Scholar] [CrossRef]
- Vilche, C.; Gely, M.; Santalla, E. Physical properties of quinoa seeds. Biosyst. Eng. 2003, 86, 59–65. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis, 20th ed.; AOAC International: Rockville, MD, USA, 2016; Volume 20. [Google Scholar]
- Marmouzi, I.; El Madani, N.; Charrouf, Z.; Cherrah, Y.; El Abbes Faouzi, M.Y. Proximate analysis, fatty acids and mineral composition of processed Moroccan Chenopodium quinoa Willd. and antioxidant properties according to the polarity. Phytotherapie 2015, 13, 110–117. [Google Scholar] [CrossRef]
- Irigoyen, R.M.T.; Giner, S.A. Extraction kinetics of saponins from quinoa seed (Chenopodium quinoa Willd.). Int. J. Food Stud. 2018, 7, 76–88. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Zhang, W.; Chen, Z. Identification and quantification of oleanolic acid and ursolic acid in Chinese herbs by liquid chromatography-ion trap mass spectrometry. Biomed. Chromatogr. 2011, 25, 1381–1388. [Google Scholar] [CrossRef] [PubMed]

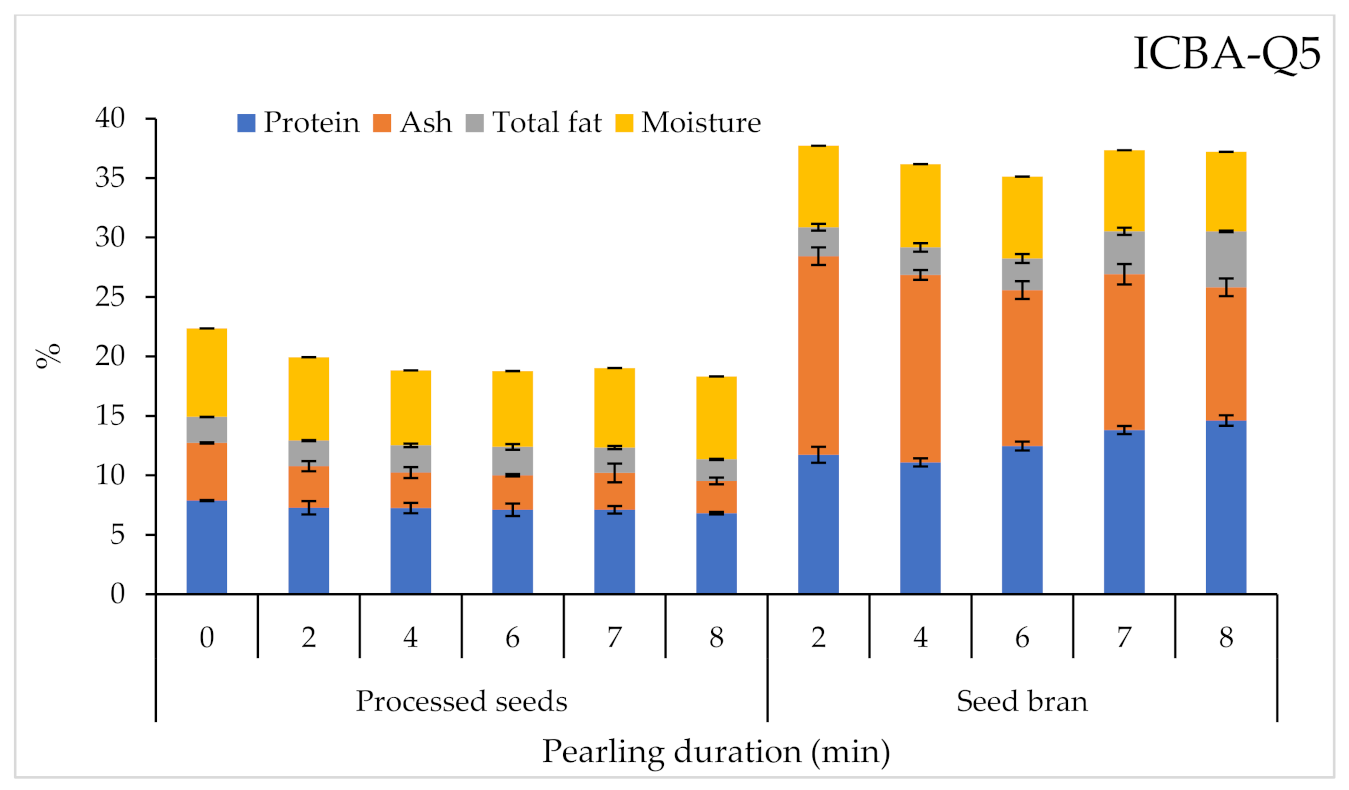
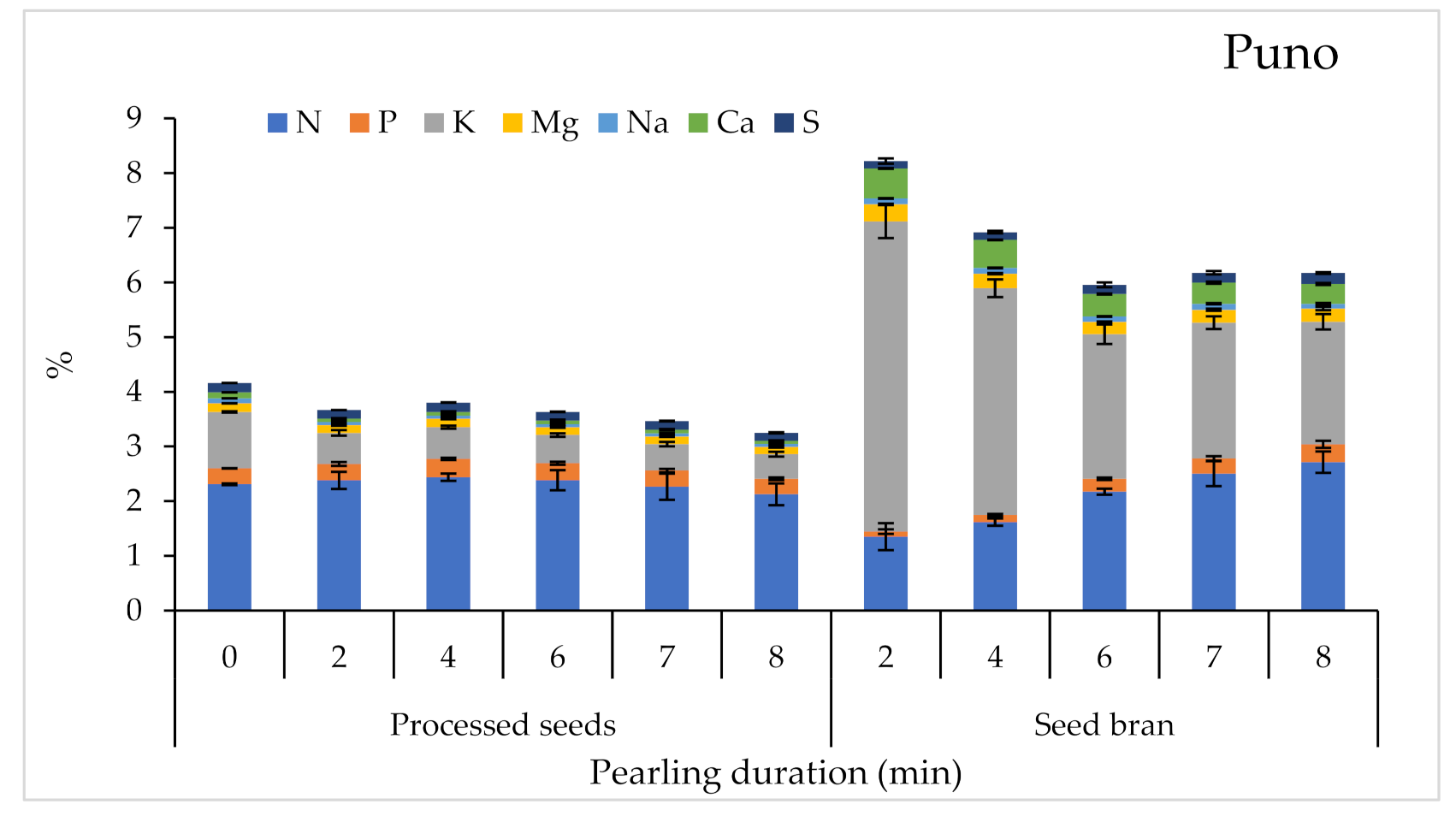
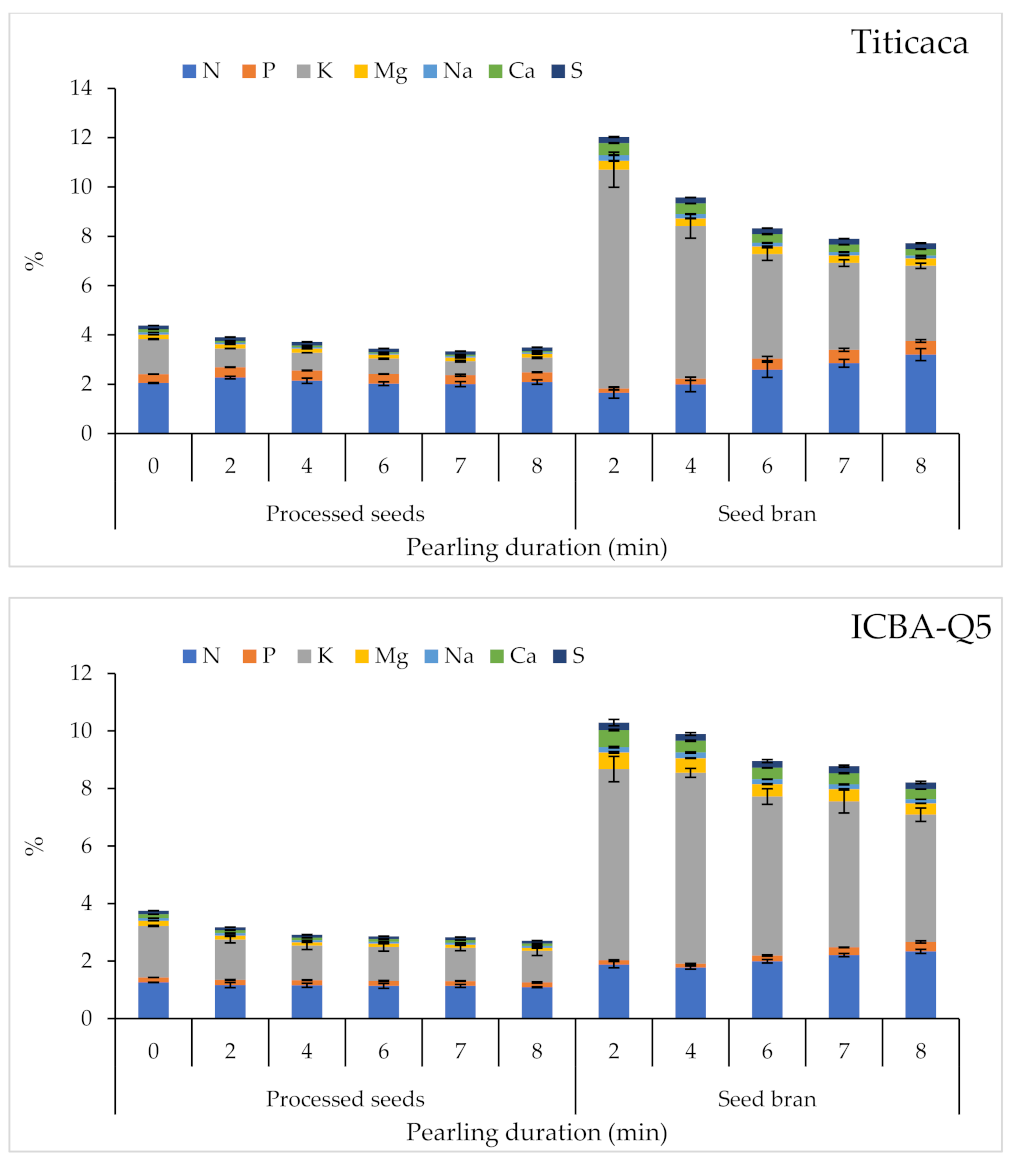
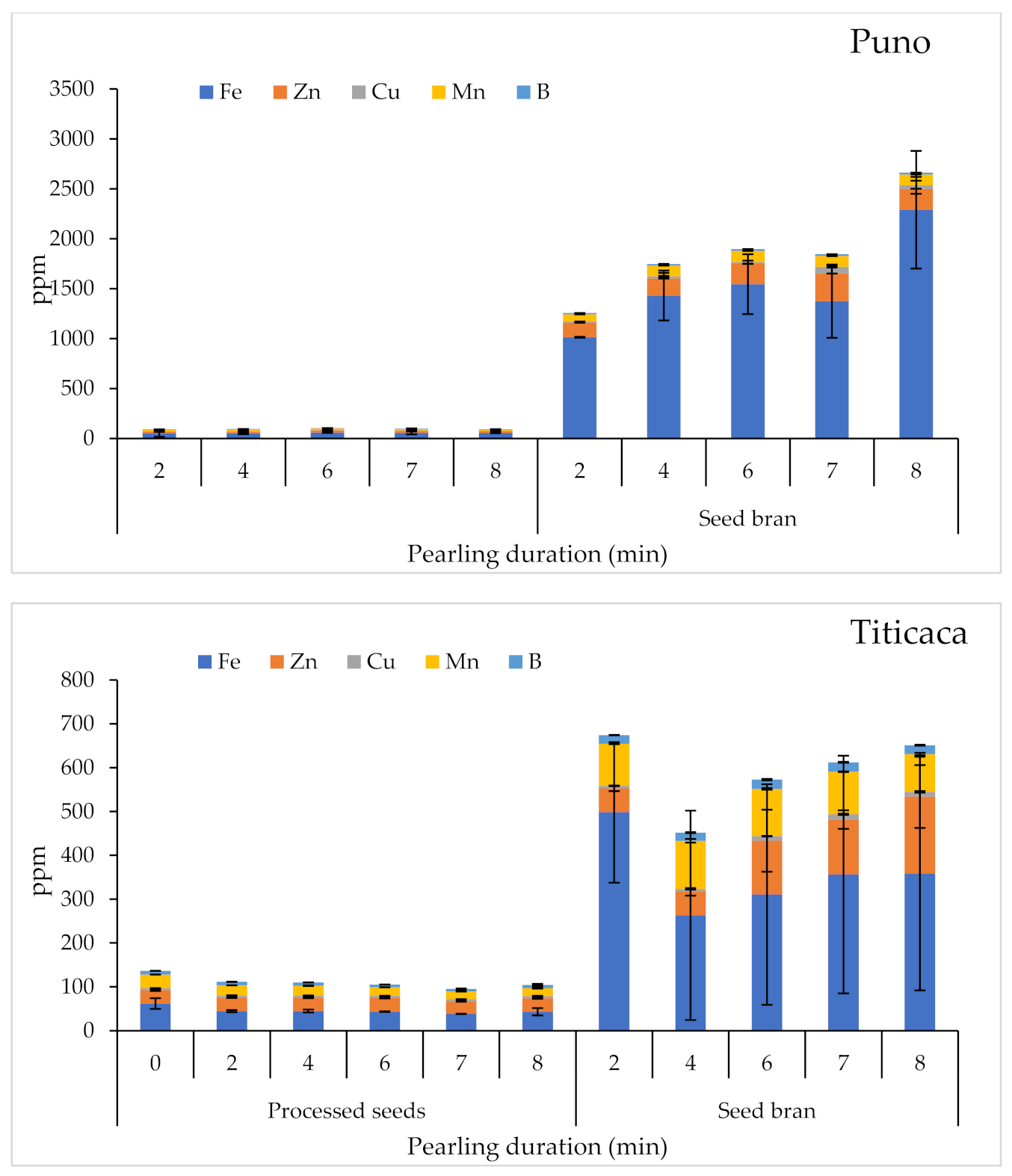
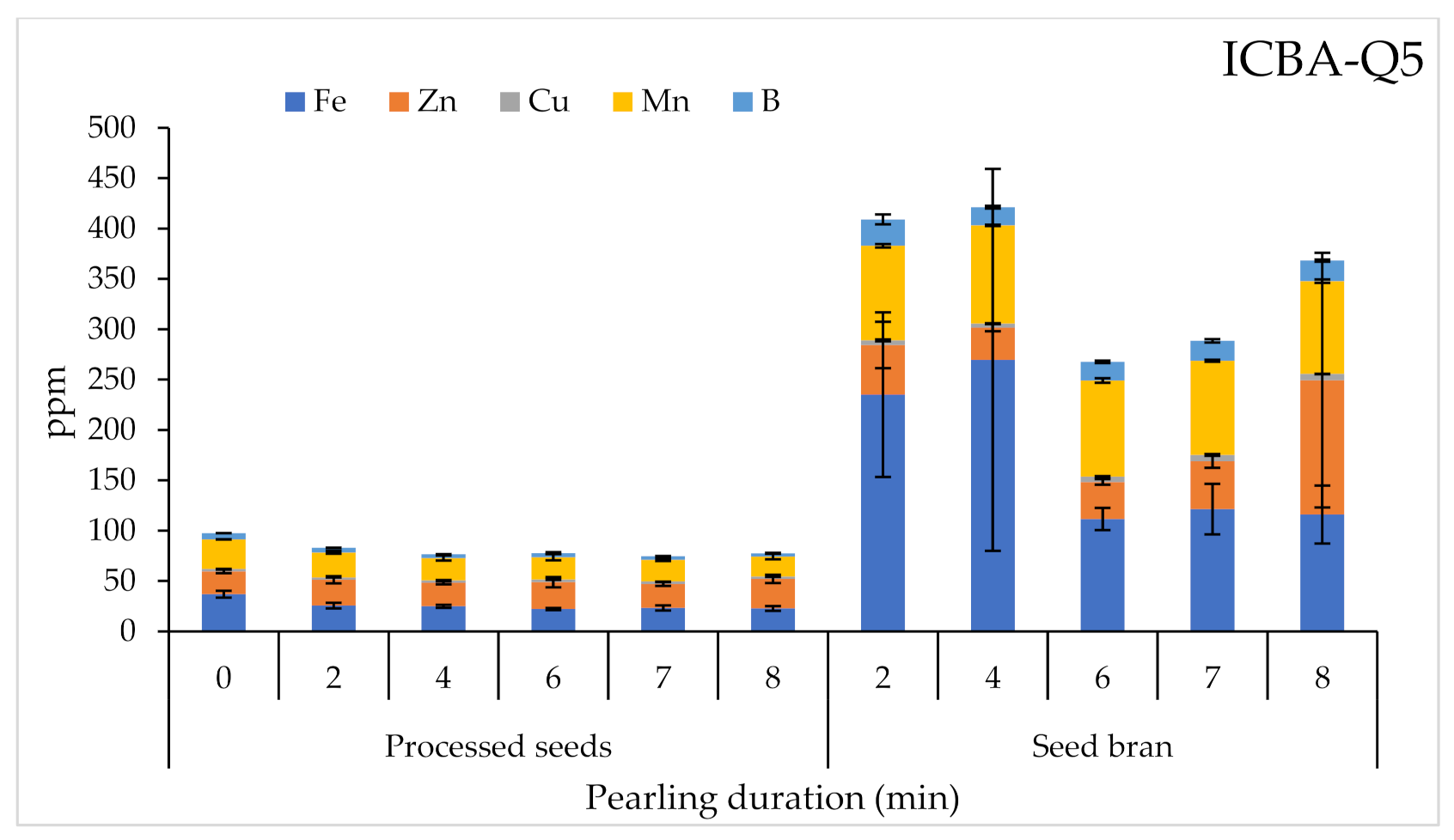
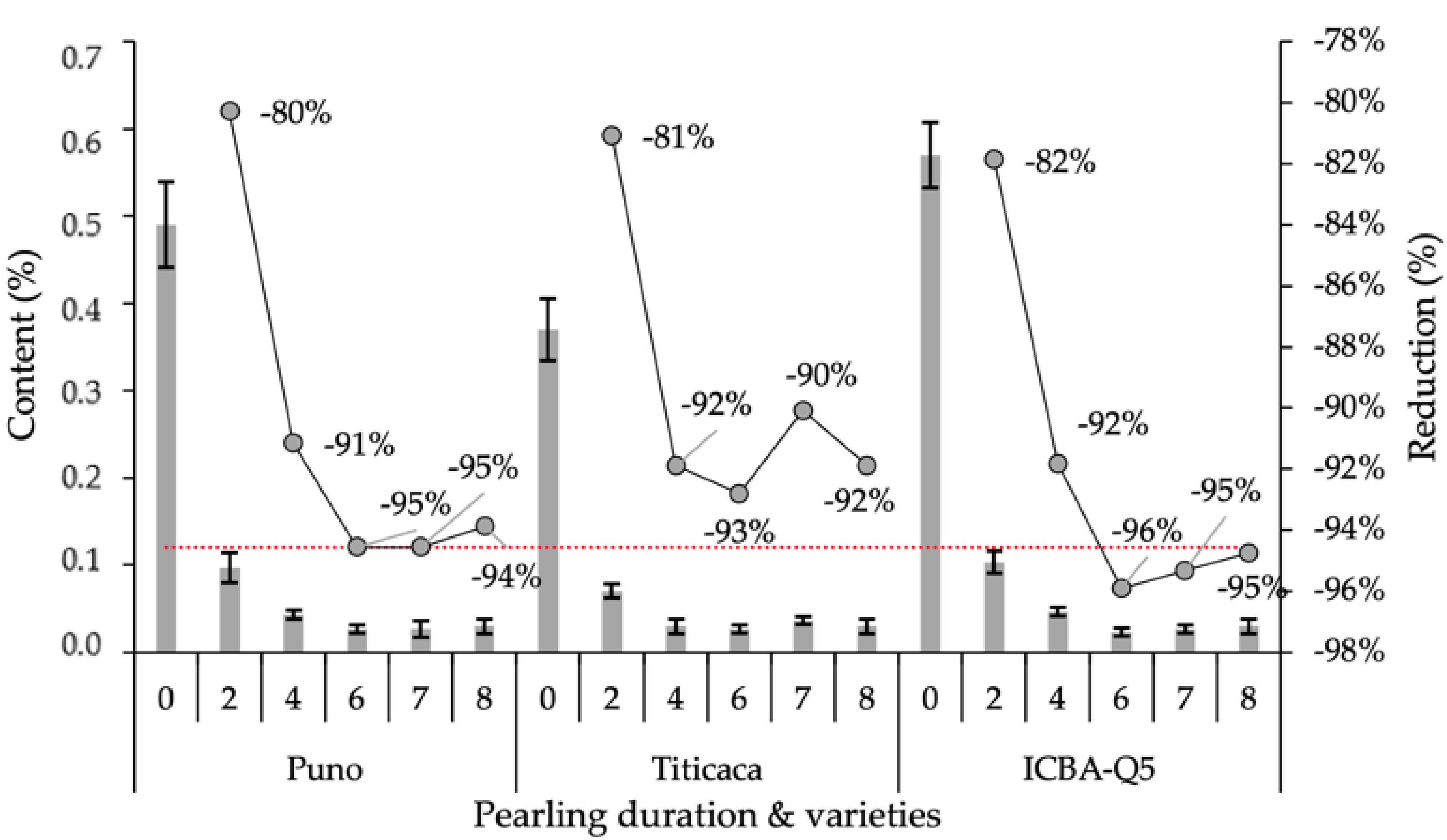
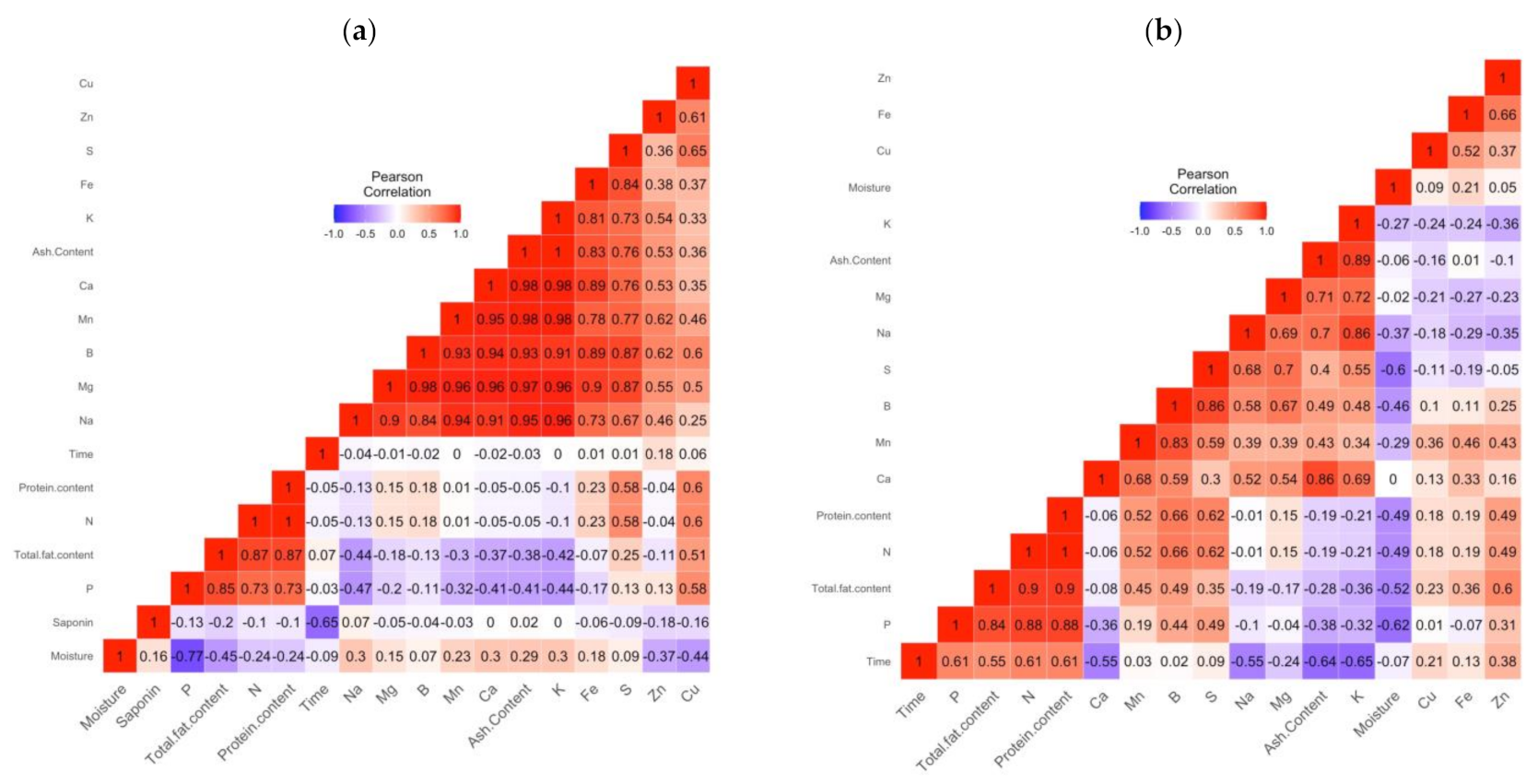
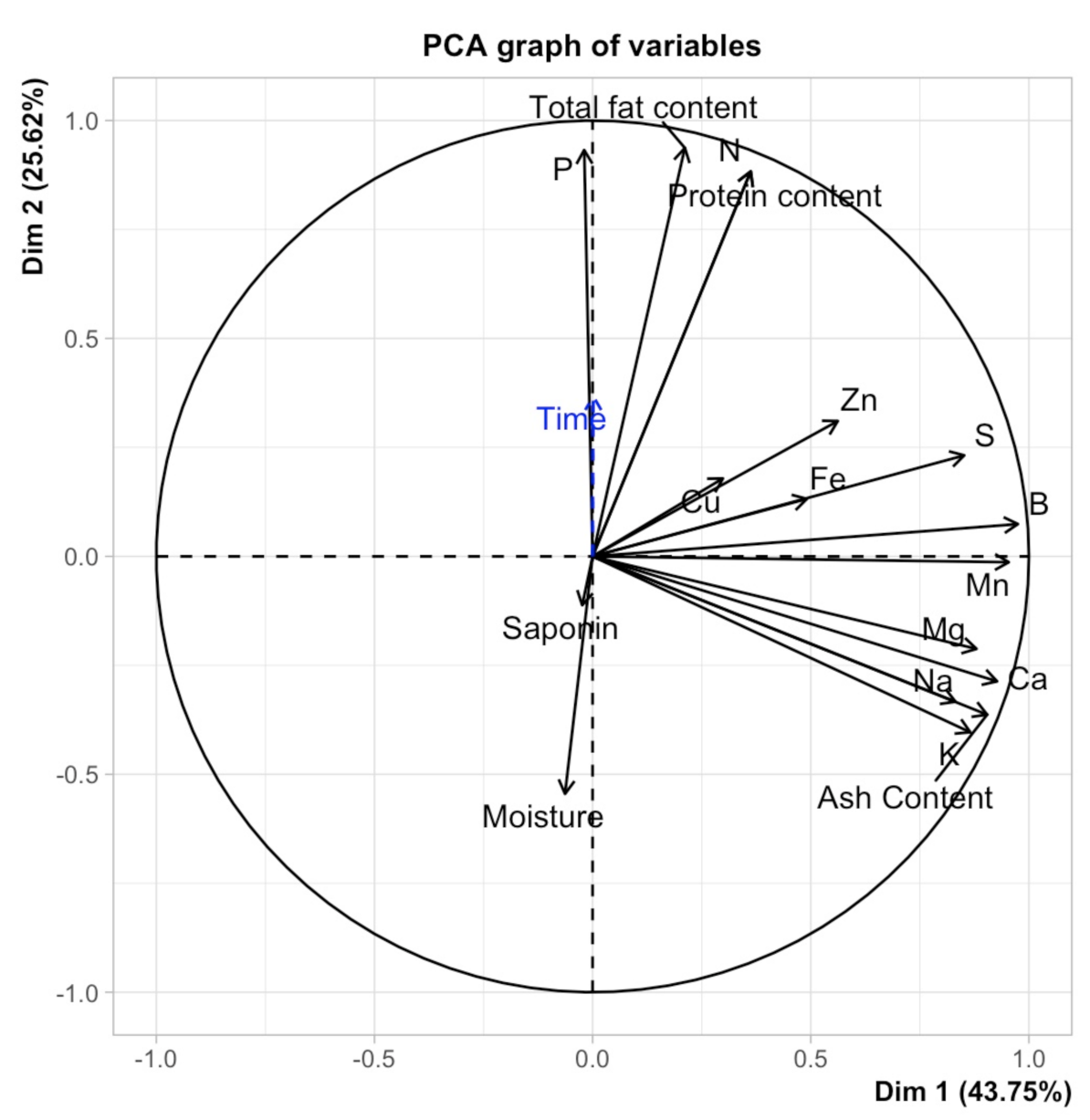

| Product | Factor | 1000 SW | PR | A | TF | M | Macro-Elements | Micro-Elements | SP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Mg | Na | Ca | S | Fe | Zn | Cu | Mn | B | |||||||||
| Processed seeds | V | *** | *** | *** | *** | *** | *** | *** | *** | NS | *** | *** | NS | NS | *** | * | ** | NS | NS | |
| PD | *** | NS | NS | NS | *** | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | *** | ||
