Is the Age of Novel Ecosystem the Factor Driving Arbuscular Mycorrhizal Colonization in Poa compressa and Calamagrostis epigejos?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Soil Sampling and Analyses
2.3. Assessment of Mycorrhizal Colonization
2.4. Statistical Analyses
3. Results
3.1. Substrate Properties
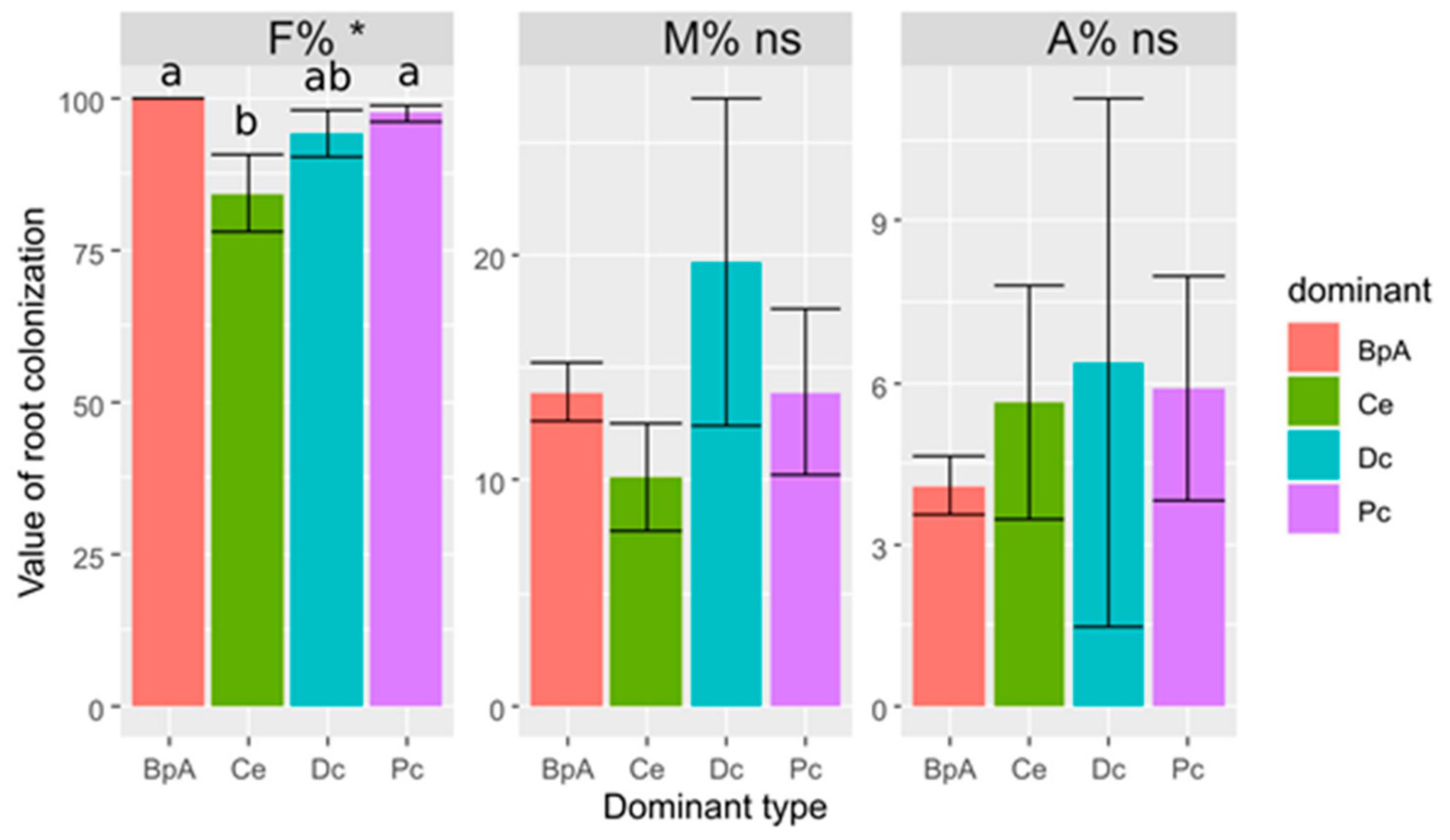
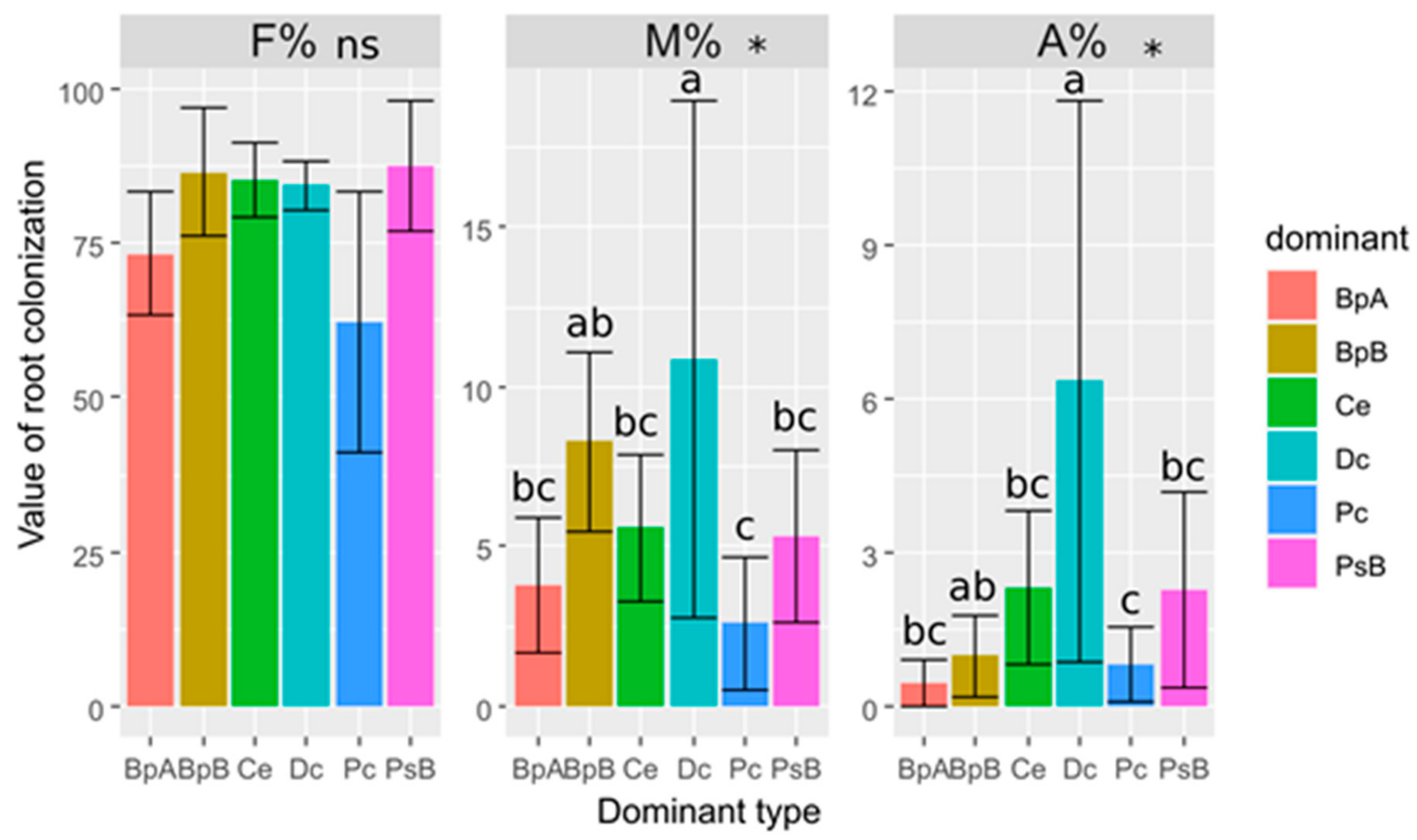
3.2. Poa compressa
3.3. Calamagrostis epigejos
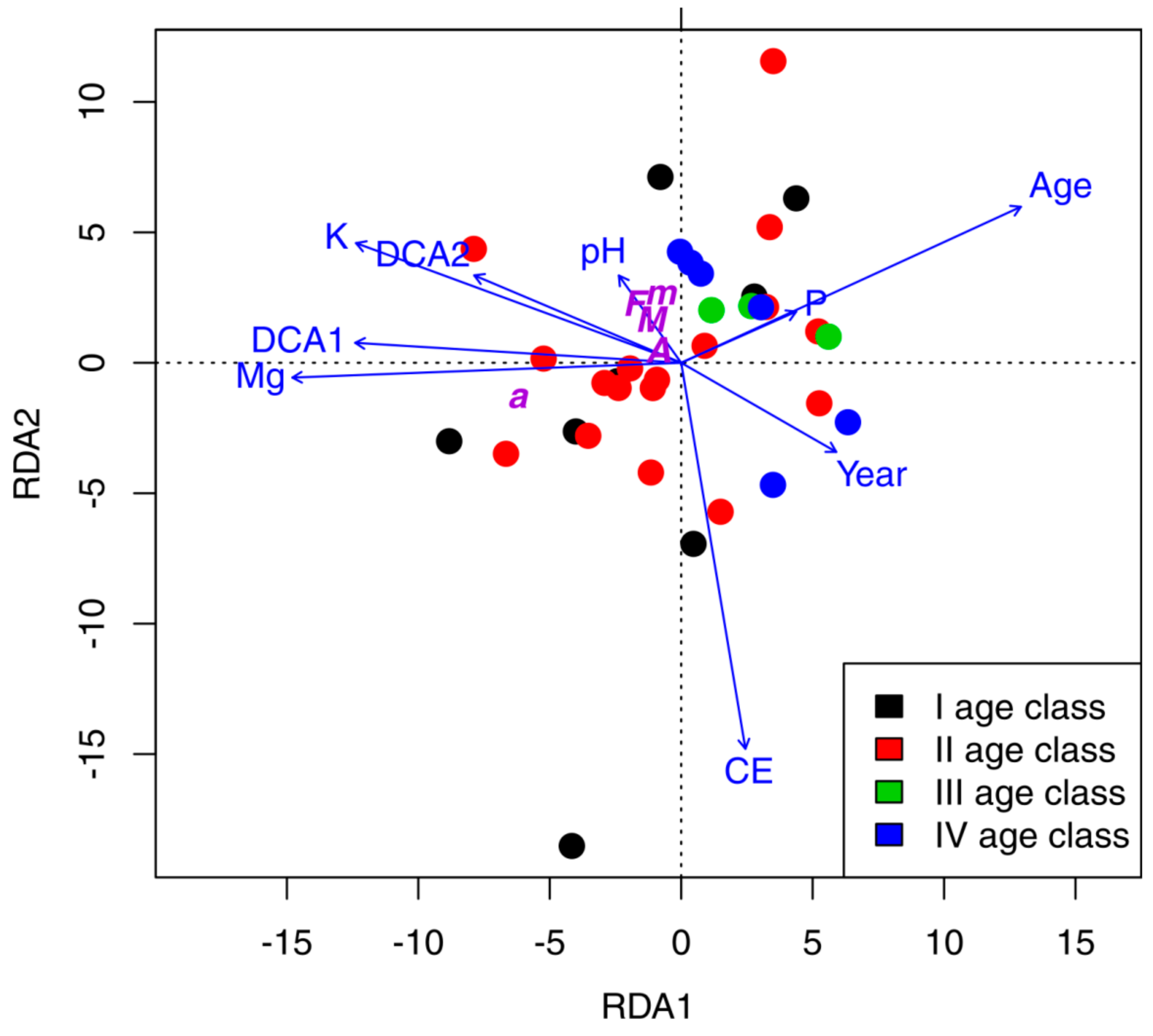
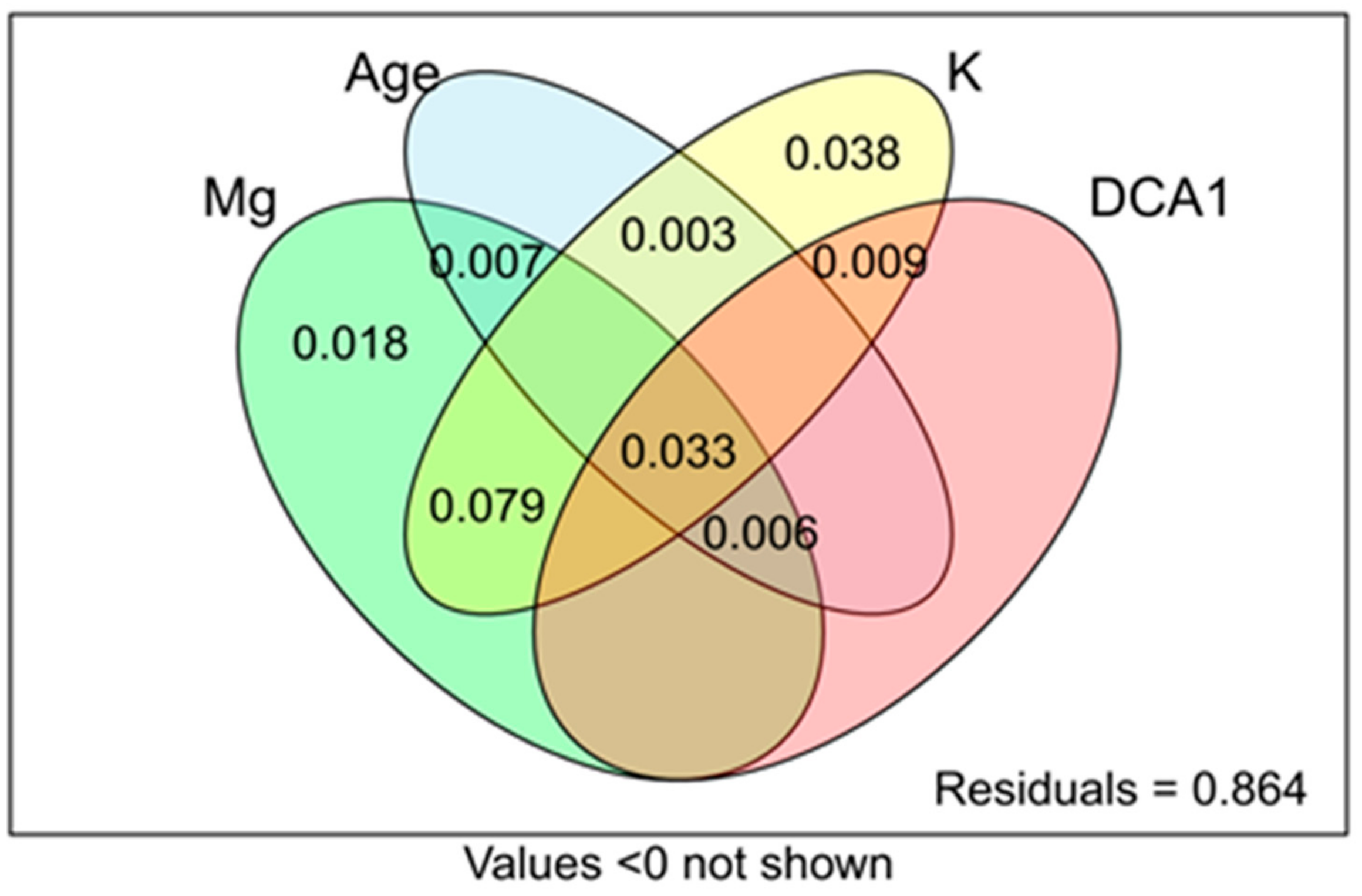
3.4. Ordination Results
4. Discussion
4.1. The Habitat Conditions and Age of the Site
4.2. The Influence of Co-Occurring and Dominant Plant Species
4.3. The Significance of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hobbs, R.J.; Higgs, E.S.; Hall, C.M. Defining Novel Ecosystems. In Intervening in the New Ecological World Order; Hobbs, R.J., Higgs, E.S., Hall, C.M., Eds.; Novel Ecosystems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Rotherham, I.D. Recombinant Ecology—A Hybrid Future? Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-49797-6. [Google Scholar]
- Woźniak, G.; Sierka, E.; Wheeler, A. Urban and Industrial Habitats: How Important They Are for Ecosystem Services. In Ecosystem Services and Global Ecology; Hufnagel, L., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Turrini, A.; Saran, M.; Giovannetti, M.; Oehl, F. Rhizoglomus venetianum, a new arbuscular mycorrhizal fungal species from a heavy metal-contaminated site, downtown Venice in Italy. Mycol. Prog. 2018, 17, 1213–1224. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Piątek, M.; Magurno, F.; Malicka, M.; Zubek, S.; Mleczko, P.; Yorou, N.S.; Jobim, K.; Vista, X.M.; et al. Rhizoglomus dalpeae, R. maiae, and R. silesianum, new species. Mycologia 2019, 111, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, G.; Dyderski, M.K.; Kompała-Bąba, A.; Jagodziński, A.M.; Pasierbiński, A.; Błońska, A.; Bierza, W.; Magurno, F.; Sierka, E. Use of remote sensing to track postindustrial vegetation development. Land Degrad. Dev. 2021, 32, 1426–1439. [Google Scholar] [CrossRef]
