Filtering Light-Emitting Diodes to Investigate Amber and Red Spectral Effects on Lettuce Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
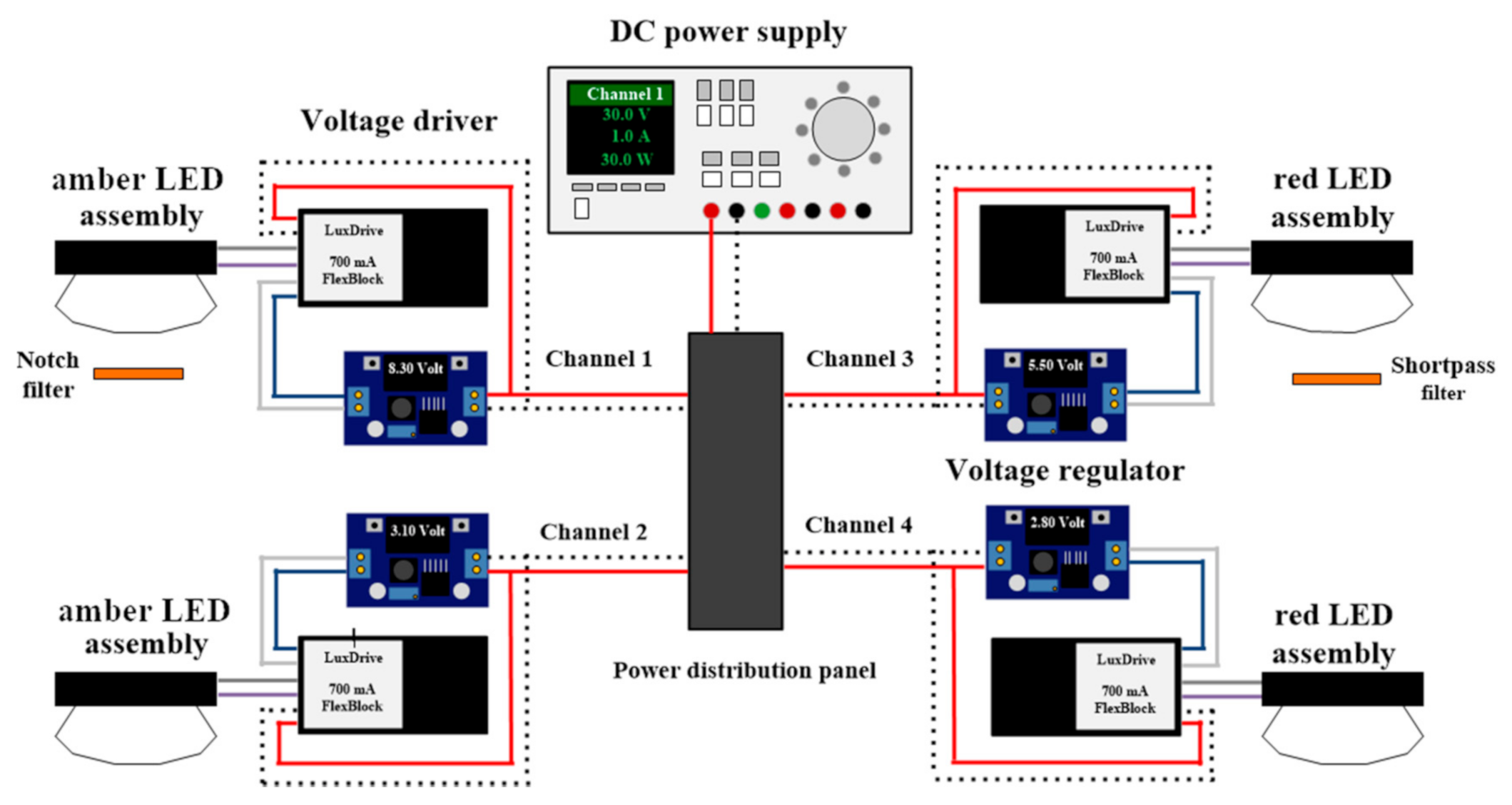
2.2. LED Lighting System
2.3. Whole Plant Photosynthetic Rate Analyses
2.4. Experimental Setup
2.5. Biomass Yield and Growth Parameter Analysis
2.6. Statistical Analysis
3. Results
3.1. LED Spectra in the 590-nm to 630-nm Range Obtained with Optical Filters
3.2. Plant Growth and Morphology
3.3. Photosynthetic Rates and Biomass Yield
4. Discussion
4.1. Photosynthetic Rate and Biomass Yield
4.2. The Impact of Light Wavelegnth and Bandwidth on Biomass Production
4.3. Plant Architecture and Leaf Coloration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dou, H.; Niu, G. Plant responses to light. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar]
- McCree, K. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Bula, R.; Morrow, R.; Tibbitts, T.; Barta, D.; Ignatius, R.; Martin, T. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Brazaityte, A.; Ulinskaite, R.; Duchovskis, P.; Samuoliene, G.; Siksnianiene, J.; Jankauskiene, J.; Sabajeviene, G.; Baranauskis, K.; Staniene, G.; Tamulaitis, G. Optimization of lighting spectrum for photosynthetic system and productivity of lettuce by using light-emitting diodes. Acta Hortic. 2006, 711, 183. [Google Scholar] [CrossRef]
- Goins, G.D.; Ruffe, L.M.; Cranston, N.A.; Yorio, N.C.; Wheeler, R.M.; Sager, J.C. Salad Crop Production under Different Wavelengths of Red Light-Emitting Diodes (Leds); 0148-7191; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in leaves of cabbage seedlings. In Proceedings of the VI International Symposium on Light in Horticulture 907, Tsukuba, Japan, 15 November 2011; pp. 179–184. [Google Scholar]
- Mitchell, C.A. Academic research perspective of LEDs for the horticulture industry. HortScience 2015, 50, 1293–1296. [Google Scholar] [CrossRef]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lefsrud, M.G.; Kopsell, D.A.; Sams, C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Stutte, G.W.; Edney, S.; Skerritt, T. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. [Google Scholar] [CrossRef]
