Increased Plant Quality, Greenhouse Productivity and Energy Efficiency with Broad-Spectrum LED Systems: A Case Study for Thyme (Thymus vulgaris L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Biomass Yield, Partitioning, and Morphology
2.2. Content and Composition of Volatile Organic Compounds (VOCs)
2.3. Productivity
2.4. Power Consumption and Biomass Efficiacy
3. Materials and Methods
3.1. Experimental Design
3.2. Lighting Systems and Illumination Conditions
3.3. Irradiance Profile Measurements
3.4. Plant Material and Growth Conditions
3.5. Harvest and Crop Managements
3.6. Energy Measurements
3.7. Chemicals
3.8. Extraction of Volatile Organic Compounds
3.9. GC-FID and GC-MS Analysis
3.10. Identification and Quantification of Volatile Organic Compounds
3.11. Statistical Analysis and Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faust, J.E.; Logan, J. Daily light integral: A research review and high-resolution maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Korczynski, P.C.; Logan, J.; Faust, J.E. Mapping monthly distribution of daily light integrals across the contiguous United States. HortTechnology 2002, 12, 12–16. [Google Scholar] [CrossRef]
- Pramuk, L.A.; Runkle, E.S. Photosynthetic daily light integral during the seedling stage influences subsequent growth and flowering of Celosia, Impatiens, Salvia, Tagetes and Viola. HortScience 2005, 40, 1336–1339. [Google Scholar] [CrossRef]
- Kong, Y.; Llewellyn, D.; Zheng, Y. Response of growth, yield, and quality of pea shoots to supplemental light-emitting diode lighting during winter greenhouse production. Can. J. Plant Sci. 2017, 98, 732–740. [Google Scholar] [CrossRef]
- Matysiak, B.; Kowalski, A. White, blue and red LED lighting on growth, morphology and accumulation of flavonoid compounds in leafy greens. Zemdirbyste 2019, 106, 281–286. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Economic analysis of greenhouse lighting: Light emitting diodes vs. high intensity discharge fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn-Bilodeau, S.; Wu, B.S.; Rufyikiri, A.S.; MacPherson, S.; Lefsrud, M. An update on plant photobiology and implications for cannabis production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Martineau, V.; Lesrud, M.; Naznin, M.T.; Kopsell, D.A. Comparison of light-emitting diode and high-pressure sodium light treatments for hydroponics growth of boston lettuce. HortScience 2012, 47, 477–482. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Dlugosz-Grochowska, O.; Kolton, A.; Żupnik, M. Effects of LED supplemental lighting on yield and some quality parameters of lamb’s lettuce grown in two winter cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Park, J.E.; Park, Y.G.; Jeong, B.R.; Hwang, S.J. Growth and Anthocyanin content of lettuce as affected by artificial light source and photoperiod in a closed-type plant production system. Korean J. Hortic. Sci. Technol. 2012, 30, 673–679. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-emitting diodes in horticulture. In Horticultural Reviews, 1st ed.; Janick, J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; Volume 43. [Google Scholar]
- McCree, K.J. The action spectrum absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo-Lewandowski, M.M.; Brown, C.S. Life cycle experiments with Arabidopsis grown under red light-emitting diodes (LEDs). Life Support Biosph. Sci. 1998, 5, 143–149. [Google Scholar] [PubMed]
- Hoenecke, M.E.; Bula, R.J.; Tibbitts, T.W. Importance of ‘blue’ photon levels for lettuce seedlings grown under red-light-emitting diodes. HortScience 1992, 27, 427–430. [Google Scholar] [CrossRef]
