The Effect of Foliar and Ground-Applied Essential Nutrients on Huanglongbing-Affected Mature Citrus Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site, Experimental Design, and Treatments
2.2. Soil Sampling, Extraction, and Nutrient Analysis
2.3. Leaf and Root Tissue Nutrient Concentration
2.4. Vegetative Growth
2.4.1. Leaf Area Index
2.4.2. Tree Canopy Volume
2.5. Statistics and Data Analysis
3. Results and Discussion
3.1. Soil Nutrient Concentration
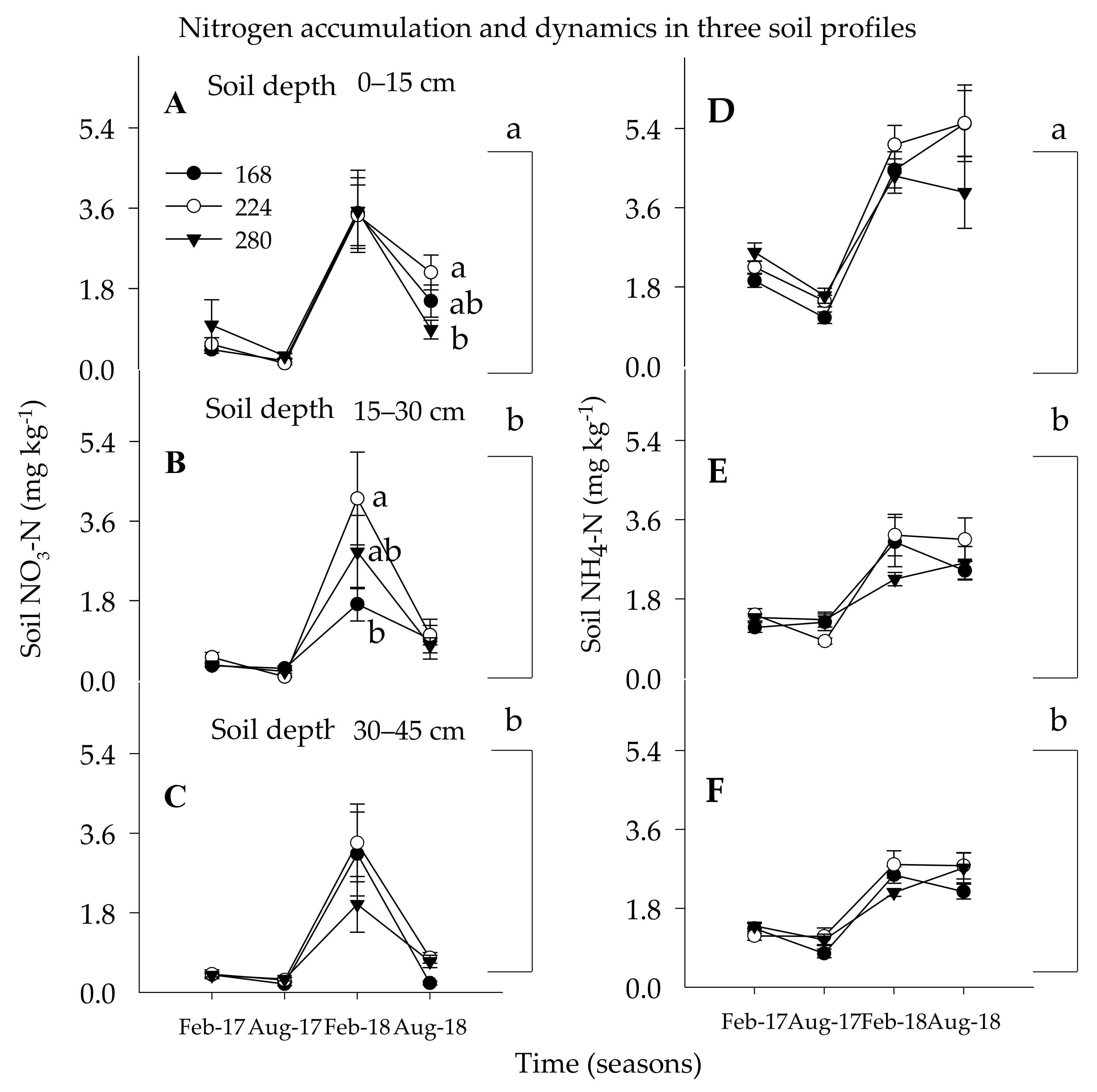
3.1.1. Soil Nitrate Nitrogen
3.1.2. Soil Ammonium Nitrogen
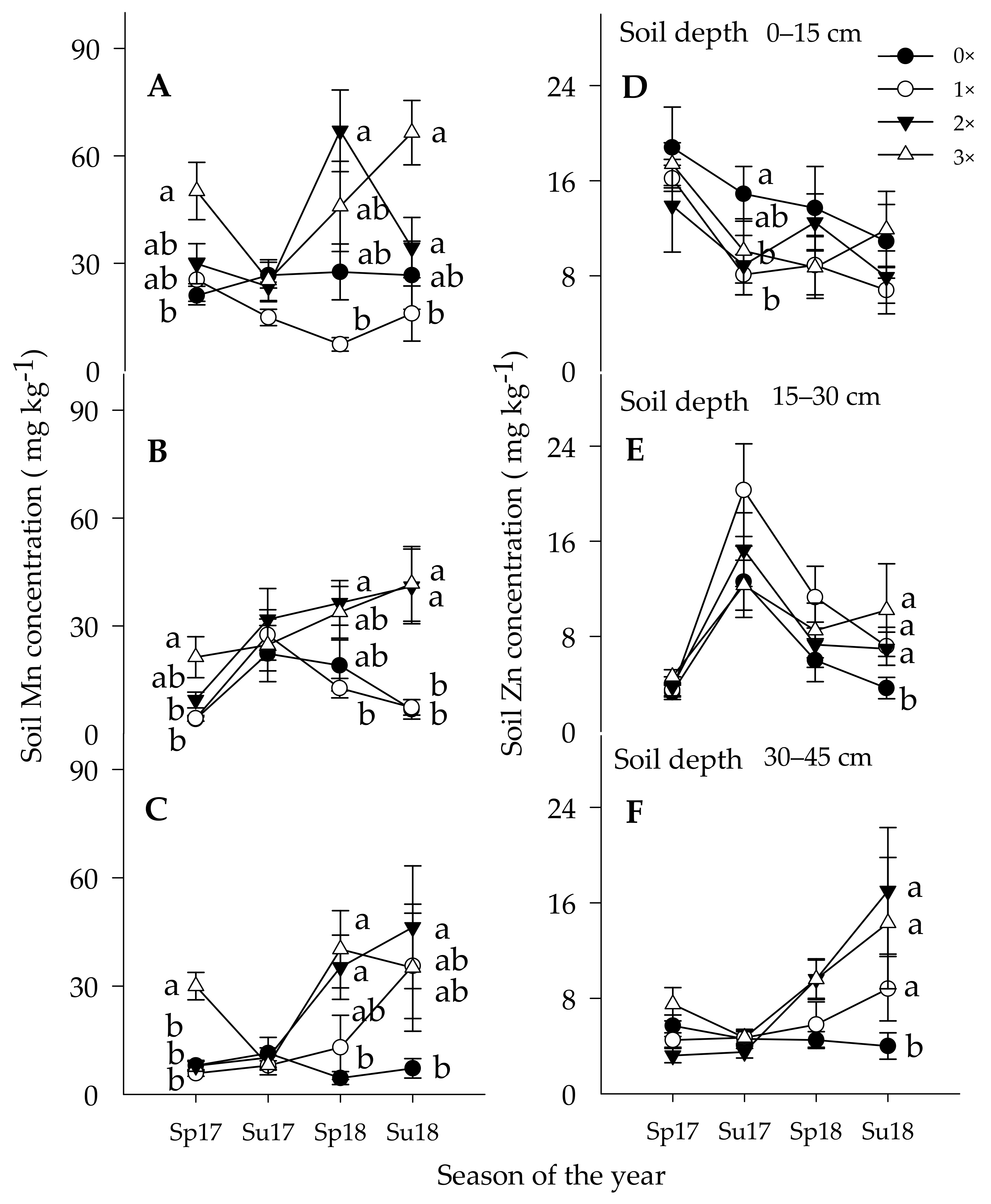
3.1.3. Soil Manganese Concentration
3.1.4. Soil Zinc Concentration
3.1.5. Other Soil Nutrients
3.2. Root Nutrient Concentration
3.2.1. Root Mn Concentration
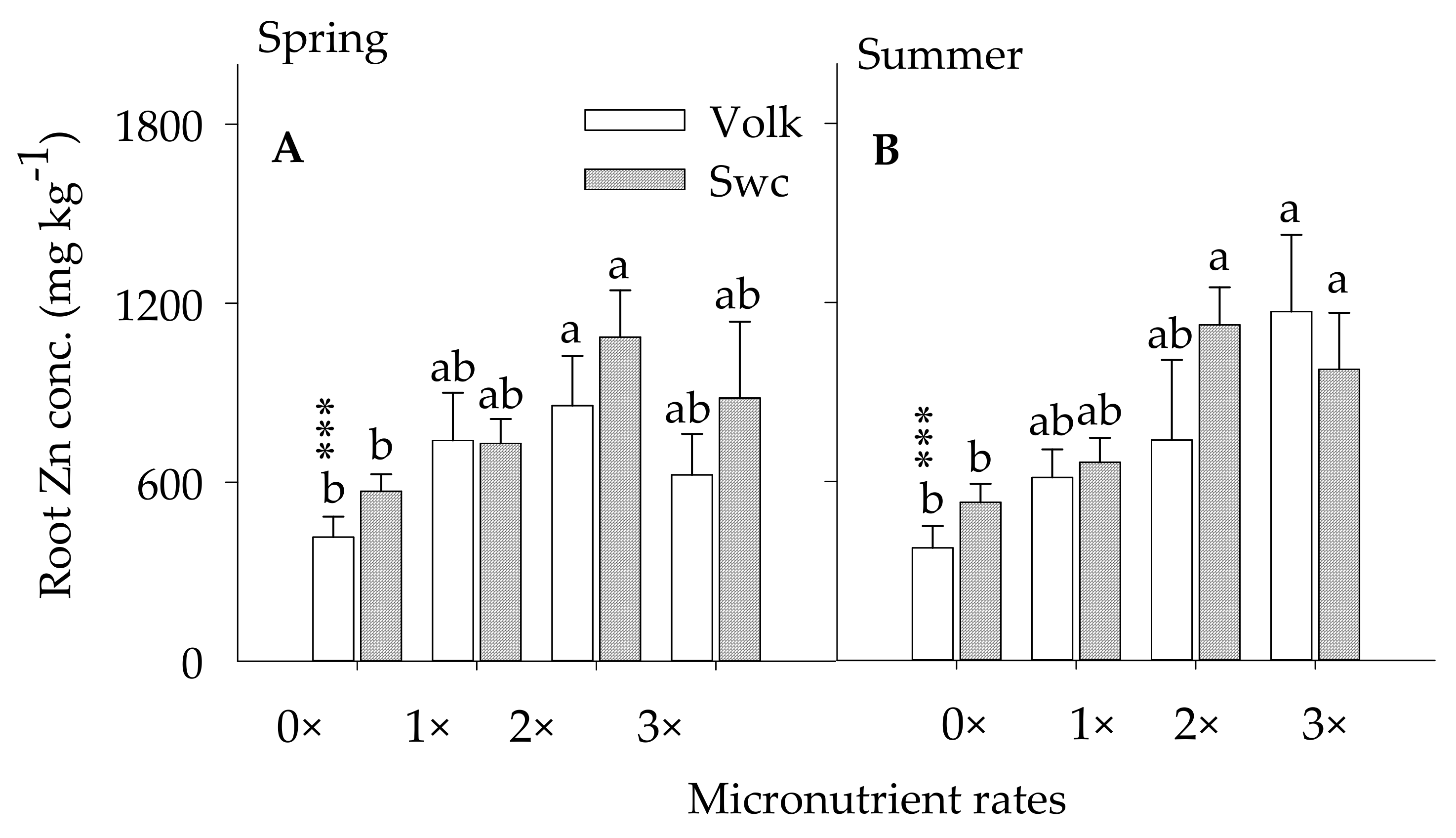
3.2.2. Root Zinc Concentration
3.3. Vegetative Growth and Leaf Nutrient Concentration
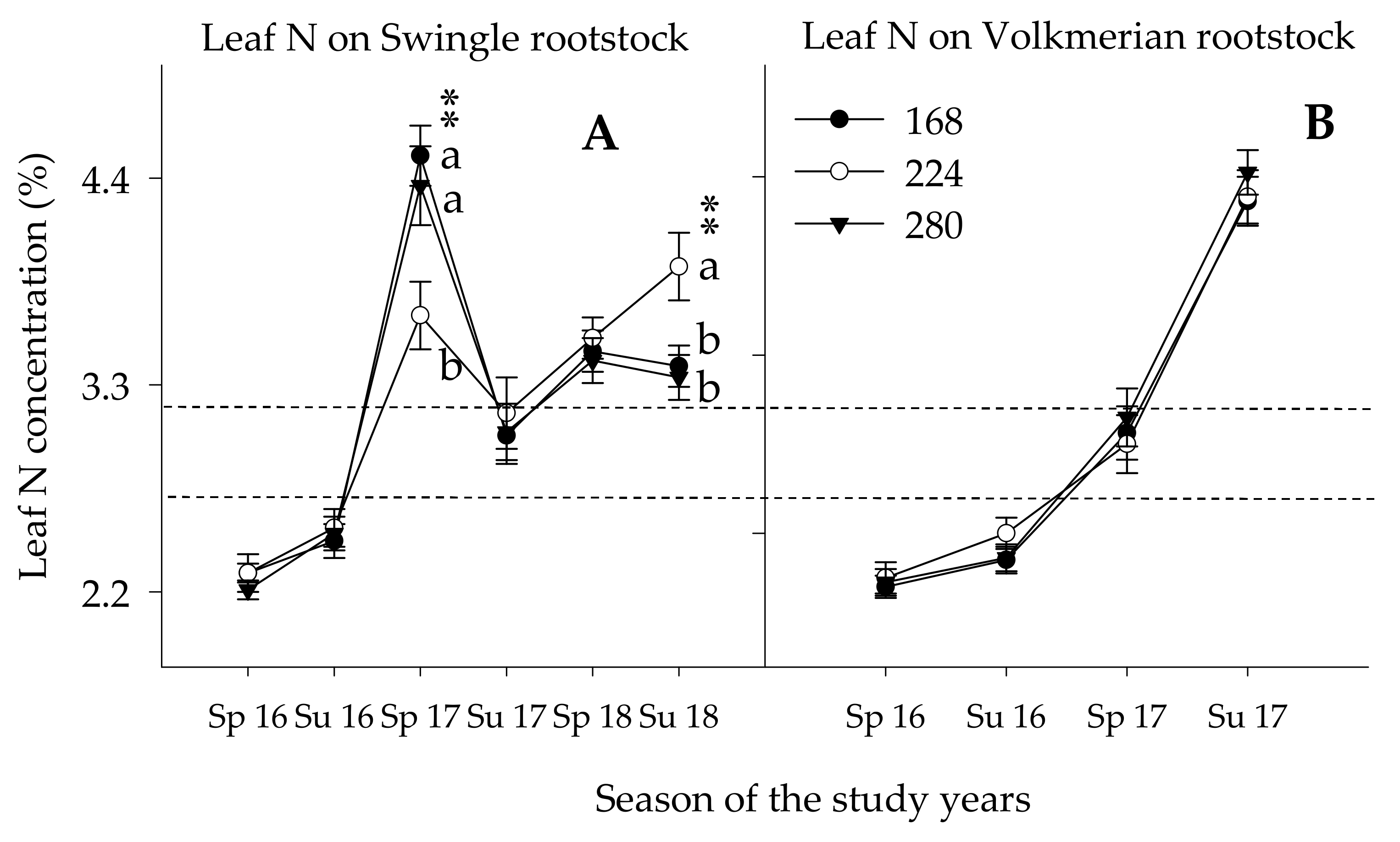
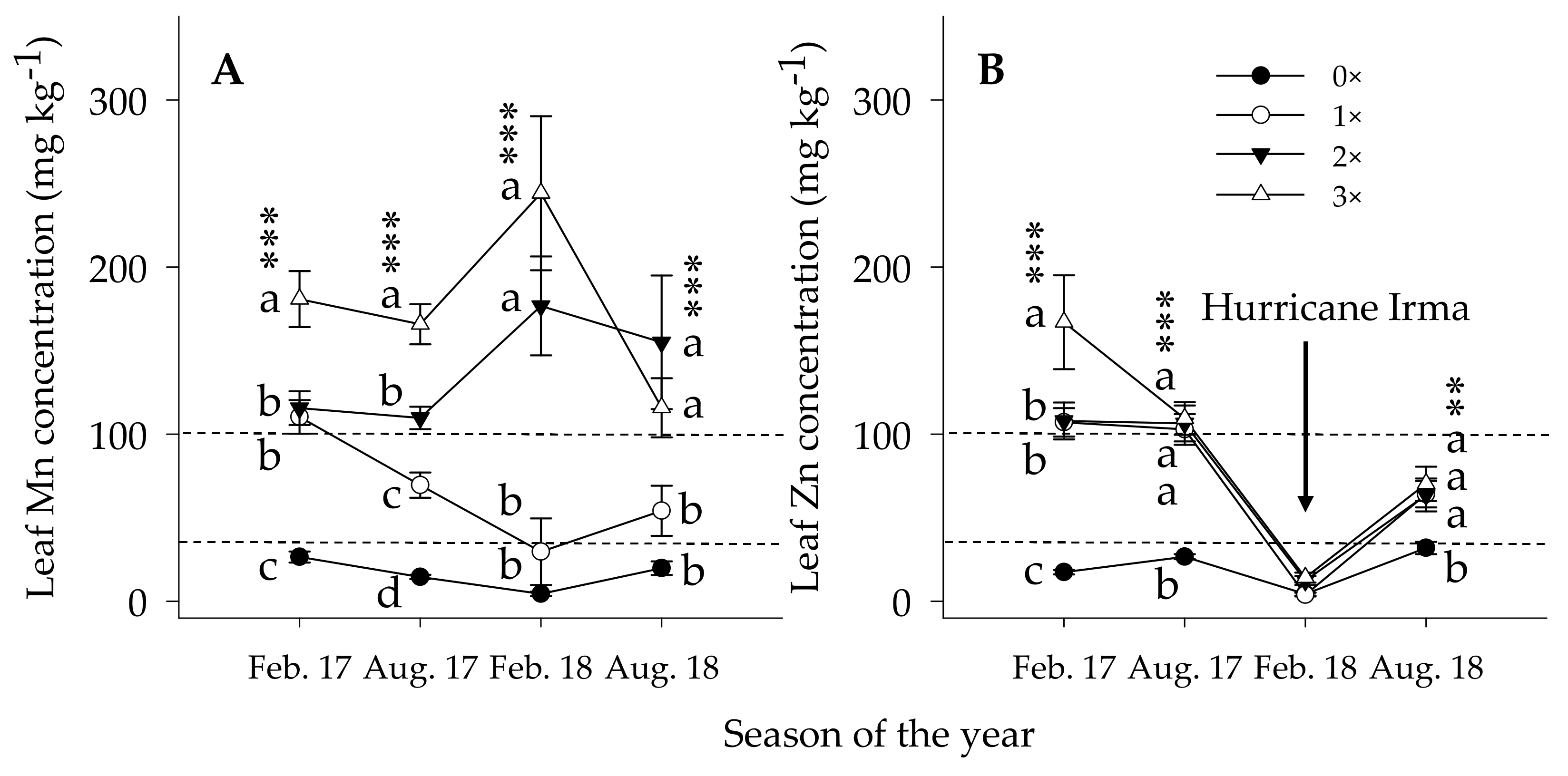
3.3.1. Leaf Nutrient Concentration
3.3.2. Leaf Area Index
3.3.3. Tree Canopy Volume
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cimò, G.; Bianco, R.L.; Gonzalez, P.; Bandaranayake, W.; Etxeberria, E.; Syvertsen, J.P. Carbohydrate and Nutritional Responses to Stem Girdling and Drought Stress with Respect to Understanding Symptoms of Huanglongbing in Citrus. HortScience 2013, 48, 920–928. [Google Scholar] [CrossRef]
- Cao, J.; Cheng, C.; Yang, J.; Wang, Q. Pathogen infection drives patterns of nutrient resorption in citrus plants. Sci. Rep. 2015, 5, 14675. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Achor, D.S.; Brlansky, R.H.; Li, Z.-G.; Gmitter, F.G. Differential anatomical responses of tolerant and susceptible citrus species to the infection of ‘Candidatus Liberibacter asiaticus’. Physiol. Mol. Plant Pathol. 2013, 83, 69–74. [Google Scholar] [CrossRef]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil 2012, 368, 73–85. [Google Scholar] [CrossRef]
