24-Epibrasinolide Modulates the Vase Life of Lisianthus Cut Flowers by Modulating ACC Oxidase Enzyme Activity and Physiological Responses
Abstract
1. Introduction
2. Results
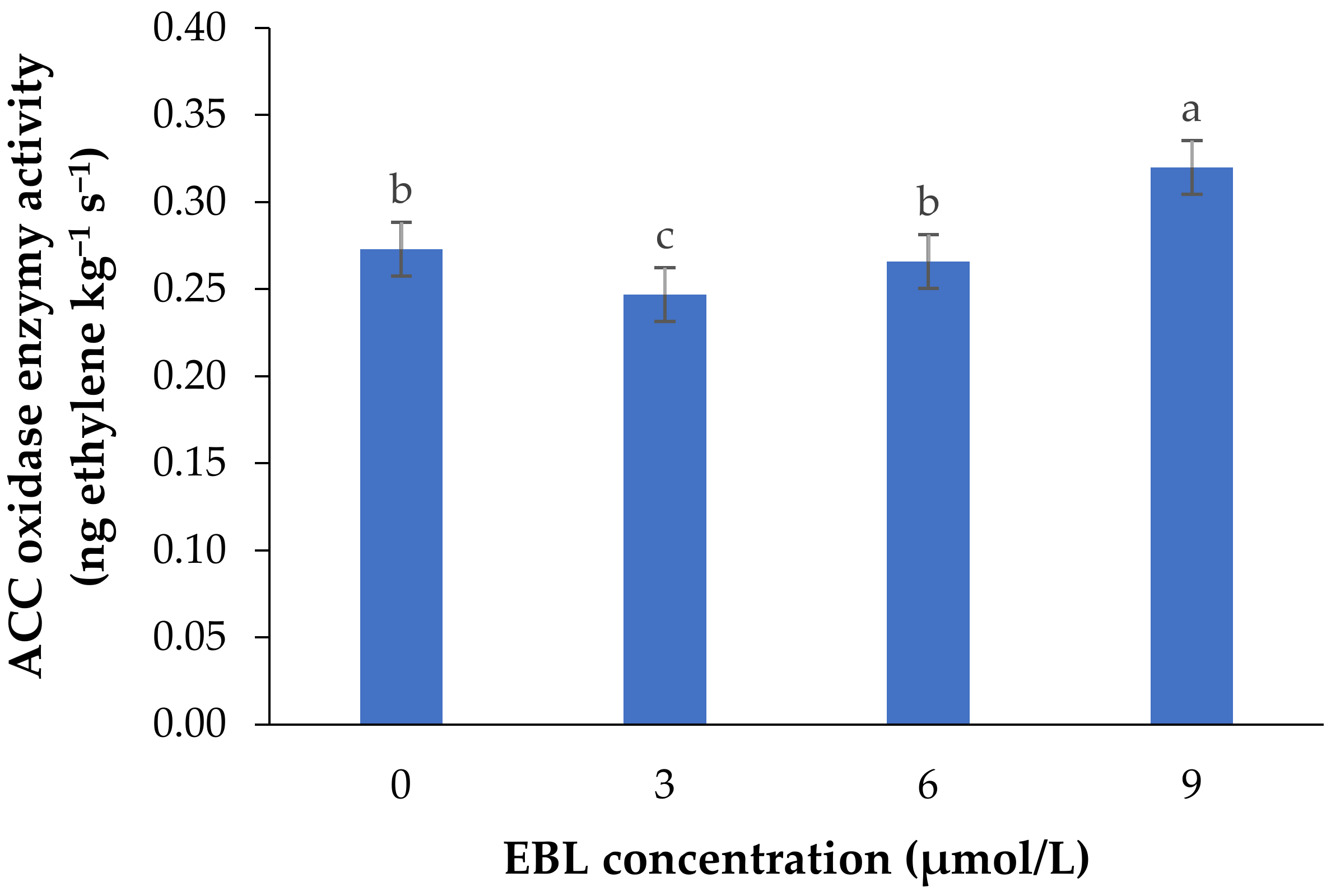
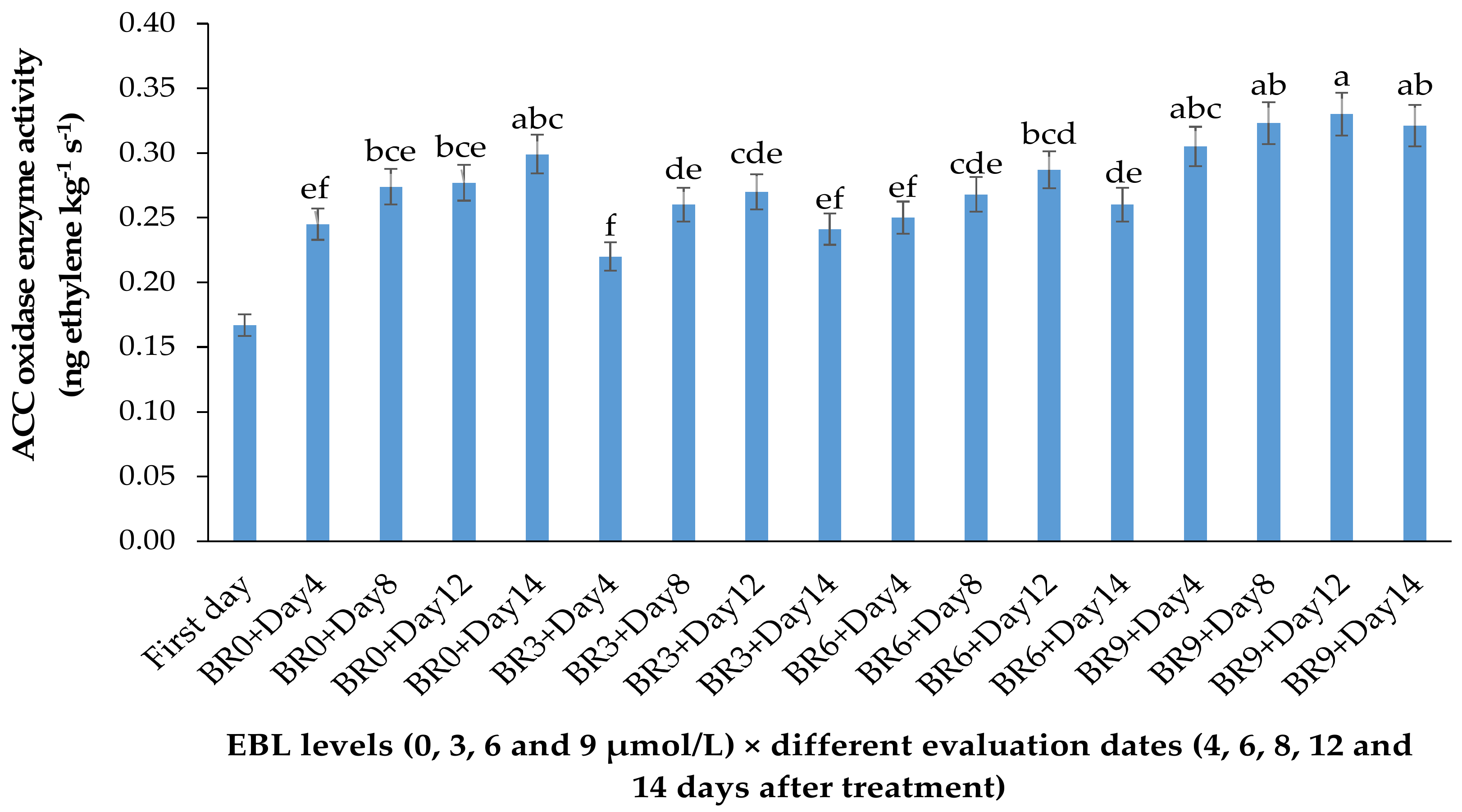
2.1. ACO Enzyme Activity
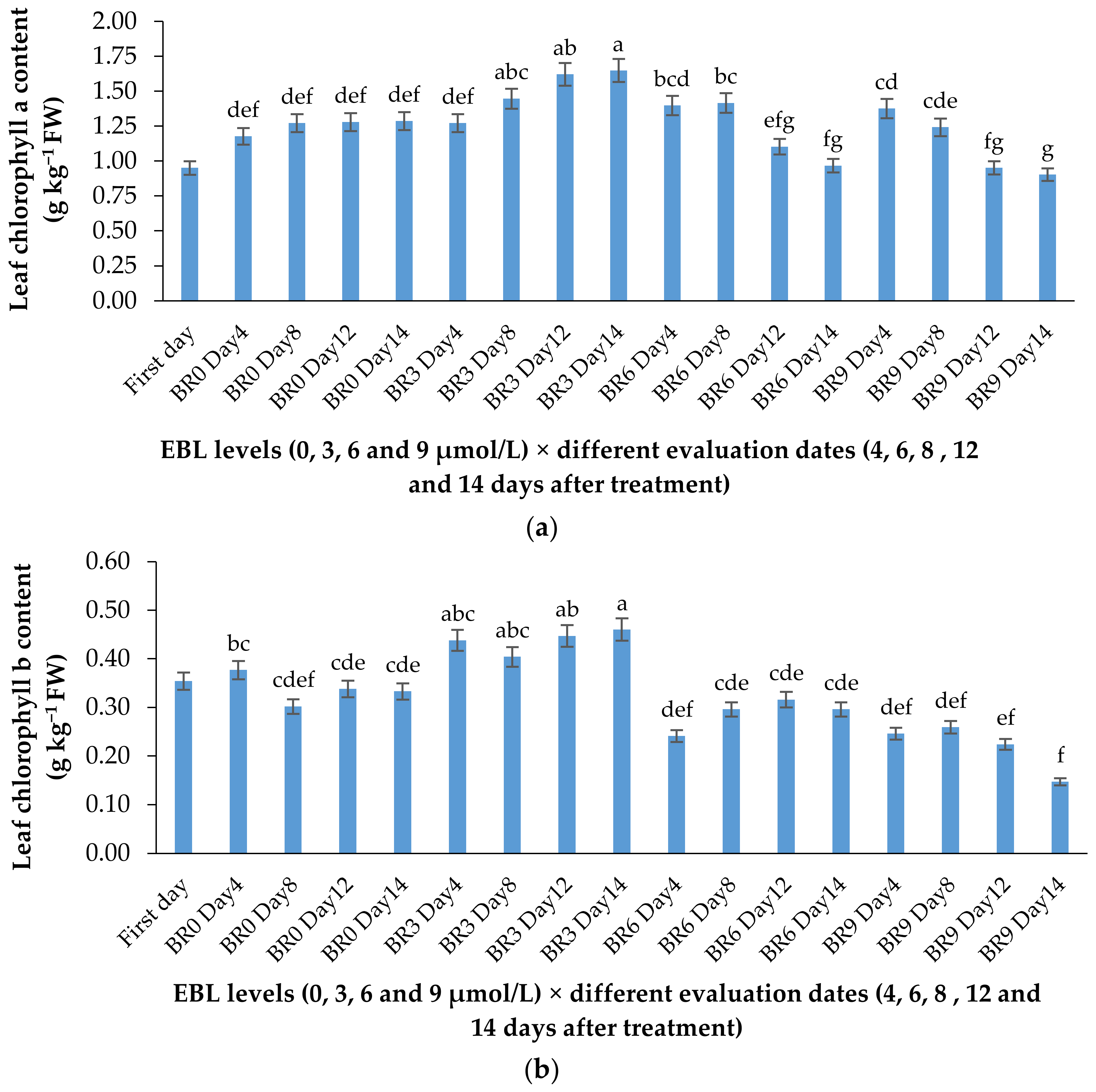
2.2. Chlorophyll a and b Content
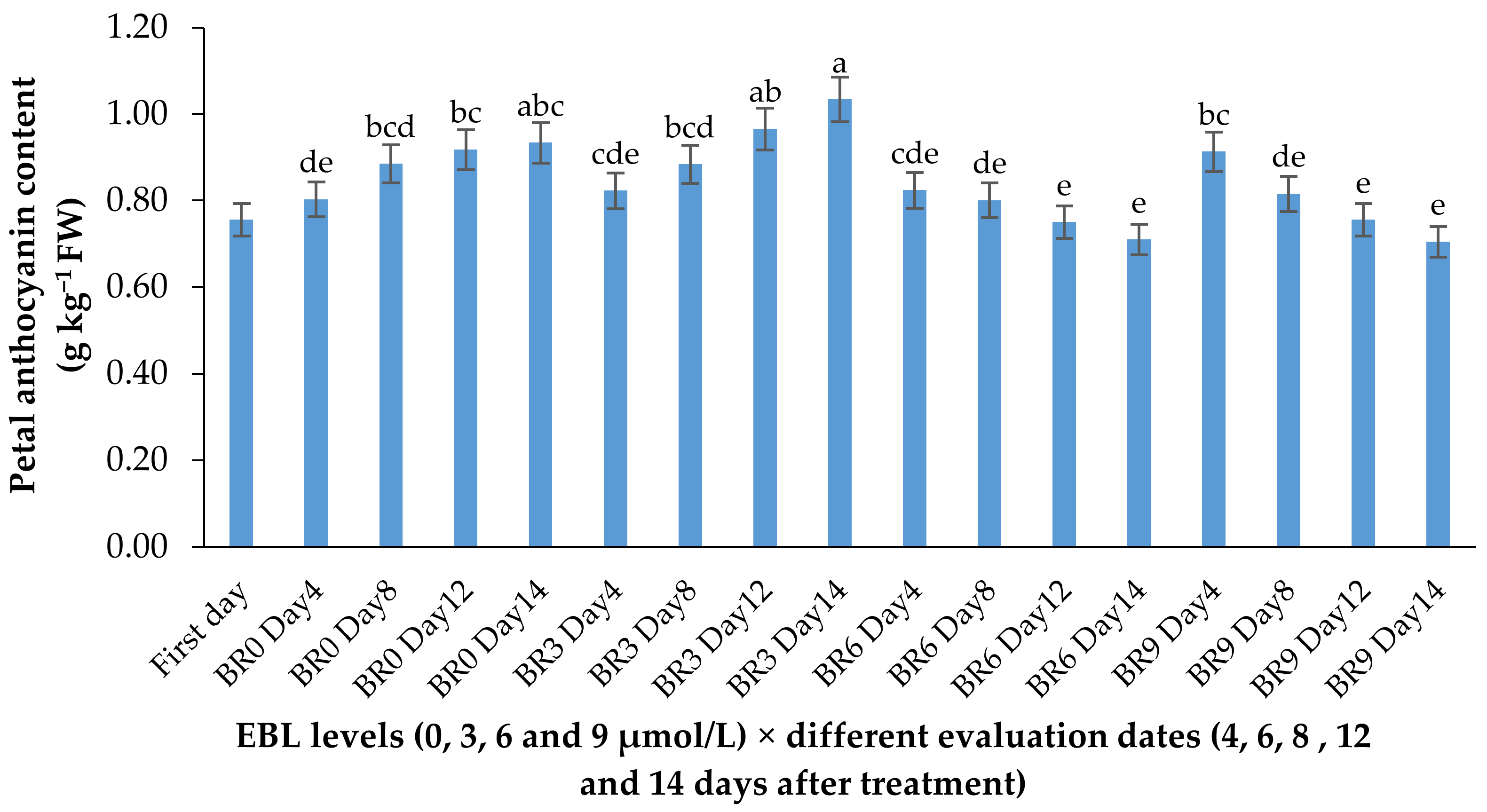
2.3. Anthocyanin Content
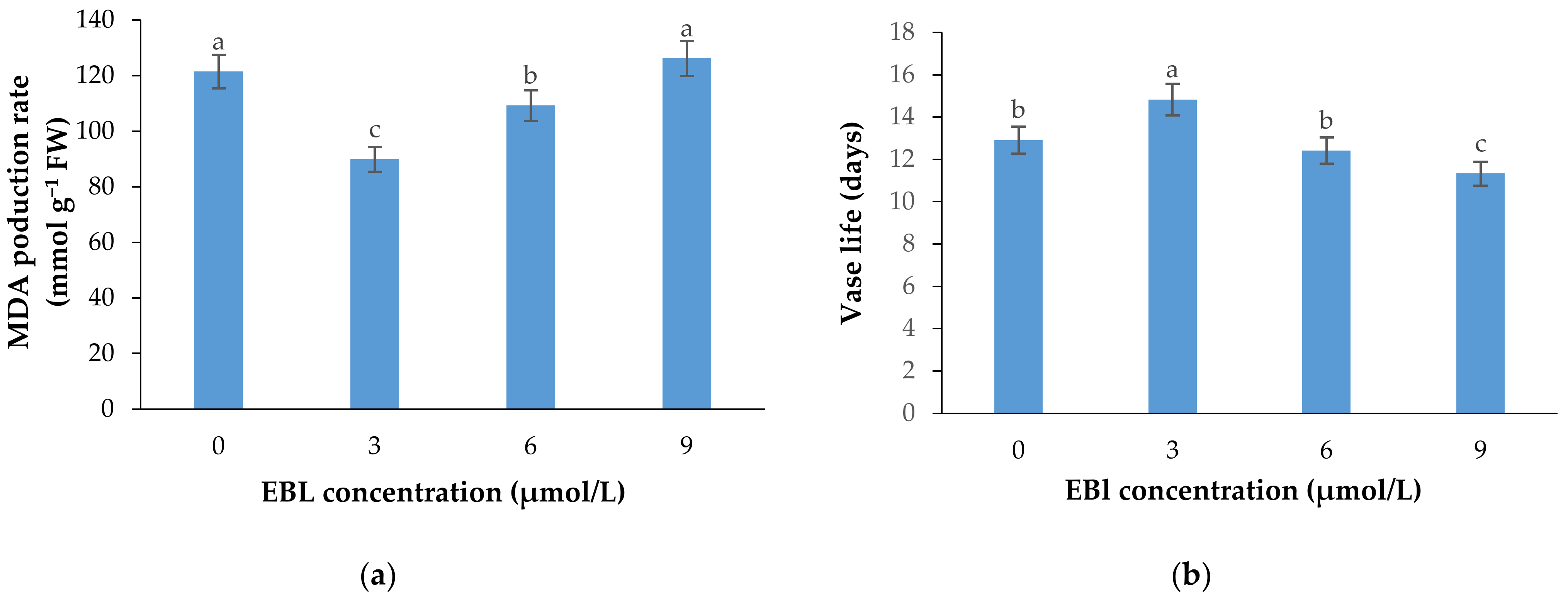
2.4. MDA Production Rate
2.5. Flower Vase Life
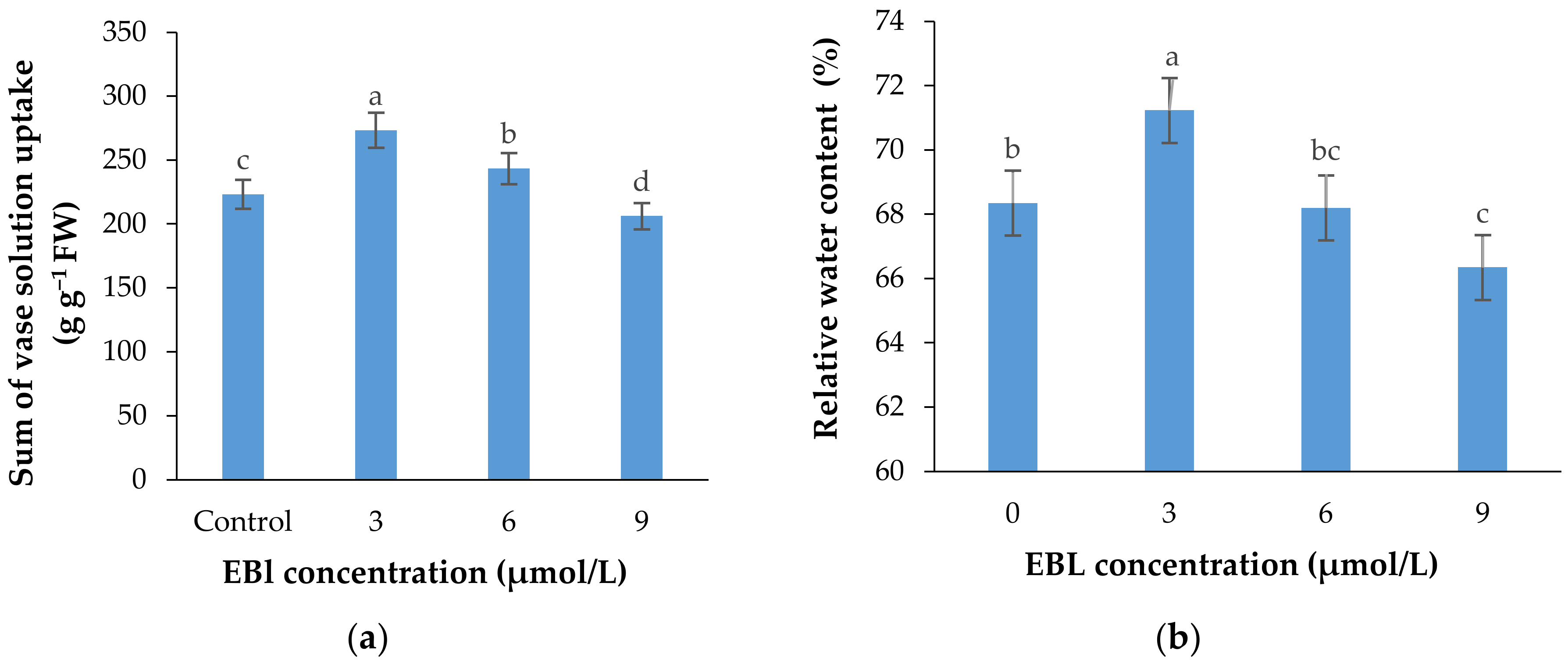
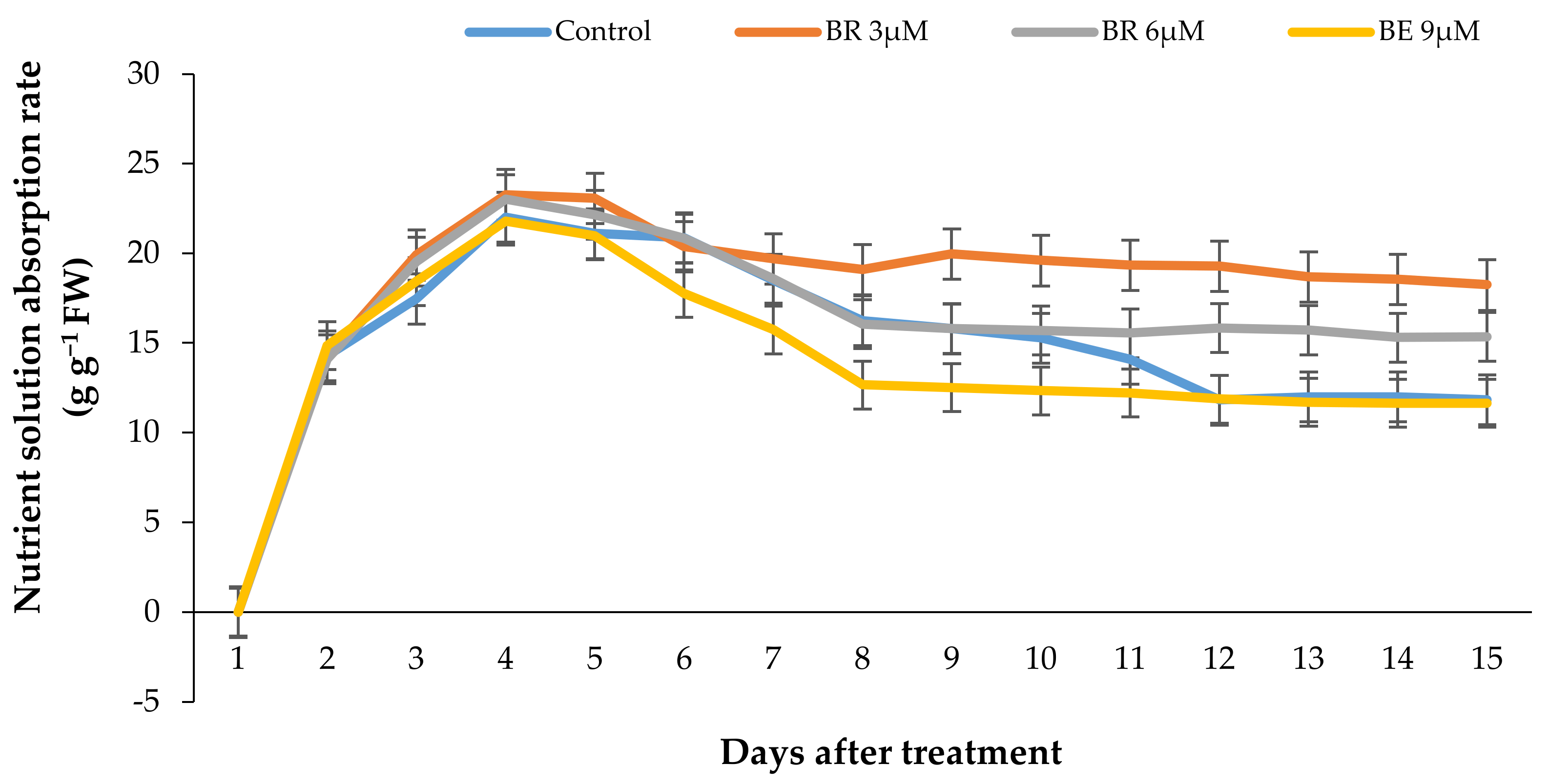
2.6. Vase Solution Uptake Rate (VSU)
2.7. Relative Water Content
3. Discussion
4. Materials and Methods
4.1. Cut Flower Preparation and Treatments
4.2. Treatment of the Flowers with EBL
4.3. ACC Oxidase Activity Assay
4.4. Chlorophyll Content Evaluation
4.5. Determination of Anthocyanin Content
4.6. MDA Production Rate
4.7. Determining the Flower Vase Life
4.8. Vase Solution Uptake Rate (VSU)
4.9. Relative Water Content