- Ahmad, M.; Grancher, N.; Heil, M.; Black, R.C.; Giovani, B.; Galland, P.; Lardemer, D. Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol. 2002, 129, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Spalding, E.P. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 2001, 26, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Hernandez, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Stutte, G.W.; Edney, S. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Schenkels, L.; Saeys, W.; Lauwers, A.; De Proft, M.P. Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Sci. Hortic. 2020, 261, 109002. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-W.; Chung, H.-Y.; Chang, M.-Y.; Fang, W. The contribution of different spectral sections to increase fresh weight of boston lettuce. HortScience 2015, 50, 1006–1010. [Google Scholar] [CrossRef]
- Novičkovas, A.; Brazaitytė, A.; Duchovskis, P.; Jankauskienė, J.; Samuolienė, G.; Virŝilė, A.; Sirtautas, R.; Bliznikas, Z.; Žukauskas, A. Solid-state lamps (LEDs) for the short-wavelength supplementary lighting in greenhouses: Experimental results with cucumber. Acta Hortic. 2012, 927, 723–730. [Google Scholar] [CrossRef]
- Folta, K.M. Green light stimulates early stem elongation, antagonizing light-mediated growth inhibition. Plant Physiol. 2004, 135, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Frechilla, S.; Talbott, L.D.; Bogomolni, R.A.; Zeiger, E. Reversal of blue light-stimulated stomatal opening by green light. Plant Cell Physiol. 2000, 41, 171–176. [Google Scholar] [CrossRef]
- Talbott, L.D.; Nikolova, G.; Ortiz, A.; Shayevich, I.; Zeiger, E. Green light reversal of blue-light-stimulated stomatal opening is found in a diversity of plant species. Am. J. Bot. 2002, 89, 366–368. [Google Scholar] [CrossRef]
- Gomez, C.; Izzo, L.G. Increasing efficiency of crop production with LEDs. AIMS 2018, 3, 135–153. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Cope, K.R.; Bugbee, B. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. HortScience 2013, 48, 504–509. [Google Scholar] [CrossRef]
- Burattini, C.; Mattoni, B.; Bisegna, F. The impact of spectral composition of white LEDs on spinach (Spinacia oleracea) growth and development. Energies 2017, 10, 1383. [Google Scholar] [CrossRef]
- Spalholz, H.; Perkins-Veazie, P.; Hernández, R. Impact of sun-simulated white light and varied blue:red spectrums on the growth, morphology, development, and phytochemical content of green- and red-leaf lettuce at different growth stages. Sci. Hortic. 2020, 264, 109195. [Google Scholar] [CrossRef]
- Gao, S.; Liu, X.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Photosynthetic characteristics and chloroplast ultrastructure of welsh onion (Allium fistulosum L.) grown under different LED wavelengths. BMC Plant Biol. 2020, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Mickens, M.A.; Torralba, M.; Robinson, S.A.; Spencer, L.E.; Romeyn, W.M.; Massa, G.D.; Wheeler, R.M. Growth of red pak choi under red and blue, supplemented white, and artificial sunlight provided by LEDs. Sci. Hortic. 2019, 245, 200–209. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and plant community implication on essential oils composition in useful wild officinal species: A pilot case study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Craver, J.K.; Boldt, J.K.; Lopez, R.G. Comparison of supplemental lighting provided by high-pressure sodium lamps or light-emitting diodes for the propagation and finishing of bedding plants in a commercial greenhouse. HortScience 2019, 54, 52–59. [Google Scholar] [CrossRef]
- Faust, J.E. First Research Report. Light Management in Greenhouses. I. Daily light Integral: A Useful Tool for the U.S. Floriculture Industry. Available online: https://www.specmeters.com/assets/1/7/A051.pdf (accessed on 17 April 2020).