- Hall, D.G.; Gottwald, T.R. Pest Management Practices Aimed at Curtailing Citrus Huanglongbing Disease. Outlooks Pest Manag. 2011, 22, 189–192. [Google Scholar] [CrossRef]
- Graham, J.H.; Johnson, E.G.; Gottwald, T.R.; Irey, M.S. Presymptomatic Fibrous Root Decline in Citrus Trees Caused by Huanglongbing and Potential Interaction with Phytophthora spp. Plant Dis. 2013, 97, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.T.; Rouse, R.E.; Ebel, R.C. Foliar Applications of Essential Nutrients on Growth and Yield of ‘Valencia’ Sweet Orange Infected with Huanglongbing. HortScience 2016, 51, 1482–1493. [Google Scholar] [CrossRef]
- Roka, F.; Muraro, R.; Morris, A.R.; Spyke, P.; Morgan, k.; Schumann, A.; Castle, W.; Stover, E. Citrus production systems to survive greening: Economic thresholds. Proc. FL State Hortic. Soc. 2009, 122, 122–126. [Google Scholar]
- Spann, T.M.; Tsagkarakis, A.E.; Syvertsen, J.P. Managing Citrus Tree Growth with Hedging and Plant Growth Regulators: Strategies for Reducing Psyllid Feeding and Huanglongbing Infection. Proc. FL State Hort. Soc. 2009, 122, 161–165. [Google Scholar]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P. Effect of Irrigation Pattern and Timing on Root Density of Young Citrus Trees Infected with Huanglongbing Disease. HortTechnology 2014, 24, 209–221. [Google Scholar] [CrossRef]
- Spann, T.M.; Schumann, A.W. The Role of Plant Nutrients in Disease Development with Emphasis on Citrus and Huanglong-bing. Proc. FL State Hortic. Sci. 2009, 122, 169–171. [Google Scholar]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M.; Mahmoud, K.A. Water and Soil Nutrient Dynamics of Huanglongbing-A ffected Citrus Trees as Impacted by Ground-Applied Nutrients. Agronomy 2020, 10, 1485. [Google Scholar] [CrossRef]
- Gottwald, T.; Graham, J.; Irey, M.; McCollum, T.; Wood, B. Inconsequential effect of nutritional treatments on huanglongbing control, fruit quality, bacterial titer and disease progress. Crop. Prot. 2012, 36, 73–82. [Google Scholar] [CrossRef]