- Wang, F. Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Chmura, D.; Molenda, T.; Błońska, A.; Woźniak, G. Sites of leachate inflows on coalmine heaps as refuges of rare moun-tainous species. Pol. J. Environ. Stud. 2011, 20, 551–557. [Google Scholar]
- Kompała-Bąba, A.; Sierka, E.; Dyderski, M.K.; Bierza, W.; Magurno, F.; Besenyei, L.; Błońska, A.; Ryś, K.; Jagodziński, A.M.; Woźniak, G. Do the dominant plant species impact the substrate and vegetation composition of post-coal mining spoil heaps? Ecol. Eng. 2020, 143, 105685. [Google Scholar] [CrossRef]
- Woźniak, G. Diversity of Vegetation on Coal-Mine Heaps of the Upper Silesia (Poland); W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2010. [Google Scholar]
- Allen, E.B.; Allen, M.F. Natural reestablishment of vesicular-arbuscular mycorrhizae following stripmine recla-mation in Wyoming. J. Appl. Ecol. 1980, 17, 139–147. [Google Scholar] [CrossRef]
- Allen, E.B. The role of mycorrhizae in mined land diversity. In Proceedings of the Third Biennial Symposium on Sur-face Coal Mine Reclamation on the Great Plains, Billings, MT, USA, 19–21 March 1984; pp. 273–295. [Google Scholar]
- Harley, J.L.; Harley, E.L. A Check-List of Mycorrhiza in the British Flora*. New Phytol. 1987, 105, 1–102. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Frouz, J.; Cajthaml, T.; Kříbek, B.; Schaeffer, P.; Bartuška, M.; Galertová, R.; Rojík, P.; Krištůfek, V. Deep, subsurface microflora after excavation respiration and biomass and its potential role in degradation of fossil organic matter. Folia Microbiol. 2011, 56, 389–396. [Google Scholar] [CrossRef]
- Markowicz, A.; Woźniak, G.; Borymski, S.; Piotrowska-Seget, Z.; Chmura, D. Links in the functional diversity between soil microorganisms and plant communities during natural succession in coal mine spoil heaps. Ecol. Res. 2015, 30, 1005–1014. [Google Scholar] [CrossRef]
- Woźniak, G.; Markowicz, A.; Borymski, S.; Piotrowska-Seget, Z.; Chmura, D.; Besenyei, L. The relationship between successional vascular plant assemblages and associated microbial communities on coal mine spoil heaps. Community Ecol. 2015, 16, 23–32. [Google Scholar] [CrossRef]