- Mathew, S. Phytochrome-mediated development in land plants: Red light sensing evolves to meet the challenges of changing light environments. Mol. Ecol. 2006, 15, 3482–3503. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Chalchat, J.C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. J. Chem. Ecol. 2003, 29, 859–880. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Gouinguene, S.P.; Turlings, T.C.J. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef]
- Spring, O.; Priester, T.; Hager, A. Light-induced accumulation of sesquiterpenes lactones in sunflower seedlings. J. Plant Physiol. 1986, 123, 79–89. [Google Scholar] [CrossRef]
- Gleizes, M.; Pauly, G.; Bernard-Dagan, C.; Jacques, P. Effects of light on terpene hydrocarbon synthesis in Pinus pinaster. Physiol. Plant. 1980, 50, 16–20. [Google Scholar] [CrossRef]
- Yamaura, T.; Tanaka, S.; Tabata, M. Participation of Phytochrome in the Photoregulation of Terpenoid Synthesis in Thyme Seedlings. Plant Cell Physiol. 1991, 32, 6. [Google Scholar] [CrossRef]
- Tanaka, S.; Yamaura, T.; Shigemoto, R.; Tabata, M. Phytochrome-mediated production of monoterpenes in thyme seedlings. Phytochemistry 1989, 28, 2955–2957. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Kegge, W.; Weldegergis, B.T.; Soler, R.; Vergeer-Van Eijk, M.; Dicke, M.; Voesenek, L.A.C.J.; Pierik, R. Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana. New Phytol. 