- Uthman, Q.; Kadyampakeni, D.; Nkedi-Kizza, P.; Barlas, N.; Atta, A.; Morgan, K. Comparative Response of Huanglongbing-Affected Sweet Orange Trees to Nitrogen and Zinc Fertilization under Microsprinkler Irrigation. Agriculture 2020, 10, 489. [Google Scholar] [CrossRef]
- Zambon, F.T.; Kadyampakeni, D.M.; Grosser, J.W. Ground Application of Overdoses of Manganese Have a Therapeutic Effect on Sweet Orange Trees Infected with Candidatus Liberibacter asiaticus. HortScience 2019, 54, 1077–1086. [Google Scholar] [CrossRef]
- Fu, X.-Z.; Xing, F.; Cao, L.; Chun, C.-P.; Ling, L.-L.; Jiang, C.-L.; Peng, L.-Z. Effects of Foliar Application of Various Zinc Fertilizers with Organosilicone on Correcting Citrus Zinc Deficiency. HortScience 2016, 51, 422–426. [Google Scholar] [CrossRef]
- Obreza, T.A.; Schumann, A. Keeping Water and Nutrients in the Florida Citrus Tree Root Zone. HortTechnology 2010, 20, 67–73. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, S. Zinc Nutrition, a Global Concern for Sustainable Citrus Production. J. Sustain. Agric. 2005, 25, 5–42. [Google Scholar] [CrossRef]
- Jimenez, A.; Torrecillas, A.; Sevilla, F.; Ortuño, M.F.; Conejero, W.; Ferreres, F.; Medina, S.F.; Galindo, A.; Gilizquierdo, A. Lime-Induced Iron Chlorosis in Citrus: Diagnosis Through Physiological and Metabolic Evidences. In Advances in Citrus Nutrition; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 321–331. [Google Scholar]
- Hippler, F.W.R.; Boaretto, R.M.; Quaggio, J.A.; Boaretto, A.E.; Abreu-Junior, C.H.; Mattos, D., Jr. Uptake and distribution of soil applied zinc by Citrus trees—addressing fertilizer use efficiency with 68Zn labeling. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Hasani, M.; Zamani, Z.; Savaghebi, G.; Fatahi, R. Effects of zinc and manganese as foliar spray on pomegranate yield, fruit quality and leaf minerals. J. Soil Sci. Plant Nutr. 2012, 12, 471–480. [Google Scholar] [CrossRef]
- Alva, A.K.; Paramasivam, S.; Fares, A.; Obreza, T.A.; Schumann, A.W. Nitrogen best management practice for citrus trees II. Nitrogen fate, transport, and components of N budget. Sci. Hortic. 2006, 109, 223–233. [Google Scholar] [CrossRef]