- Kumar, A.; Raghuwanshi, R.; Upadhyay, R.S. Vesicular-arbuscular mycorrhizal association in naturally revegetated coal mine spoil. Trop. Ecol. 2003, 44, 253–256. [Google Scholar]
- Li-Ping, W.; Kui-Mei, Q.; Shi-Long, H.; Bo, F. Fertilizing reclamation of arbuscular mycorrhizal fungi on coal mine complex substrate. Procedia Earth Planet. Sci. 2009, 1, 1101–1106. [Google Scholar] [CrossRef][Green Version]
- Daft, M.J.; Nicolson, T.H. Arbuscular Mycorrhizas in Plants Colonizing Coal Wastes in Scotland. New Phytol. 1974, 73, 1129–1138. [Google Scholar] [CrossRef]
- Qian, K.; Wang, L.; Yin, N. Effects of AMF on soil enzyme activity and carbon sequestration capacity in reclaimed mine soil. Int. J. Min. Sci. Technol. 2012, 22, 553–557. [Google Scholar] [CrossRef]
- Khan, A.G. Vesicular-Arbuscular Mycorrhizas in Plants Colonizing Black Wastes From Bituminous Coal Mining in the Illawarra Region Of New South Wales. New Phytol. 1978, 81, 53–63. [Google Scholar] [CrossRef]
- Diaz, G.; Honrubia, M. Infectivity of mine soils from South-East Spain. Agric. Ecosyst. Environ. 1990, 29, 85–89. [Google Scholar] [CrossRef]
- Smyth, C.R.; Box, P.O. Establishment and growth of mycorrhizal and Rhizobium inoculated high-elevation native legumes on an unamended coal mine spoil dump in South-eastern British Columbia. In Proceedings of the 21st Annual British Colum-bia Mine Reclamation Symposium in Cranbrook, Cranbrook, Canada, 22–25 September 1997; pp. 32–45. [Google Scholar]
- Entry, J.A.; Rygiewicz, P.T.; Watrud, L.S.; Donnelly, P.K. Influence of adverse soil conditions on the formation and function of Arbuscular mycorrhizas. Adv. Environ. Res. 2002, 7, 123–138. [Google Scholar] [CrossRef]
- Allen, E.B.; Allen, M.F. Water Relations of Xeric Grasses in the Field: Interactions of Mycorrhizas and Competition. New Phytol. 1986, 104, 559–571. [Google Scholar] [CrossRef]
- Bradshaw, A. Restoration of mined lands—using natural processes. Ecol. Eng. 1997, 8, 255–269. [Google Scholar] [CrossRef]
- Frouz, J.; Prach, K.; Pižl, V.; Háněl, L.; Starý, J.; Tajovský, K.; Materna, J.; Balík, V.; Kalčík, J.; Řehounková, K. Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. Eur. J. Soil Biol. 2008, 44, 109–121. [Google Scholar] [CrossRef]