2013, 200, 861–874. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Sabzalian, M.R. Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem. 2020, 307, 125521. [Google Scholar] [CrossRef] [PubMed]
- Poel, B.R.; Runkle, E.S. Seedling growth is similar under supplemental greenhouse lighting from high-pressure sodium lamps or light-emitting diodes. HortScience 2017, 52, 388–394. [Google Scholar] [CrossRef]
- Wallace, C.; Both, A.J. Evaluating operating characteristics of light sources for horticultural applications. Acta Hortic. 2016, 1134, 435–443. [Google Scholar] [CrossRef]
- Hernandez, E.; Hernandez, M.B.; Mattson, N.S. Quality, yield, and biomass efficacy of several hydroponic lettuce (Lactuca sativa L.) cultivars in response to high pressure sodium lights or light emitting diodes for greenhouse supplemental lighting. Horticulturae 2020, 6, 7. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red:blue ratio in LED lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- WetterKontor. Available online: https://www.wetterkontor.de/de/wetter/deutschland/rueckblick.asp?id=20&datum0=09.10.2018&datum1=31.12.2018&jr=2021&mo=3&datum=08.03.2021&t=8&part=0 (accessed on 10 March 2021).
- Yeh, N.; Chung, J.P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Barta, D.J.; Tibbitts, T.W.; Bula, R.J.; Morrow, R.C. Evaluation of light emitting diode characteristics for a space-based plant irradiation source. Adv. Space Res. 1992, 12, 141–149. [Google Scholar] [CrossRef]