- Aubert, B. Huanglongbing (HLB) a Diagnosis and strategies for control in Reunion Island A Case Study. IRCHLB Proc. 2008, 2008, 1–34. [Google Scholar]
- Morgan, K.; Scholberg, J.; Obreza, T.; Wheaton, T. Size, Biomass, and Nitrogen Relationships with Sweet Orange Tree Growth. J. Am. Soc. Hortic. Sci. 2006, 131, 149–156. [Google Scholar] [CrossRef]
- Paramasivam, S.; Alva, A.K.; Fares, A.; Sajwan, K.S. Estimation of Nitrate Leaching in an Entisol under Optimum Citrus Production. Soil Sci. Soc. Am. J. 2001, 65, 914–921. [Google Scholar] [CrossRef]
- Kiggundu, N.; Migliaccio, K.W.; Schaffer, B.; Li, Y.; Crane, J.H. Water savings, nutrient leaching, and fruit yield in a young avocado orchard as affected by irrigation and nutrient management. Irrig. Sci. 2011, 30, 275–286. [Google Scholar] [CrossRef]
- Syvertsen, J.; Smith, M. Nitrogen Uptake Efficiency and Leaching Losses from Lysimeter-grown Citrus Trees Fertilized at Three Nitrogen Rates. J. Am. Soc. Hortic. Sci. 1996, 121, 57–62. [Google Scholar] [CrossRef]
- Morgan, K.T. Nutrient Status and Root Density of Huanglongbing-A ffected Trees: Consequences of Irrigation Water Bicarbonate and Soil pH Mitigation with Acidification. Agronomy 2019, 9, 746. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, S. Citrus Decline: Soil Fertility and Plant Nutrition. J. Plant Nutr. 2009, 32, 197–245. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M. Effect of Essential Nutrients on Roots Growth and Lifespan of Huanglongbing Affected Citrus Trees. Plants 2020, 9, 483. [Google Scholar] [CrossRef]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P. Manganese adsorption, availability, and uptake in citrus under microsprinkler irrigation. Age 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Tian, S.; Lu, L.; Labavitch, J.M.; Webb, S.M.; Yang, X.; Brown, P.H.; He, Z. Spatial imaging of Zn and other elements in Huanglong-bing-affected grapefruit by synchrotron-based micro X-ray fluorescence investigation. J. Exp. Bot. 2014, 65, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as Essential and Toxic Element for Plants: Transport, Accumulation and Resistance Mecanisms. J. Soil Sci. Plant Nat. 2010, 10, 476–494. [Google Scholar] [CrossRef]