- Tropek, R.; Cizek, O.; Kadlec, T.; Klecka, J. Habitat Use ofHipparchia Semele (Lepidoptera) in Its Artificial Stronghold: Necessity of the Resource-Based Habitat View in Restoration of Disturbed Sites. Pol. J. Ecol. 2017, 65, 385–399. [Google Scholar] [CrossRef]
- Rawlik, M.; Kasprowicz, M.; Jagodziński, A.M. Differentiation of herb layer vascular flora in reclaimed areas depends on the species composition of forest stands. For. Ecol. Manag. 2018, 409, 541–551. [Google Scholar] [CrossRef]
- Błońska, A.; Kompała-Bąba, A.; Sierka, E.; Besenyei, L.; Magurno, F.; Bierza, W.; Frydecka, K.; Woźniak, G.B. Impact of Selected Plant Species on Enzymatic Activity of Soil Substratum on Post-Mining Heaps. J. Ecol. Eng. 2019, 20, 138–144. [Google Scholar] [CrossRef]
- Frouz, J.; Keplin, B.; Pižl, V.; Tajovský, K.; Starý, J.; Lukešová, A.; Nováková, A.; Balík, V.; Háněl, L.; Materna, J.; et al. Soil biota and upper soil layer development in two contrasting post-mining chronosequences. Ecol. Eng. 2001, 17, 275–284. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, H.M.; Urbi, Z.; Islam, S. Environmental Impact of Coal Mining: A case study on Barapukuria Coal Mining Industry, Dinajpur, Bangladesh. Middle-East J. Sci. Res. 2014, 21, 268–274. [Google Scholar] [CrossRef]
- Frouz, J. (Ed.) Soil Biota and Ecosystem Development in Post Mining Sites; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Frouz, J.; Pižl, V.; Cienciala, E.; Kalčík, J. Carbon storage in post-mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry 2009, 94, 111–121. [Google Scholar] [CrossRef]
- Martín-Moreno, C.; Duque, J.F.M.; Ibarra, J.M.N.; Rodríguez, N.H.; Santos, M.; Ángel, S.; Castillo, L.S. Effects of Topography and Surface Soil Cover on Erosion for Mining Reclamation: The Experimental Spoil Heap at El Machorro Mine (Central Spain). Land Degrad. Dev. 2013, 27, 145–159. [Google Scholar] [CrossRef]