- Van Iersel, M.W.; Gianino, D. An adaptive control approach for light-emitting diode lights can reduce the energy costs of supplemental lighting in greenhouses. HortScience 2017, 52, 72–77. [Google Scholar] [CrossRef]
- Harbick, K.; Albright, L.D.; Mattson, N.S. Electrical savings comparison of supplemental lighting control systems in greenhouse environments. In Proceedings of the American Society of Agricultural and Biological Engineers (ASABE) Annual International Meeting, Orlando, FL, USA, 17–20 July 2016. Paper Number 162460478. [Google Scholar] [CrossRef]
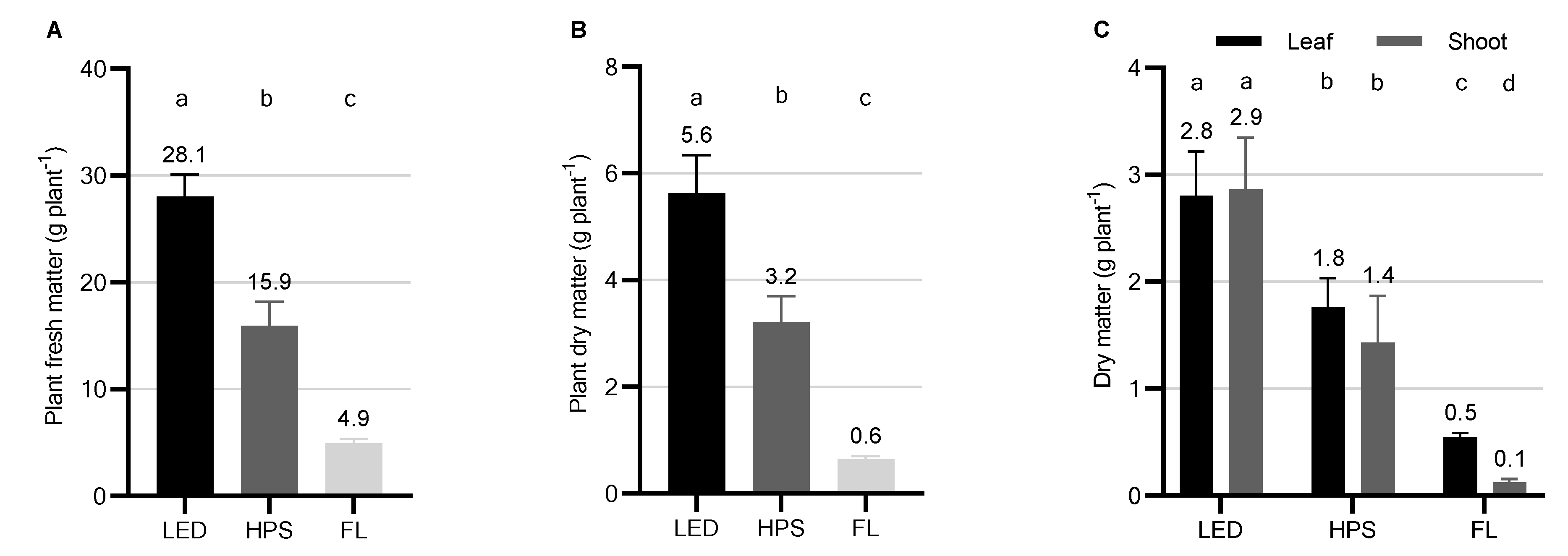
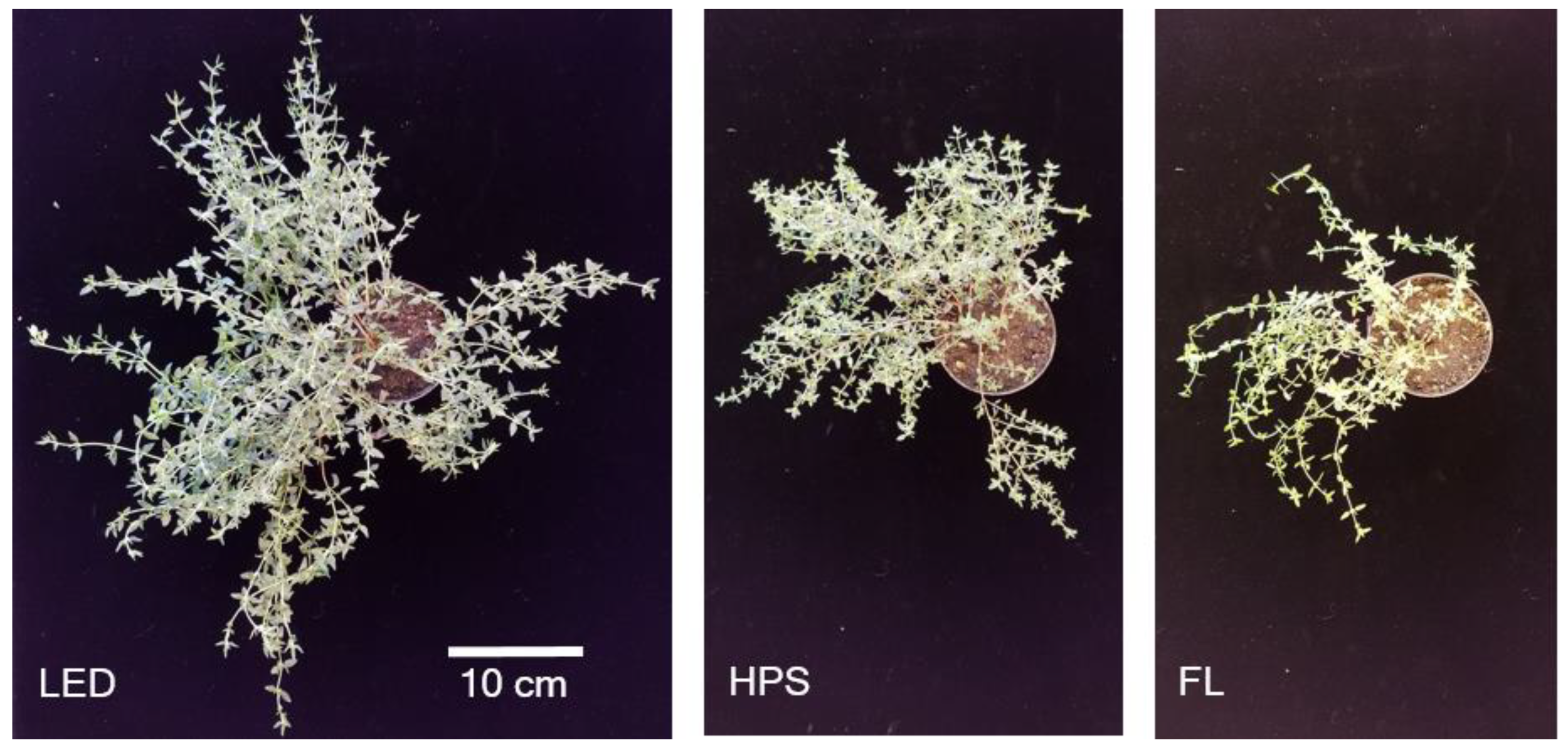
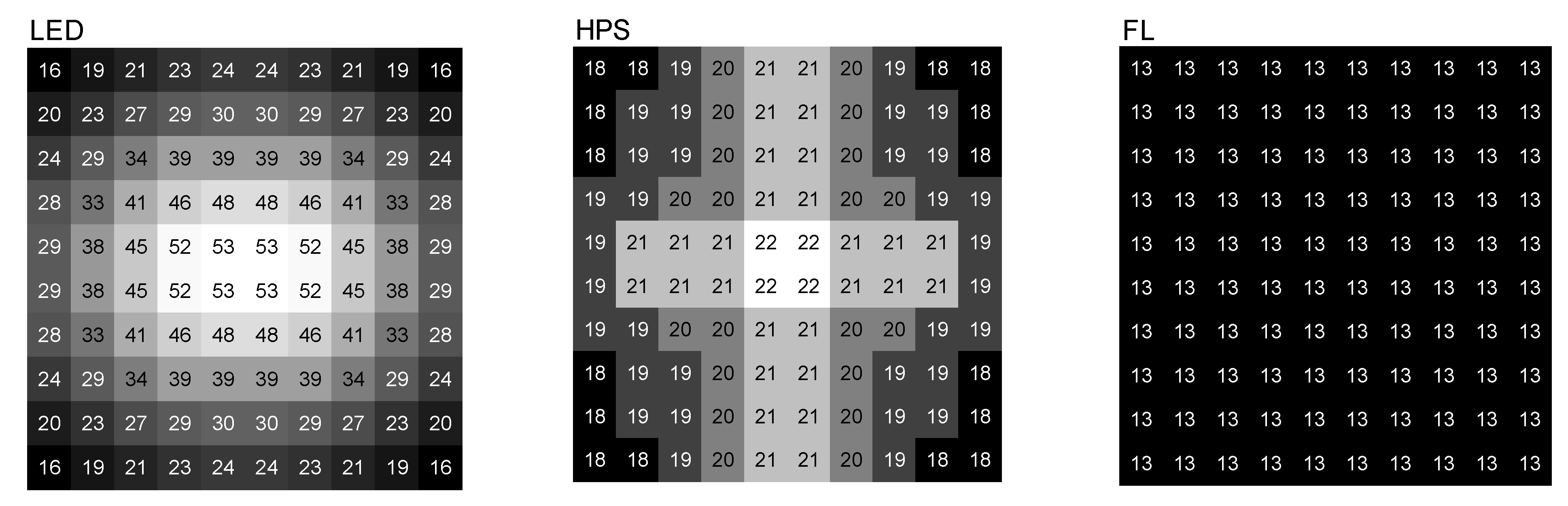
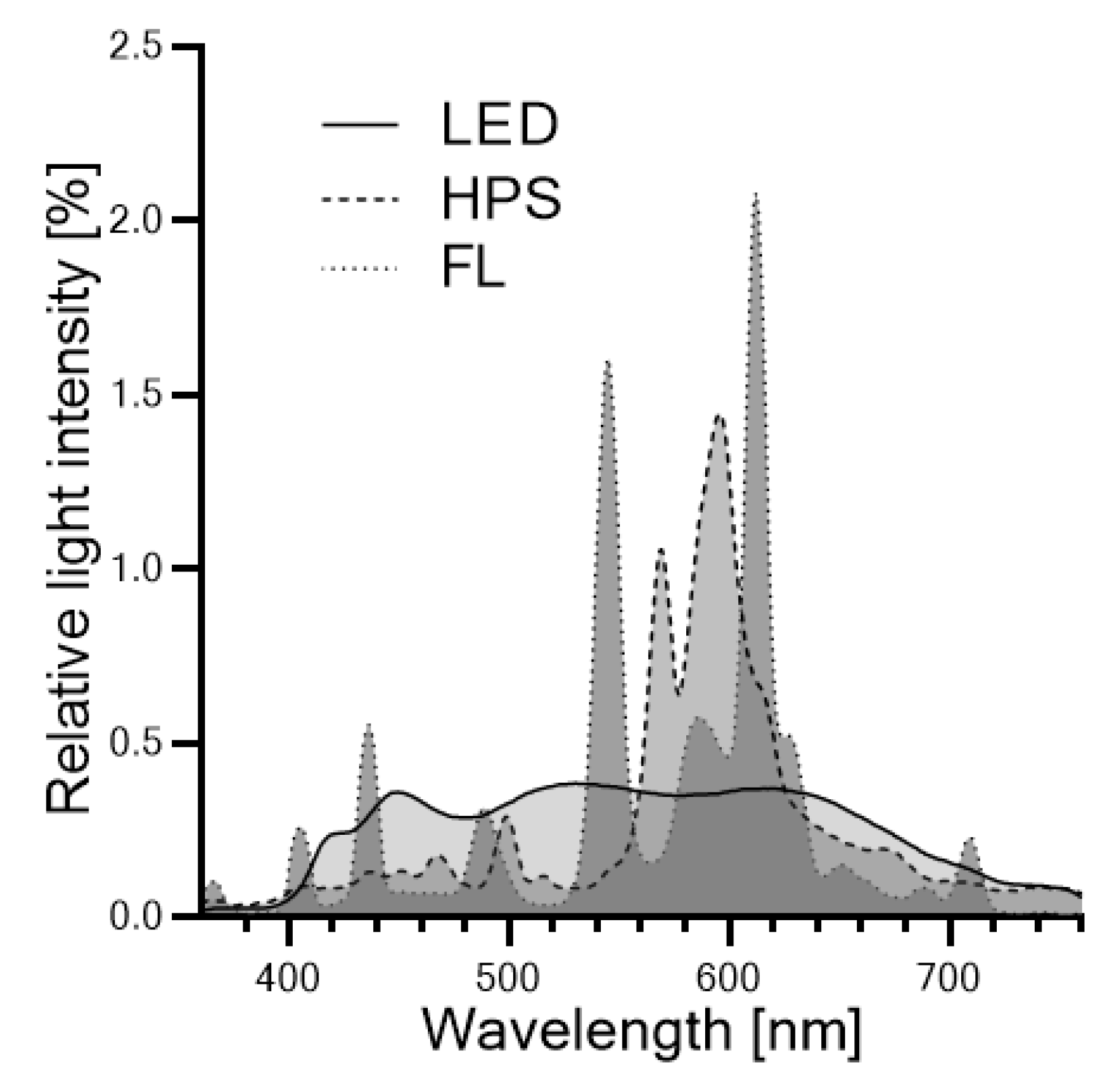
| Parameter * | Supplemental Light Fixtures ** | |||||
|---|---|---|---|---|---|---|
| LED | HPS | FL | ||||
| µmol m−2 s−1 | % *** | µmol m−2 s−1 | % *** | µmol m−2 s−1 | % *** | |
| PPFD (400–700 nm) | 212 | 91.2 | 132 | 92.5 | 57 | 95 |
| PFD (360–760 nm) | 232 | 100 | 143 | 100 | 60 | 100 |
| PFD-Ultraviolet (360–399 nm) | 1.7 | 0.7 | 1.6 | 1.1 | 0.6 | 1.0 |
| PFD-Blue (400–519 nm) | 65.7 | 28.4 | 16.9 | 11.9 | 8.2 | 13.6 |
| PFD-Green (520–559 nm) | 33.5 | 14.5 | 7.0 | 4.9 | 13.5 | 22.4 |
| PFD-Yellow (560–624 nm) | 56.9 | 24.5 | 83.6 | 58.7 | 27.9 | 46.4 |