| V × PD | NS | NS | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | ||
| p-value | 0.223 | 0.439 | 0.855960 | 0.133 | 0.022630 | 0.439 | 0.358 | 0.904432 | 0.9115 | 0.939080 | 0.968891 | 0.889 | 0.961 | 0.145 | 0.7194 | 0.73797 | 0.9343 | 0.0469 | ||
| Seed bran | V | - | *** | NS | *** | *** | *** | *** | ** | * | *** | *** | *** | *** | *** | ** | *** | ** | - | |
| PD | - | *** | *** | *** | *** | *** | *** | *** | NS | *** | *** | NS | NS | *** | NS | NS | NS | - | ||
| V × PD | - | *** | NS | *** | ** | *** | *** | * | NS | ** | NS | NS | * | NS | NS | NS | NS | - | ||
| p-value | 6.09 × 10−5 | 0.164 | 1.01 × 10−7 | 0.001727 | 6.09 × 10−5 | 5.82 × 10−6 | 0.01169 | 0.9239 | 0.00407 | 0.737929 | 0.095 | 0.0317 | 0.568709 | 0.25700 | 0.097694 | 0.1639 | - | |||
| Variety/Product | PD | Proximate Analysis | Macro-Nutrients | Micro-Nutrients | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | A | TF | M | N | P | K | Mg | Na | Ca | S | Fe | Zn | Cu | Mn | B | |||
| Puno | ||||||||||||||||||
| Processed seeds | 0 | a | a | b | a | a | a | a | a | a | a | a | a | b | a | a | a | |
| 2 | a | b | ab | ab | a | a | b | ab | ab | b | a | a | ab | a | bc | ab | ||
| 4 | a | b | a | b | a | a | b | ab | b | b | a | a | ab | a | ab | ab | ||
| 6 | a | b | a | bc | a | a | b | ab | b | b | a | a | ab | a | bc | ab | ||
| 7 | a | b | a | c | a | a | b | ab | ab | b | a | a | ab | a | bc | ab | ||
| 8 | a | b | ab | bc | a | a | b | b | ab | b | a | a | ab | a | c | b | ||
| Seed bran | 2 | d | a | c | a | c | a | a | a | a | a | c | a | a | a | b | a | |
| 4 | cd | b | c | a | c | a | b | ab | a | a | bc | a | a | a | ab | b | ||
| 6 | bc | c | b | a | b | a | c | b | a | b | ab | a | a | a | a | b | ||