- Collier, M.J.; Devitt, C. Novel ecosystems: Challenges and opportunities for the Anthropocene. Anthr. Rev. 2016, 3, 231–242. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Waters, C.N.; Zalasiewicz, J.; Summerhayes, C.; Barnosky, A.D.; Poirier, C.; Gałuszka, A.; Cearreta, A.; Edgeworth, M.; Ellis, E.C.; Ellis, M.; et al. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 2016, 351, aad2622. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, G.; Cohn, E. Monitoring of Spontaneous Vegetation Dynamics on Post Coal Mining Waste Sites in Upper Silesia, Poland. In Geotechnical and Environmental Aspects of Waste Disposal Sites—Proceedings of the 4th International Sympo-sium on Geotechnics Related to the Environment; Sarsby, R.W., Felton, A.J., Eds.; Taylor and Francis Group: London, UK, 2007; pp. 289–294. [Google Scholar]
- Błońska, A.; Kompała-Bąba, A.; Sierka, E.; Bierza, W.; Magurno, F.; Besenyei, L.; Ryś, K.; Woźniak, G. Diversity of Vegetation Dominated by Selected Grass Species on Coal-Mine Spoil Heaps in Terms of Reclamation of Post-Industrial Areas. J. Ecol. Eng. 2019, 20, 209–217. [Google Scholar] [CrossRef]
- Schulz, D. Recultivation of mining waste dumps in the Ruhr area, Germany. Water Air Soil Pollut. 1996, 91, 89–98. [Google Scholar] [CrossRef]
- Piekarska-Stachowiak, A.; Szary, M.; Ziemer, B.; Besenyei, L.; Woźniak, G. An application of the plant functional group concept to restoration practice on coal mine spoil heaps. Ecol. Res. 2014, 29, 843–853. [Google Scholar] [CrossRef]
- Dobrzański, B.; Udziak, S.; Klimowicz, Z.; Melke, J. Badanie Gleb w Laboratorium i w Polu. Przewodnik do Ćwiczeń z Gleboznawstwa dla Studentów Biologii i Geografii. Wyd; Uniwersytetu Marii Curie-Skłodowskiej: Lublin, Poland, 1992. [Google Scholar]
- Fotyma, E.; Wilkos, G.; Pietruch, C. Test Glebowy Azotu Mineralnego Możliwości Praktycznego Wykorzystania; Mat. szkol. 69/98; IUNG: Puławy, Poland, 1998. (In Polish) [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycor-rhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazii-Pearson, V. Mesure du taux de mycorhization VA d’un systéme radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionelle. In Physiological and genetical aspects of mycorrhizae: Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, France, 1–5 July 1985; 1986; pp. 217–221. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 May 2021).