- Gómez, C.; Morrow, R.C.; Bourget, C.M.; Massa, G.D.; Mitchell, C.A. Comparison of intracanopy light-emitting diode towers and overhead high-pressure sodium lamps for supplemental lighting of greenhouse-grown tomatoes. HortTechnology 2013, 23, 93–98. [Google Scholar] [CrossRef]
- Martineau, V.; Lefsrud, M.; Naznin, M.T.; Kopsell, D.A. Comparison of light-emitting diode and high-pressure sodium light treatments for hydroponics growth of Boston lettuce. HortScience 2012, 47, 477–482. [Google Scholar] [CrossRef]
- Bergstrand, K.-J.; Schüssler, H. Growth, development and photosynthesis of some horticultural plants as affected by different supplementary lighting technologies. Eur. J. Hortic. Sci. 2013, 119–125. [Google Scholar]
- Dueck, T.; Janse, J.; Eveleens, B.; Kempkes, F.; Marcelis, L. Growth of tomatoes under hybrid LED and HPS lighting. In Proceedings of the International Symposium on Advanced Technologies and Management Towards Sustainable Greenhouse Ecosystems: Greensys2011 952, Chalkidiki, Greece, 5–11 June 2011; pp. 335–342. [Google Scholar]
- Gajc-Wolska, J.; Kowalczyk, K.; Metera, A.; Mazur, K.; Bujalski, D.; Hemka, L. Effect of supplementary lighting on selected physiological parameters and yielding of tomato plants. Folia Hortic. 2013, 25, 153–159. [Google Scholar] [CrossRef]
- Dougher, T.A.; Bugbee, B. Evidence for yellow light suppression of lettuce growth. Photochem. Photobiol. 2001, 73, 208–212. [Google Scholar] [CrossRef]
- Vänninen, I.; Pinto, D.; Nissinen, A.; Johansen, N.; Shipp, L. In the light of new greenhouse technologies: 1. Plant-mediated effects of artificial lighting on arthropods and tritrophic interactions. Ann. Appl. Biol. 2010, 157, 393–414. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Yeh, N.G.; Wu, C.-H.; Cheng, T.C. Light-emitting diodes—Their potential in biomedical applications. Renew. Sustain. Energy Rev. 2010, 14, 2161–2166. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Wu, B.-S.; Hitti, Y.; MacPherson, S.; Orsat, V.; Lefsrud, M.G. Comparison and perspective of conventional and LED lighting for photobiology and industry applications. Environ. Exp. Bot. 2019, 171, 103953. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Harbinson, J.; Van Ieperen, W. Light distribution in leaf chambers and its consequences for photosynthesis measurements. Photosynthetica 2010, 48, 219–226. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Barnes, C.; Tibbitts, T.; Sager, J.; Deitzer, G.; Bubenheim, D.; Koerner, G.; Bugbee, B. Accuracy of quantum sensors measuring yield photon flux and photosynthetic photon flux. HortScience 1993, 28, 1197–1200. [Google Scholar] [CrossRef]
- Langhans, R.W.; Tibbitts, T. Plant Growth Chamber Handbook; Project NCR-101; Iowa State University: Ames, IA, USA, 1997. [Google Scholar]
- Arsovski, A.A.; Galstyan, A.; Guseman, J.M.; Nemhauser, J.L. Photomorphogenesis. Arab. Book 2012, 10, e0147. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.C.; Giger, W., Jr. Re-evaluation of published data on the relative photosynthetic efficiency of intermittent and continuous light. Agric. Meteorol. 1980, 22, 289–302. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-n.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Heo, J.; Lee, C.; Chakrabarty, D.; Paek, K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Evers, J.B.; Huth, N.I.; Renton, M. Light extinction in spring wheat canopies in relation to crop configuration and solar angle. In Proceedings of the Plant Growth Modeling And Applications, Washigton, DC, USA, 9–13 November 2009; pp. 107–110. [Google Scholar]
- Yavari, N.; Tripathi, R.; Wu, B.-S.; MacPherson, S.; Singh, J.; Lefsrud, M. The effect of light quality on plant physiology, photosynthetic, and stress response in Arabidopsis thaliana leaves. PLoS ONE 2021, 16, e0247380. [Google Scholar] [CrossRef] [PubMed]
- Britz, S.J. Photoregulation of root: Shoot ratio in soybean seedlings. Photochem. Photobiol. 1990, 52, 151–159. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Vogelmann, T.C. Do changes in light direction affect absorption profiles in leaves? Funct. Plant Biol. 2010, 37, 403–412. [Google Scholar] [CrossRef]