- Xue, S.; Chen, Y.; Reeves, R.D.; Baker, A.J.; Lin, Q.; Fernando, D.R. Manganese uptake and accumulation by the hyperaccumulator plant Phytolacca acinosa Roxb. (Phytolaccaceae). Environ. Pollut. 2004, 131, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mina, J.M. Plant Nutrition and Defense Mechanism: Frontier Knowledge. In Advances in Citrus Nutrition; Springer: New York, NY, USA, 2012; pp. 1–12. [Google Scholar]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P. Boron availability and uptake in huanglongbing-affected citrus trees on a Florida entisol. J. Plant Nutr. 2020, 43, 1248–1258. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Shyam, S. Boron nutrition in citrus-current status and future strategies–a review. Agric. Rev. Res. Commun. Cent. India 2005, 26, 173–186. [Google Scholar]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef]
- Elrashidi, M.A.; O’Connor, G.A. Boron Sorption and Desorption in Soils. Soil. Sci. Soc. Am. J. 1982, 46, 27. [Google Scholar] [CrossRef]
- Morgan, K.T.; Hanlon, E.A. Improving Citrus Nitrogen Uptake Efficiency: Understanding Citrus Nitrogen Requirements 1 Citrus Fruit Crop Requirement for. EDIS 2006, 2006, 1–5. [Google Scholar]
- Quiñones, A.; Martínez-Alcántara, B.; Legaz, F. Influence of irrigation system and fertilization management on seasonal distribution of N in the soil profile and on N-uptake by citrus trees. Agric. Ecosyst. Environ. 2007, 122, 399–409. [Google Scholar] [CrossRef]
- Obreza, T.A.; Morgan, K.T. Recommended Fertilizer Rates and Timing. In Nutrition of Florida Citrus Trees, Elec. data info. Source, SL 253; University of Florida Institute of Food and Agricultural Sciences: Gainesville, GL, USA, 2008; pp. 48–57. [Google Scholar]