- Rydlová, J.; Püschel, D.; Dostálová, M.; Janoušková, M.; Frouz, J. Nutrient limitation drives response of Calamagrostis epigejos to arbuscular mycorrhiza in primary succession. Mycorrhiza 2016, 26, 757–767. [Google Scholar] [CrossRef]
- Endresz, G.; Somodi, I.; Kalapos, T. Arbuscular mycorrhizal colonisation of roots of grass species differing in invasiveness. Community Ecol. 2013, 14, 67–76. [Google Scholar] [CrossRef]
- Rydlová, J.; Vosátka, M. Associations of dominant plant species with arbuscular mycorrhizal fungi during vegetation development on coal mine spoil banks. Folia Geobot. Phytotaxon. 2001, 36, 85–97. [Google Scholar] [CrossRef]
- Malcová, R.; Albrechtová, J.; Vosátka, M. The role of the extraradical mycelium network of arbuscular mycorrhizal fungi on the establishment and growth of Calamagrostis epigejos in industrial waste substrates. Appl. Soil Ecol. 2001, 18, 129–142. [Google Scholar] [CrossRef]
- Bąba, W.; Błońska, A.; Kompała-Bąba, A.; Małkowski, Ł.; Ziemer, B.; Sierka, E.; Nowak, T.; Woźniak, G.; Besenyei, L. Arbuscular mycorrhizal fungi (AMF) root colonization dynamics of Molinia caerulea (L.) Moench. in grasslands and post-industrial sites. Ecol. Eng. 2016, 95, 817–827. [Google Scholar] [CrossRef]
- Püschel, D.; Rydlová, J.; Vosátka, M. The development of arbuscular mycorrhiza in two simulated stages of spoil-bank succession. Appl. Soil Ecol. 2007, 35, 363–369. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Błońska, A.; Kompała-Bąba, A.; Woźniak, G. Effects of Calamagrostis epigejos, Chamaenerion palustre and Tussilago farfara on nutrient availability and microbial activity in the surface layer of spoil heaps after hard coal mining. Ecol. Eng. 2015, 83, 328–337. [Google Scholar] [CrossRef]
- Endresz, G.; Mojzes, A.; Kalapos, T. Deficit watering reduces plant growth to a smaller extent with arbuscular mycorrhizal association than without it for non-invasive grass species but not for invasive grass species. Appl. Ecol. Environ. Res. 2015, 13, 551–567. [Google Scholar] [CrossRef]
- Blanke, V.; Wagner, M.; Renker, C.; Lippert, H.; Michulitz, M.; Kuhn, A.J.; Buscot, F. Arbuscular mycorrhizas in phosphate-polluted soil: Interrelations between root colonization and nitrogen. Plant Soil 2011, 343, 379–392. [Google Scholar] [CrossRef]
- Gucwa-Przepióra, E.; Chmura, D.; Sokołowska, K. AM and DSE colonization of invasive plants in urban habitat: A study of Upper Silesia (southern Poland). J. Plant Res. 2016, 129, 603–614. [Google Scholar] [CrossRef]
- Gucwa-Przepióra, E.; Nadgórska-Socha, A.; Fojcik, B.; Chmura, D. Enzymatic activities and arbuscular mycorrhizal colonization of Plantago lanceolata and Plantago major in a soil root zone under heavy metal stress. Environ. Sci. Pollut. Res. 2016, 23, 4742–4755. [Google Scholar] [CrossRef]
- Bhadalung, N.; Suwanarit, A.; Dell, B.; Nopamornbodi, O.; Thamchaipenet, A.; Rungchuang, J. Effects of long-term NP-fertilization on abundance and diversity of arbuscular mycorrhizal fungi under a maize cropping system. Plant Soil 2005, 270, 371–382. [Google Scholar] [CrossRef]
- Ju, X.; Kou, C.; Christie, P.; Dou, Z.; Zhang, F. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497–506. [Google Scholar] [CrossRef]
- Acosta, J.; Cano, A.F.; Arocena, J.; Debela, F.; Martínez-Martínez, S. Distribution of metals in soil particle size fractions and its implication to risk assessment of playgrounds in Murcia City (Spain). Geoderma 2009, 149, 101–109. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Azcón-Aguilar, C.; Barea, J.M.; Gianinazzi, S.; Gianinazzi-Pearson, V. (Eds.) Mycorrhizas—Functional Processes and Ecological Impact; Springer: Berlin/Heidelberg, Germmany, 2009. [Google Scholar] [CrossRef]
- Kasowska, D. Mycorrhizal status of plants in two successional stages on spoil heaps from fire loam mining in Lower Silesia (SW Poland). Acta Soc. Bot. Pol. 2002, 71, 155–161. [Google Scholar] [CrossRef][Green Version]
- Gould, A.B.; Hendrix, J.W.; Ferriss, R.S. Relationship of mycorrhizal activity to time following reclamation of surface mine land in western Kentucky. I. Propagule and spore population densities. Can. J. Bot. 1996, 74, 247–261. [Google Scholar] [CrossRef]
- Enkhtuya, B.; Pöschl, M.; Vosátka, M. Native Grass Facilitates Mycorrhizal Colonisation and P Uptake of Tree Seedlings in Two Anthropogenic Substrates. Water Air Soil Pollut. 2005, 166, 217–236. [Google Scholar] [CrossRef]
- Janoušková, M.; Seddas, P.; Libor, M.; van Tuinen, D.; Dvořáčková, A.; Tollot, M.; Gianinazzi-Pearson, V.; Vosátka, M.; Gollotte, A. Development and activity of Glomus intraradices as affected by co-existence with Glomus claroideum in one root system. Mycorrhiza 2009, 19, 393–402. [Google Scholar] [CrossRef]
- John, J.; Lundholmb, J.; Kernaghanc, G. Colonization of green roof plants by mycorrhizal and root endophytic fungi. Ecol. Eng. 2014, 71, 651–659. [Google Scholar] [CrossRef]