| 7 | ab | c | ab | a | ab | b | c | b | a | b | a | a | a | a | ab | b | ||
| 8 | a | c | a | a | a | b | c | b | a | b | a | a | a | a | ab | b | ||
| ICBA-Q5 | ||||||||||||||||||
| Processed seeds | 0 | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | |
| 2 | a | a | a | ab | a | a | a | a | a | a | a | a | a | a | a | a | ||
| 4 | a | a | a | ab | a | a | a | a | a | a | a | a | a | a | a | a | ||
| 6 | a | a | a | ab | a | a | a | a | a | a | a | a | a | a | a | a | ||
| 7 | a | a | a | b | a | a | a | a | a | a | a | a | a | a | a | a | ||
| 8 | a | a | a | b | a | a | a | a | a | a | a | a | a | a | a | a | ||
| Seed bran | 2 | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | |
| 4 | a | a | a | a | a | a | a | a | ab | a | a | a | a | a | a | a | ||
| 6 | a | a | a | a | a | a | a | a | b | a | a | a | a | a | a | a | ||
| 7 | a | a | a | a | a | a | a | a | ab | a | a | a | a | a | a | a | ||
| 8 | a | a | a | a | a | a | a | a | ab | a | a | a | a | a | a | a | ||
| Titicaca | ||||||||||||||||||
| Processed seeds | 0 | a | Ash | b | a | a | a | a | a | a | a | a | a | b | a | a | a | |
| 2 | a | b | ab | ab | a | a | b | ab | ab | b | a | a | ab | a | bc | ab | ||
| 4 | a | b | ab | b | a | a | b | ab | b | b | a | a | ab | a | ab | ab | ||
| 6 | a | b | ab | bc | a | a | b | ab | b | b | a | a | ab | a | bc | ab | ||
| 7 | a | b | ab | c | a | a | b | ab | ab | b | a | a | ab | a | bc | ab | ||
| 8 | a | b | ab | bc | a | a | b | b | ab | b | a | a | ab | a | c | b | ||
| Seed bran | 2 | d | a | c | a | d | b | a | a | a | a | a | a | b | c | ab | a | |
| 4 | cd | b | c | a | cd | b | b | b | b | b | a | a | b | bc | a | a | ||
| 6 | bc | c | b | a | bc | a | c | b | bc | c | a | a | ab | ab | ab | a | ||