- Mehlich, A.; Carolina, N. Communications in Soil Science and Plant Analysis Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 2008, 15, 1409–1416. [Google Scholar] [CrossRef]
- Mylavarapu, R.S.; Sanchez, J.F.; Nguyen, J.H.; Bartos, J.M. Evaluation of Mehlich-1 and Mehlich-3 extraction procedures for plant nutrients in acid mineral soils of Florida*. Commun. Soil Sci. Plant Anal. 2002, 33, 807–820. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P.; Mahmoud, K. Ammonium and Nitrate Distribution in Soil Using Drip and Microsprinkler Irrigation for Citrus Production. Soil Sci. Soc. Am. J. 2014, 78, 645–654. [Google Scholar] [CrossRef]
- Brunetto, G.; De Melo, G.W.B.; Toselli, M.; Quartieri, M.; Tagliavini, M. THE Role of Mineral Nutrition on Yields and Fruit Quality in Grapevine, Pear and Apple. Rev. Bras. de Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen Nutrition of Fruit Trees to Reconcile Productivity and Environmental Concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef]
- Hue, N.V.; Uchida, R.; Ho, M.C. Sampling and analysis of soils and plant tissues: How to take representative samples, how the samples are tested. In Plant Nutrient Management in Hawaii’s Soils: Approaches for Tropical and Subtropical Agriculture; University of Hawaii: Honolulu, HI, USA, 2000; pp. 23–30. [Google Scholar]
- Lavon, R.; Salomon, R.; Goldschmidt, E.E. Effect of Potassium, Magnesium, and Calcium Deficiencies on Nitrogen Constituents and Chloroplast Components in Citrus Leaves. J. Am. Soc. Hortic. Sci. 1999, 124, 158–162. [Google Scholar] [CrossRef]
- Werner, L.P.; Jull, L.G. Fertilizer nitrogen uptake and partitioning in young and mature common hackberry (Celtis occidentalis) trees. Arboric. Urban For. 2013, 39, 85–93. [Google Scholar]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W. Biomass, nutrient accumulation and tree size relationships for drip- and microsprinkler-irrigated orange trees. J. Plant Nutr. 2015, 39, 589–599. [Google Scholar] [CrossRef]