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. Green light drives CO2 fixation deep within leaves. Plant Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Pocock, T. Light-emitting diodes and the modulation of specialty crops: Light sensing and signaling networks in plants. HortScience 2015, 50, 1281–1284. [Google Scholar] [CrossRef]
- Casal, J.J. Shade avoidance. Arab. Book 2012, 10, e0157. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Metallo, R.M.; Kopsell, D.A.; Sams, C.E.; Bumgarner, N.R. Influence of blue/red vs. white LED light treatments on biomass, shoot morphology, and quality parameters of hydroponically grown kale. Sci. Hortic. 2018, 235, 189–197. [Google Scholar] [CrossRef]
- Mickens, M.; Torralba, M.; Robinson, S.; Spencer, L.; Romeyn, M.; Massa, G.; Wheeler, R. Growth of red pak choi under red and blue, supplemented white, and artificial sunlight provided by LEDs. Sci. Hortic. 2019, 245, 200–209. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Shimizu, H.; Saito, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Light environment optimization for lettuce growth in plant factory. Ifac Proc. Vol. 2011, 44, 605–609. [Google Scholar] [CrossRef]
- Wu, H.-C.; Lin, C.-C. Red light-emitting diode light irradiation improves root and leaf formation in difficult-to-propagate Protea cynaroides L. plantlets in vitro. HortScience 2012, 47, 1490–1494. [Google Scholar] [CrossRef]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.-R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Krueger, C.G.; Johnson, W.D.; Raskin, I. Development and phytochemical characterization of high polyphenol red lettuce with anti-diabetic properties. PLoS ONE 2014, 9, e91571. [Google Scholar] [CrossRef]
- Maier, A.; Hoecker, U. COP1/SPA ubiquitin ligase complexes repress anthocyanin accumulation under low light and high light conditions. Plant Signal. Behav. 2015, 10, e970440. [Google Scholar] [CrossRef]
- Landi, M.; Agati, G.; Fini, A.; Guidi, L.; Sebastiani, F.; Tattini, M. Unveiling the shade nature of cyanic leaves: A view from the “blue absorbing side” of anthocyanins. PlantCell Environ. 2021, 44, 1119–1129. [Google Scholar] [CrossRef]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. A review of strawberry photobiology and fruit flavonoids in controlled environments. Front. Plant Sci. 2021, 12, 611893. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Owen, W.G.; Lopez, R.G. End-of-production supplemental lighting with red and blue light-emitting diodes (LEDs) influences red pigmentation of four lettuce varieties. HortScience 2015, 50, 676–684. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
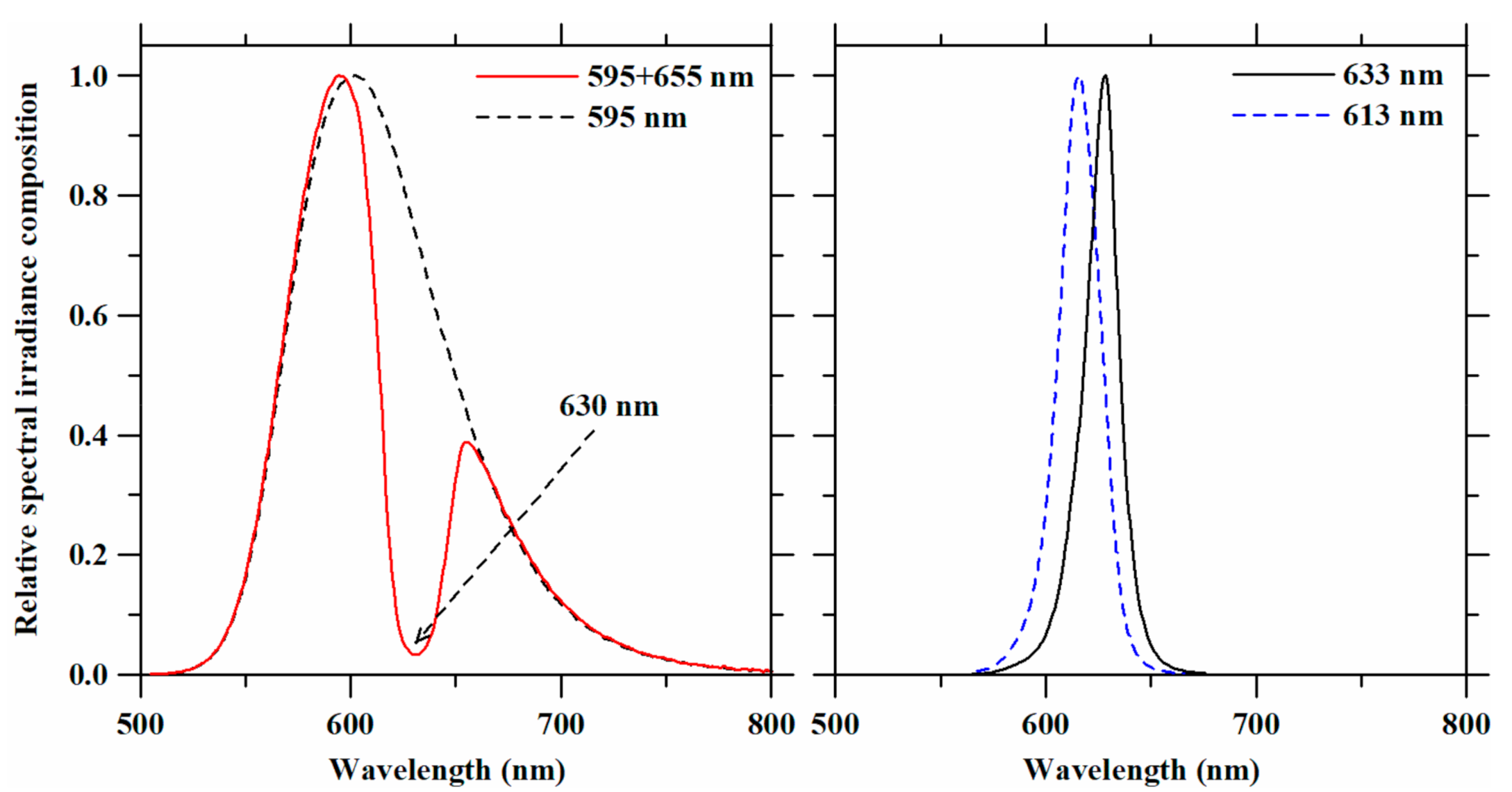


| Light Treatments | Peak Wavelength (nm) | FWHM (nm) | Irradiance Level (W·m−2) | PPFD (µmol·m−2·s−1) | YPF (µmol·m−2·s−1) | |
|---|---|---|---|---|---|---|
| 595 + 655 nm | 1st peak | 595 | 48.90 | 50 (37.5 + 12.5) | 250 (187.5 + 62.5) | 240 |
| 2nd peak | 655 | 41.44 | ||||
| 595 nm | 602 | 74.07 | 50 | 256 | 242 | |
| 633 nm | 633 | 20.24 | 50 | 267 | 257 | |
| 613 nm | 613 | 22.12 | 50 | 243 | 239 | |
| LED Treatment | Photosynthetic Rate (μmol CO2 m−2 sec−1) | Shoot Fresh Mass (g) | Shoot Dry Mass (g) | Leaf Area (cm2) |
|---|---|---|---|---|
| 595 + 655 nm | 2.66 ± 0.16 ab | 27.16 ± 2.98 c | 1.16 ± 0.10 e | 419.5 ± 115.34 g |
| 595 nm | 2.87 ± 0.08 a | 27.12 ± 2.68 c | 1.16 ± 0.11 e | 400.7 ± 66.25 g |
| 633 nm | 2.34 ± 0.17 ab | 11.99 ± 1.54 d | 0.50 ± 0.08 f | 220.3 ± 45.13 h |
| 613 nm | 2.31 ± 0.02 b | 5.76 ± 1.75 d | 0.25 ± 0.08 f | 136.6 ± 22.04 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.-S.; MacPherson, S.; Lefsrud, M. Filtering Light-Emitting Diodes to Investigate Amber and Red Spectral Effects on Lettuce Growth. Plants 2021, 10, 1075. https://doi.org/10.3390/plants10061075
Wu B-S, MacPherson S, Lefsrud M. Filtering Light-Emitting Diodes to Investigate Amber and Red Spectral Effects on Lettuce Growth. Plants. 2021; 10(6):1075. https://doi.org/10.3390/plants10061075
Chicago/Turabian StyleWu, Bo-Sen, Sarah MacPherson, and Mark Lefsrud. 2021. "Filtering Light-Emitting Diodes to Investigate Amber and Red Spectral Effects on Lettuce Growth" Plants 10, no. 6: 1075. https://doi.org/10.3390/plants10061075
APA StyleWu, B.-S., MacPherson, S., & Lefsrud, M. (2021). Filtering Light-Emitting Diodes to Investigate Amber and Red Spectral Effects on Lettuce Growth. Plants, 10(6), 1075. https://doi.org/10.3390/plants10061075