- Maier, W.; Hammer, K.; Dammann, U.; Schulz, B.; Strack, D. Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae. Planta 1997, 202, 36–42. [Google Scholar] [CrossRef]
- Moynahan, O.S.; Zabinski, C.A.; Gannon, J.E. Microbial Community Structure and Carbon-Utilization Diversity in a Mine Tailings Revegetation Study. Restor. Ecol. 2002, 10, 77–87. [Google Scholar] [CrossRef]
- Kasowska, D.; Koszelnik-Leszek, A. Ecological Features of Spontaneous Vascular Flora of Serpentine Post-Mining Sites in Lower Silesia. Arch. Environ. Prot. 2014, 40, 33–52. [Google Scholar] [CrossRef][Green Version]
- Kompała-Bąba, A.; Bierza, W.; Błońska, A.; Sierka, E.; Magurno, F.; Chmura, D.; Besenyei, L.; Radosz, Ł.; Woźniak, G. Vegetation diversity on coal mine spoil heaps – how important is the texture of the soil substrate? Biologia 2019, 74, 419–436. [Google Scholar] [CrossRef]
- Box, J. Nature Conservation and Post-Industrial Landscapes. Ind. Archaeol. Rev. 1999, 21, 137–146. [Google Scholar] [CrossRef]
- Poulin, M.J.; Bel-Rhlid, R. Flavonoids released by carrot (Daucus carota) seedlings stimulate hyphal development of vesicular-arbuscular mycorrhizal fungi in the presence of optimal CO2 enrichment. J. Chem. Ecol. 1993, 19, 2317–2327. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Nagahashi, G.; Pfeffer, P.E.; Kayser, W.M.; Reider, C. On-farm production and utilization of arbuscular mycorrhizal fungus inoculum. Can. J. Plant Sci. 2005, 85, 15–21. [Google Scholar] [CrossRef]
- Mudrák, O.; Hermová, M.; Tesnerová, C.; Rydlová, J.; Frouz, J. Above-ground and below-ground competition between the willow Salix caprea and its understorey. J. Veg. Sci. 2016, 27, 156–164. [Google Scholar] [CrossRef]
- Woźniak, G.; Chmura, D.; Błońska, A.; Tokarska- Guzik, B.; Sierka, E. Applicability of functional groups concept in analysis of spatiotemporal vegetation changes on manmade habitats. Pol. J. Environ. Stud. 2011, 20, 623–631. [Google Scholar]
- Tropek, R.; Kadlec, T.; Hejda, M.; Kocarek, P.; Skuhrovec, J.; Malenovsky, I.; Vodka, S.; Spitzer, L.; Banar, P.; Konvicka, M. Technical reclamations are wasting the conservation potential of post-mining sites. A case study of black coal spoil dumps. Ecol. Eng. 2012, 43, 13–18. [Google Scholar] [CrossRef]
- Woźniak, G.; Pasierbiński, A.; Rostański, A. The diversity of spontaneous woodland vegetation on coals mine heaps of Up-per-Silesian industrial region. Arch. Environ. Prot. 2003, 29, 93–105. (In Polish) [Google Scholar]
- Ardejani, F.D.; Shokri, B.J.; Bagheri, M.; Soleimani, E. Investigation of pyrite oxidation and acid mine drainage characterization associated with Razi active coal mine and coal washing waste dumps in the Azad shahr–Ramian region, northeast Iran. Environ. Earth Sci. 2010, 61, 1547–1560. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Huang, G.; Dong, C. Enhancement of economic and ecological sustainability through integrated management of coal and electricity in north China. Procedia Environ. Sci. 2012, 13, 467–497. [Google Scholar] [CrossRef][Green Version]
- Jochimsen, M.E. Vegetation development and species assemblages in a long-term reclamation project on mine spoil. Ecol. Eng. 2001, 17, 187–198. [Google Scholar] [CrossRef]
- Frouz, J.; Elhottová, D.; Pižl, V.; Tajovský, K.; Šourková, M.; Picek, T.; Maly, S. The effect of litter quality and soil faunal composition on organic matter dynamics in post-mining soil: A laboratory study. Appl. Soil Ecol. 2007, 37, 72–80. [Google Scholar] [CrossRef]
- Błońska, A.; Kidawa, J.; Molenda, T.; Chmura, D. Hydrogeochemical Conditions of the Development of Anthropogenic Carbonate Swamps: A Case Study of an Abandoned Polish Sandpit. Pol. J. Environ. Stud. 2019, 29, 561–569. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Wrońska-Pilarek, D.; Jagodziński, A.M. Ecological lands for conservation of vascular plant diversity in the urban environment. Urban Ecosyst. 2017, 20, 639–650. [Google Scholar] [CrossRef]
- Magurno, F.; Malicka, M.; Posta, K.; Wozniak, G.; Lumini, E.; Piotrowska-Seget, Z. Glomalin gene as molecular marker for functional diversity of arbuscular mycorrhizal fungi in soil. Biol. Fertil. Soils 2019, 55, 411–417. [Google Scholar] [CrossRef]
- Prach, K.; Pyšek, P. Using spontaneous succession for restoration of human-disturbed habitats: Experience from Central Europe. Ecol. Eng. 2001, 17, 55–62. [Google Scholar] [CrossRef]
- Rahmonov, O.; Szymczyk, A. Relations between vegetation and soil in initial succession phases inpost-sand excavations. Ekológia (Bratislava) 2010, 29, 412–429. [Google Scholar] [CrossRef]
| Age of Heap | Dominant Plant Species | Tested Individuals | ||
|---|---|---|---|---|
| P. compressa | C. epigejos | |||
| Class I | P. compressa | four paches | 40 | |
| Class I | P. compressa | three paches | 30 | |
| Class II | C. epigejos | five paches | 50 | |
| Class II | D. carota | four paches | 40 | |
| Class II | C. epigejos | four paches | 40 | |
| Class II | D. carota | three paches | 30 | |