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahrami, S.N.; Zakizadeh, H.; Hamidoghli, Y.; Ghasemnezhad, M. Salicylic acid retards petal senescence in cut lisianthus (Eustoma grandiflorum ‘Miarichi Grand White’) flowers. Hortic. Environ. Biotechnol. 2013, 54, 519–523. [Google Scholar] [CrossRef]
- Harbaugh, B.K. Lisianthus (Eustoma grandiflorum). In Flower Breeding and Genetics Issues, Challenges and Opportunities for the 21st Century; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 644–663. [Google Scholar]
- Kazemi, M.; Asadi, M.; Aghdasi, S. Postharvest life of cut lisianthus flowers as affected by silicon, malic acid and acetylsalicylic acid. Res. J. Soil Biol. 2012, 4, 15–20. [Google Scholar] [CrossRef]
- De Beer, J.; Petersen, N. Post-harvest physiology of cut flowers: A problem-based, cooperative learning activity for the biology classroom. Am. Biol. Teach. 2017, 79, 578–583. [Google Scholar] [CrossRef]
- Su, J.; Nie, Y.; Zhao, G.; Cheng, D.; Wang, R.; Chen, J.; Zhang, S.; Shen, W. Endogenous hydrogen gas delays petal senescence and extends the vase life of lisianthus cut flowers. Postharvest Biol. Technol. 2019, 147, 148–155. [Google Scholar] [CrossRef]