| PFD-Red (625–700 nm) | 55.3 | 23.9 | 24.3 | 17.1 | 7.6 | 12.7 |
| PFD-Far Red (701–760 nm) | 18.7 | 8.1 | 9.0 | 6.4 | 2.4 | 3.9 |
| R/FR ratio (660/730 nm) ‡ | 2.8 | 2.4 | 0.1 | |||
| DLI (mol m−2 d−1) ± | 10.6/11.7 | 6.6/7.2 | 2.9/3.0 | |||
| Compound | RI * | Light-Emitting Diode (LED) | High-Pressure Sodium Lamp (HPS) | Fluorescent Light (FL) | |||
|---|---|---|---|---|---|---|---|
| % ** | µg 100 mg−1 LDM *** | % | µg 100 mg−1 LDM | % | µg 100 mg−1 LDM | ||
| monoterpene hydrocarbons | |||||||
| α-pinene | 938.0 ± 0.4 | 0.8 ± 0.1 | 14.03 ± 0.8 a | 0.8 ± 0.1 | 11.41 ± 1.1 b | 1.0 ± 0.1 | 7.22 ± 1.1 c |
| sabinene | 977.8 ± 0.4 | 1.4 ± 0.4 | 34.21 ± 8.5 a | 1.5 ± 0.6 | 33.57 ± 6.4 a | 1.3 ± 0.5 | 13.95 ± 1.5 b |
| myrcene | 991.8 ± 0.3 | 1.6 ± 0.2 | 35.16 ± 1.8 a | 1.5 ± 0.2 | 28.50 ± 3.6 a | 1.8 ± 0.2 | 17.19 ± 1.6 b |
| α-terpinene | 1020.8 ± 0.3 | 1.9 ± 0.4 | 39.47 ± 4.3 a | 2.1 ± 0.7 | 33.43 ± 3.2 a | 2.2 ± 0.8 | 19.72 ± 2.1 b |
| p-cymene | 1029.1 ± 0.5 | 8.5 ± 2.2 | 157.70 ± 27.4 a | 8.3 ± 2.5 | 123.90 ± 8.0 a | 6.9 ± 2.5 | 55.16 ± 4.7 b |
| limonene | 1033.1 ± 0.3 | 0.6 ± 0.1 | 9.81 ± 0.7 a | 0.6 ± 0.1 | 8.22 ± 0.8 a | 0.6 ± 0.1 | 4.59 ± 0.5 b |
| γ-terpinene | 1064.4 ± 0.8 | 15.2 ± 5.4 | 323.20 ± 56.7 a | 16.0 ± 4.7 | 259.90 ± 49.0 ab | 20.1 ± 6.3 | 179.50 ± 22.6 b |
| oxygenated monoterpenes | |||||||
| cis-sabinene hydrate | 1071.7 ± 0.4 | 1.3 ± 0.1 | 28.41 ± 2.3 a | 1.4 ± 0.2 | 25.38 ± 1.6 a | 1.3 ± 0.3 | 12.31 ± 0.8 b |