- Ebel, R.C.; Hamido, S.; Morgan, K.T. Interaction of Huanglongbing and Foliar Applications of Copper on Growth and Nutrient Acquisition of Citrus sinensis cv. Valencia. HortScience 2019, 54, 297–302. [Google Scholar] [CrossRef]
- Bréda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef]
- Alva, A.K.; Paramasivam, S. Nitrogen Management for High Yield and Quality of Citrus in Sandy Soils. Soil Sci. Soc. Am. J. 1998, 62, 1335–1342. [Google Scholar] [CrossRef]
- Morgan, K.T.; Wheaton, T.A.; Castle, W.S.; Parsons, L.R. Response of Young and Maturing Citrus Trees Grown on a Sandy Soil to Irrigation Scheduling, Nitrogen Fertilizer Rate, and Nitrogen Application Method. HortScience 2009, 44, 145–150. [Google Scholar] [CrossRef]
- Mattos, D.; Graetz, D.A.; Alva, A.K. Biomass Distribution and Nitrogen-15 Partitioning in Citrus Trees on a Sandy Entisol. Soil Sci. Soc. Am. J. 2014, 67, 555. [Google Scholar] [CrossRef]
- Khakural, B.R.; Alva, A.K. Communications in Soil Science and Plant Analysis Transformation of urea and ammonium nitrate in an entisol and a spodosol under citrus production Transformation of Urea and Ammonium Nitrate in an Entisol and a Spodosol Under. Commun. Soil Sci. Plant Anal. 2008, 27, 3045–3057. [Google Scholar] [CrossRef]
- Sato, S.; Morgan, K.T.; Ozores-hampton, M.; Simonne, E.H. Spatial and Temporal Distributions in Sandy Soils with Seepage Irrigation: I. Ammonium and Nitrate. Soil Sci. Soc. Am. J. 2009, 73, 1044–1052. [Google Scholar] [CrossRef]
- Khan, A.S.; Ullah, W.; Malik, A.U.; Ahmad, R.; Saleem, B.A.; Rajwana, I.A. Exogenous applications of boron and zinc influence leaf nutrient status, tree growth and fruit quality of feutrell’s early (Citrus reticulata Blanco). Pak. J. Agric. Sci. 2012, 49, 113–119. [Google Scholar]
- Eller, F.; Brix, H. Influence of low calcium availability on cadmium uptake and translocation in a fast-growing shrub and a metal-accumulating herb. AoB Plants 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Mattos, D., Jr.; Quaggio, J.A.; Cantarella, H.; Alva, A.K. Nutrient Content of Biomass Components. Sci. Agrícola 2003, 60, 155–160. [Google Scholar] [CrossRef]