- In, B.C.; Ha, S.T.T.; Lee, Y.S.; Lim, J.H. Relationships between the longevity, water relations, ethylene sensitivity, and gene expression of cut roses. Postharvest Biol. Technol. 2017, 131, 74–83. [Google Scholar] [CrossRef]
- Hurr, B.M.; Huber, D.J.; Vallejos, C.E. Features of programmed cell death precede watersoaking development in ethylene-treated immature cucumber fruit. Postharvest Biol. Technol. 2010, 58, 13–20. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Li, H.; Lin, X.; Lin, S.; Joyce, D.C.; He, S. Alleviation of effects of exogenous ethylene on cut ‘Master’ carnation flowers with nano-silver and silver thiosulfate. Postharvest Biol. Technol. 2018, 143, 86–91. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Asghari, M.; Zahedipour, P. 24-Epibrassinolide Acts as a Growth-Promoting and Resistance-Mediating Factor in Strawberry Plants. J. Plant Growth Regul. 2016, 35, 722–729. [Google Scholar] [CrossRef]
- Coll, Y.; Coll, F.; Amorós, A.; Pujol, M. Brassinosteroids roles and applications: An up-date. Biologia 2015, 70, 726–732. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Bajguz, A.; Zhou, J.; Bhardwaj, R. Analysis of Brassinosteroids in Plants. J. Plant Growth Regul. 2017, 36, 1002–1030. [Google Scholar] [CrossRef]