| linalool | 1100.9 ± 0.5 | 2.6 ± 0.7 | 61.92 ± 11.3 a | 2.7 ± 0.8 | 52.22 ± 11.2 a | 2.3 ± 0.9 | 21.25 ± 1.9 b |
| borneol | 1173.6 ± 0.3 | 0.9 ± 0.4 | 44.44 ± 2.6 a | 1.0 ± 0.6 | 46.50 ± 5.9 ab | 1.5 ± 0.7 | 31.75 ± 3.7 b |
| thymol | 1297.6 ± 1.8 | 54.6 ± 6.9 | 1134.00 ± 86.3 a | 52.9 ± 6.4 | 917.10 ± 142.9 a | 50.2 ± 7.9 | 429.90 ± 57.3 b |
| carvacrol | 1304.6 ± 1.1 | 2.4 ± 0.4 | 60.82 ± 4.9 a | 2.3 ± 0.5 | 48.82 ± 8.2 a | 2.0 ± 0.6 | 21.42 ± 2.9 b |
| sesquiterpene hydrocarbons | |||||||
| β-caryophyllene | 1436.55 ± 0.5 | 3.1 ± 1.3 | 47.69 ± 7.1 a | 3.67 ± 1.1 | 49.50 ± 7.0 a | 3.32 ± 1.1 | 21.91 ± 3.4 b |
| % of total extract ** | 94.93 ± 1.50 | 94.68 ± 2.22 | 94.53 ± 1.95 | ||||
| Total VOCs [% g−1 LDM] **** | 2.7 ± 0.22 a | 2.3 ± 0.25 a | 1.1 ± 0.10 b | ||||
| Light Fixture * | FM ** per Square Meter [g m−2] *** | DM ** per Square Meter [g m−2] *** | VOC ** per Square Meter [mg m−2] **** |
|---|---|---|---|
| LED | 897.9 ± 64.65a | 180.2 ± 24.69 a | 2472 ± 626.4 a |
| HPS | 509.4 ± 72.88 b | 102.6 ± 16.87 b | 1273 ± 334.0 a |
| FL | 158.0 ± 6.73 c | 20.62 ± 2.06 c | 199.1 ± 30.98 b |
| Light Fixture * | Power Consumption per Meter2 [W m−2] | Power Consumption for Thyme Cultivation [kWh m−2] | Power Savings Compared to HPS [%] | Fresh Thyme Production ** [g kWh−1 m−2] | Dry Thyme Production ** [mg kWh−1 m−2] | VOC Production *** [mg kWh−1 m−2] |