| 7 | ab | c | ab | a | ab | a | c | b | c | d | a | a | ab | a | ab | a | ||
| 8 | a | c | a | a | a | a | c | b | c | e | a | a | a | a | b | a | ||
| 0.8 mg/mL | 1 mL of the stock solution and 0.3 mL of ethanol. | 1 mL of the diluted solution is taken. | |
| 0.5 mg/mL | 0.5 mL of the stock solution and 0.5 mL of ethanol. | 1 mL of the diluted solution is taken. | |
| 0.3 mg/mL | 0.3 mL of the stock solution and 0.7 mL of ethanol. | 1 mL of the diluted solution is taken. | |
| 0.2 mg/mL | 0.5 of the stock solution and 4.5 mL of ethanol. | 1 mL of the diluted solution is taken. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafik, S.; Rahmani, M.; Rodriguez, J.P.; Andam, S.; Ezzariai, A.; El Gharous, M.; Karboune, S.; Choukr-Allah, R.; Hirich, A. How Does Mechanical Pearling Affect Quinoa Nutrients and Saponin Contents? Plants 2021, 10, 1133. https://doi.org/10.3390/plants10061133
Rafik S, Rahmani M, Rodriguez JP, Andam S, Ezzariai A, El Gharous M, Karboune S, Choukr-Allah R, Hirich A. How Does Mechanical Pearling Affect Quinoa Nutrients and Saponin Contents? Plants. 2021; 10(6):1133. https://doi.org/10.3390/plants10061133
Chicago/Turabian StyleRafik, Sifeddine, Mohamed Rahmani, Juan Pablo Rodriguez, Said Andam, Amine Ezzariai, Mohamed El Gharous, Salwa Karboune, Redouane Choukr-Allah, and Abdelaziz Hirich. 2021. "How Does Mechanical Pearling Affect Quinoa Nutrients and Saponin Contents?" Plants 10, no. 6: 1133. https://doi.org/10.3390/plants10061133
APA StyleRafik, S., Rahmani, M., Rodriguez, J. P., Andam, S., Ezzariai, A., El Gharous, M., Karboune, S., Choukr-Allah, R., & Hirich, A. (2021). How Does Mechanical Pearling Affect Quinoa Nutrients and Saponin Contents? Plants, 10(6), 1133. https://doi.org/10.3390/plants10061133