- Asghari, M.; Rezaei-Rad, R. 24-Epibrassinolide enhanced the quality parameters and phytochemical contents of table grape. J. Appl. Bot. Food Qual. 2018, 91, 226–231. [Google Scholar]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef]
- Symons, G.M.; Davies, C.; Shavrukov, Y.; Dry, I.B.; Reid, J.B.; Thomas, M.R. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 2006, 140, 150–158. [Google Scholar] [CrossRef]
- Luan, L.Y.; Zhang, Z.W.; Xi, Z.M.; Huo, S.S.; Ma, L.N. Brassinosteroids regulate anthocyanin biosynthesis in the ripening of grape berries. South Afr. J. Enol. Vitic. 2013, 34, 196–203. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Ban, Q.; Han, S.; Rao, J. Role of Brassinosteroids in Persimmon (Diospyros kaki L.) Fruit Ripening. J. Agric. Food Chem. 2018, 66, 2637–2644. [Google Scholar] [CrossRef]
- Zhu, T.; Tan, W.R.; Deng, X.G.; Zheng, T.; Zhang, D.W.; Lin, H.H. Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biol. Technol. 2015, 100, 196–204. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Z.; Qin, G.; Tian, S. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2010, 56, 50–55. [Google Scholar] [CrossRef]
- Hubert, O.; Mbéguié-A-Mbéguié, D. Expression patterns of ethylene biosynthesis genes from bananas during fruit ripening and in relationship with finger drop. AoB Plants 2012, 2012, pls041. [Google Scholar] [CrossRef]
- Yin, X.R.; Xie, X.L.; Xia, X.J.; Yu, J.Q.; Ferguson, I.B.; Giovannoni, J.J.; Chen, K.S. Involvement of an ethylene response factor in chlorophyll degradation during citrus fruit degreening. Plant J. 2016, 86, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.P.; Yu, J.Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Benefits of brassinosteroid crosstalk. Trends Plant Sci. 2012, 17, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Shimizu-Yumoto, H.; Ichimura, K. Combination pulse treatment of 1-naphthaleneacetic acid and aminoethoxyvinylglycine greatly improves postharvest life in cut Eustoma flowers. Postharvest Biol. Technol. 2010, 56, 104–107. [Google Scholar] [CrossRef]
- Zheng, J.; An, Y.; Wang, L. 24-Epibrassinolide enhances 5-ALA-induced anthocyanin and flavonol accumulation in calli of ‘Fuji’ apple flesh. Plant Cell Tissue Organ Cult. 2018, 134, 319–330. [Google Scholar] [CrossRef]