| Class III | B. pendula B | two paches | 20 | |
| Class III | P.sylvestris B | two paches | 20 | |
| Class IV | B. pendula A | three paches | 30 | |
| Class IV | B. pendula A | three paches | 30 | |
| Heap Age Class | Vegetation Type Sampled | The Studied Species Indivi-duals | pH (KCl) | P mg 100 g−1 | K mg 100 g−1 | Mg mg 100 g−1 | orgC % | N-NH4 mg 100 g−1 | Conductivity µS cm−1 |
|---|---|---|---|---|---|---|---|---|---|
| Class I | Poa compressa | C. epigejos | 6.47 ± 0.18 | 3.57 ± 0.77 | 13.97 ± 3.62 | 26.67 ± 3.06 | 10.00 ± 0.46 | 3.28 ± 2.70 | 175.00 ± 32.65 |
| Class II | Calamagrostis epigejos | C. epigejos | 5.51 ± 0.68 | 4.02 ± 0.22 | 15.63 ± 1.65 | 32.50 ± 3.73 | 10.10 ± 0.74 | 1.38 ± 0.20 | 136.00 ± 21.72 |
| Class II | Daucus carota | C. epigejos | 5.73 ± 0.49 | 6.60 ± 0.61 | 16.13 ± 5.48 | 16.27 ± 7.23 | 15.63 ± 6.82 | 3.10 ± 0.49 | 82.67 ± 5.36 |
| Class III | Betula pendula B | C. epigejos | 5.30 ± 0.89 | 6.20 ± 1.65 | 17.83 ± 0.62 | 22.27 ± 3.56 | 9.00 ± 1.72 | 2.50 ± 0.78 | 111.00 ± 21.38 |
| Class IV | Betula pendula A | C. epigejos | 4.60 ± 0.18 | 4.77 ± 1.33 | 9.87 ± 2.55 | 10.97 ± 5.40 | 7.57 ± 3.58 | 2.74 ± 1.35 | 74.33 ± 9.84 |
| Class III | Pinus sylvestris B | C. epigejos | 6.75 ± 0.07 | 4.70 ± 0.85 | 4.15 ± 0.50 | 15.10 ± 1.98 | 18.70 ± 0.57 | 5.92 ± 0.54 | 123.00 ± 18.38 |
| Class I | Poa compressa | P. compressa | 6.27 ± 0.17 | 4.08 ± 0.32 | 15.70 ± 0.34 | 32.00 ± 2.02 | 6.90 ± 0.44 | 1.86 ± 0.20 | 166.50 ± 23.09 |
| Class II | Calamagrostis epigejos | P. compressa | 4.59 ± 0.62 | 5.60 ± 0.95 | 16.05 ± 4.60 | 18.88 ± 4.25 | 13.22 ± 5.86 | 3.46 ± 0.97 | 242.30 ± 78.59 |
| Class II | Daucus carota | P. compressa | 6.23 ± 0.36 | 4.94 ± 0.72 | 19.50 ± 2.22 | 27.68 ± 2.72 | 7.38 ± 1.21 | 2.93 ± 1.08 | 198.00 ± 97.75 |
| Class IV | Betula pendula A | P. compressa | 6.55 ± 1.13 | 4.40 ± 1.76 | 13.47 ± 0.18 | 23.67 ± 4.76 | 10.00 ± 3.63 | 2.60 ± 0.74 | 73.00 ± 3.79 |
| Code | Description | Df | AIC | Pseudo-F | p-Value |
|---|---|---|---|---|---|
| Mg | Mg (mg/100 g) | 1 | 240.56 | 4.3205 | 0.015 |
| K | K2O (mg/100 g) | 1 | 241.78 | 3.0345 | 0.020 |
| Age | Mg (mg/100 g) | 1 | 241.36 | 3.4667 | 0.025 |
| DCA1 | DCA axis 1 | 1 | 241.82 | 2.9982 | 0.035 |
| CE | Calamagrostis epigejos | 1 | 243.54 | 1.2636 | NS |
| PC | Poa compressa | 1 | 243.54 | 1.2636 | NS |
| DCA2 | DCA axis 2 | 1 | 243.62 | 1.1863 | NS |
| Year | Year of study | 1 | 244.14 | 0.6832 | NS |
| P | P2O5 (mg/100 g) | 1 | 244.36 | 0.4712 | NS |
| pH | pH | 1 | 244.69 | 0.1569 | NS |
| Species | Soil | Spoil | Method | AMF | References |
|---|---|---|---|---|---|
| Calamagrostis epigejos | loess, perlite, Czech Republik | coal mine | Trouvelot | 94% | [55] |
| loess, clay, perlite, Czech Republik | coal mine | Trouvelot | 80% | [55] | |
| clay, Czech Republik | contains fly ash from a power station burning brown coal | Giovanetti and Mosse | present, no data | [68] | |
| loess, Czech Republik | coal mine | Trouvelot | 75–97% | [69] | |
| gravel, sand, loess, | fireloam strip mine | McGonigle et al. | present, no data | [66] | |
| caolinite-montmorilonite-illite clays | industral areas | Giovanetti and Mosse | present, no data | [52] | |
| clay, Czech Republik | coal mine | Giovanetti and Mosse | present | [50] | |
| Poa compressa | calcareous slope with thin-layered rendzina soil | exposed to emissions of a nearby phosphate fertilizer factory | McGonigle et al. | present 12–42% | [58] |
| peat-vermiculite | - | McGonigle et al. | present | [70] | |
| clay | - | - | 40% | [71] | |
| mulched | coal mine | McGonigle et al. | 5–95% | [72] | |
| clay, rocks | serpentine open-pit mine | McGonigle et al. | present | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, G.; Chmura, D.; Małkowski, E.; Zieleźnik-Rusinowska, P.; Sitko, K.; Ziemer, B.; Błońska, A. Is the Age of Novel Ecosystem the Factor Driving Arbuscular Mycorrhizal Colonization in Poa compressa and Calamagrostis epigejos? Plants 2021, 10, 949. https://doi.org/10.3390/plants10050949
Woźniak G, Chmura D, Małkowski E, Zieleźnik-Rusinowska P, Sitko K, Ziemer B, Błońska A. Is the Age of Novel Ecosystem the Factor Driving Arbuscular Mycorrhizal Colonization in Poa compressa and Calamagrostis epigejos? Plants. 2021; 10(5):949. https://doi.org/10.3390/plants10050949
Chicago/Turabian StyleWoźniak, Gabriela, Damian Chmura, Eugeniusz Małkowski, Paulina Zieleźnik-Rusinowska, Krzysztof Sitko, Barbara Ziemer, and Agnieszka Błońska. 2021. "Is the Age of Novel Ecosystem the Factor Driving Arbuscular Mycorrhizal Colonization in Poa compressa and Calamagrostis epigejos?" Plants 10, no. 5: 949. https://doi.org/10.3390/plants10050949
APA StyleWoźniak, G., Chmura, D., Małkowski, E., Zieleźnik-Rusinowska, P., Sitko, K., Ziemer, B., & Błońska, A. (2021). Is the Age of Novel Ecosystem the Factor Driving Arbuscular Mycorrhizal Colonization in Poa compressa and Calamagrostis epigejos? Plants, 10(5), 949. https://doi.org/10.3390/plants10050949