|---|---|---|---|---|---|---|
| LED | 257.7 | 454.6 | 31.3 | 1.92 ± 0.15 a | 396.3 ± 54.31 a | 5.4 ± 1.4a |
| HPS | 374.9 | 661.3 | na | 0.77 ± 0.11 b | 155.2 ± 25.5 b | 1.9 ± 0.5 b |
| FL | 299.4 | 528.1 | 20.1 | 0.30 ± 0.03 c | 39.1 ± 3.9 c | 0.4 ± 0.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabbert, J.M.; Schulz, H.; Krähmer, A. Increased Plant Quality, Greenhouse Productivity and Energy Efficiency with Broad-Spectrum LED Systems: A Case Study for Thyme (Thymus vulgaris L.). Plants 2021, 10, 960. https://doi.org/10.3390/plants10050960
Tabbert JM, Schulz H, Krähmer A. Increased Plant Quality, Greenhouse Productivity and Energy Efficiency with Broad-Spectrum LED Systems: A Case Study for Thyme (Thymus vulgaris L.). Plants. 2021; 10(5):960. https://doi.org/10.3390/plants10050960
Chicago/Turabian StyleTabbert, Jenny Manuela, Hartwig Schulz, and Andrea Krähmer. 2021. "Increased Plant Quality, Greenhouse Productivity and Energy Efficiency with Broad-Spectrum LED Systems: A Case Study for Thyme (Thymus vulgaris L.)" Plants 10, no. 5: 960. https://doi.org/10.3390/plants10050960
APA StyleTabbert, J. M., Schulz, H., & Krähmer, A. (2021). Increased Plant Quality, Greenhouse Productivity and Energy Efficiency with Broad-Spectrum LED Systems: A Case Study for Thyme (Thymus vulgaris L.). Plants, 10(5), 960. https://doi.org/10.3390/plants10050960