- Xi, Z.M.; Zhang, Z.W.; Huo, S.S.; Luan, L.Y.; Gao, X.; Maa, L.N.; Fang, Y.L. Regulating the secondary metabolism in grape berry using exogenous 24-epibrassinolide for enhanced phenolics content and antioxidant capacity. Food Chem. 2013, 141, 3056–3065. [Google Scholar] [CrossRef]
- Yuan, L.B.; Peng, Z.H.; Zhi, T.T.; Zho, Z.; Liu, Y.; Zhu, Q.; Xiong, X.Y.; Ren, C.M. Brassinosteroid enhances cytokinin-induced anthocyanin biosynthesis in Arabidopsis seedlings. Biol. Plant. 2015, 59, 99–105. [Google Scholar] [CrossRef]
- Fernández-Maculet, J.C.; Yang, S.F. Extraction and partial characterization of the ethylene-forming enzyme from apple fruit. Plant Physiol. 1992, 99, 751–754. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Wagner, G.J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979, 64, 88–93. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Asrar, A.W.A. Effects of some preservative solutions on vase life and keeping quality of snapdragon (Antirrhinum majus L.) cut flowers. J. Saudi Soc. Agric. Sci. 2012, 11, 29–35. [Google Scholar] [CrossRef]
- Damunupola, J.W. Xylem Flow in Cut Acacia Holosericea Stems; University of Queensland: Brisbane, Australia, 2009. [Google Scholar]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Reigosa Roger, M.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]

| Mean Squares | |||||||
|---|---|---|---|---|---|---|---|
| ACC Oxidase Activity (ng ethylene kg−1 s−1) | Chlorophyll a (g kg−1 FW) | Chlorophyll b (g kg−1 FW) | Anthocyanin Content (g kg−1 FW) | MDA Production Rate (mmol g−1 FW) | Vase Life (Days) | Relative Water Content (%) | |
| EBL | 0.350 ** | 0.727 ** | 0.215 ** | 0.054 ** | 4962.530 ** | 116.27 ** | 102.848 ** |
| Vase life duration | 0.070 ** | 0.19 ** | 0.008 ** | 0.002 ns | 2836.572 ** | 0.727 ** | 1115.706 ** |
| EBL × VLD | 0.018 ns | 0.21 ** | 0.015 ** | 0.027 ** | 236.571 ns | 0.16 ** | 15.85 ** |
| Error | 0.015 | 0.016 | 0.002 | 0.009 | 145.092 | 0.29 | 23.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darvish, M.; Shirzad, H.; Asghari, M.; Noruzi, P.; Alirezalu, A.; Pateiro, M.; Takshe, A.; Lorenzo, J.M. 24-Epibrasinolide Modulates the Vase Life of Lisianthus Cut Flowers by Modulating ACC Oxidase Enzyme Activity and Physiological Responses. Plants 2021, 10, 995. https://doi.org/10.3390/plants10050995
Darvish M, Shirzad H, Asghari M, Noruzi P, Alirezalu A, Pateiro M, Takshe A, Lorenzo JM. 24-Epibrasinolide Modulates the Vase Life of Lisianthus Cut Flowers by Modulating ACC Oxidase Enzyme Activity and Physiological Responses. Plants. 2021; 10(5):995. https://doi.org/10.3390/plants10050995
Chicago/Turabian StyleDarvish, Mohammad, Habib Shirzad, Mohammadreza Asghari, Parviz Noruzi, Abolfazl Alirezalu, Mirian Pateiro, Aseel Takshe, and José Manuel Lorenzo. 2021. "24-Epibrasinolide Modulates the Vase Life of Lisianthus Cut Flowers by Modulating ACC Oxidase Enzyme Activity and Physiological Responses" Plants 10, no. 5: 995. https://doi.org/10.3390/plants10050995
APA StyleDarvish, M., Shirzad, H., Asghari, M., Noruzi, P., Alirezalu, A., Pateiro, M., Takshe, A., & Lorenzo, J. M. (2021). 24-Epibrasinolide Modulates the Vase Life of Lisianthus Cut Flowers by Modulating ACC Oxidase Enzyme Activity and Physiological Responses. Plants, 10(5), 995. https://doi.org/10.3390/plants10050995