- Paramasivam, S.; Alva, A.K.; Hostler, K.H.; Easterwood, G.W.; Southwell, J.S. Fruit nutrient accumulation of four orange varieties during fruit development. J. Plant Nutr. 2000, 23, 313–327. [Google Scholar] [CrossRef]
- Azam, F.; Malik, K.A.; Sajjad, M.I. Transformation sin soil and availability to plants o f is N applied as inorganic fertilizer and legume residues Introduction Nitrogen applied to soil as plant residues or in the form or inorganic fertilizers may be taken up by plant. Plant Soil 1985, 86, 3–13. [Google Scholar] [CrossRef]
- Hamido, S.A.; Morgan, K.T.; Ebel, R.C.; Kadyampakeni, D.M. Improved Irrigation Management of Sweet Orange with Huanglongbing. HortScience 2017, 52, 916–921. [Google Scholar] [CrossRef]
- Jezek, M.; Geilfus, C.-M.; Bayer, A.; Mühling, K.-H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Colaço, A.F.; Trevisan, R.G.; Molin, J.P.; Rosell-Polo, J.R.; Escolà, A. Orange tree canopy volume estimation by manual and LiDAR-based methods. Adv. Anim. Biosci. 2017, 8, 477–480. [Google Scholar] [CrossRef]
- Wheaton, T.; Whitney, J.; Castle, W.; Muraro, R.; Browning, H.; Tucker, D. Citrus Scion and Rootstock, Topping Height, and Tree Spacing Affect Tree Size, Yield, Fruit Quality, and Economic Return. J. Am. Soc. Hortic. Sci. 1995, 120, 861–870. [Google Scholar] [CrossRef]

| Method and Rate of Application (kg ha−1 year−1) | |||||||
|---|---|---|---|---|---|---|---|
| Soil | Foliar | Soil | |||||
| Treatments | N rate 1 | Mn 2 | Zn 3 | B 4 | Mn 5 | Zn 5 | B 6 |
| 0× | 168 | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ |
| 224 | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ | |
| 280 | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ | |
| 1× | 168 | 9 | 9 | 2.3 | ̶ | ̶ | ̶ |
| 224 | 9 | 9 | 2.3 | ̶ | ̶ | ̶ | |
| 280 | 9 | 9 | 2.3 | ̶ | ̶ | ̶ | |
| 2× | 168 | 9 | 9 | 2.3 | 9 * | 9 * | 2.3 * |
| 224 | 9 | 9 | 2.3 | 9 | 9 | 2.3 | |
| 280 | 9 | 9 | 2.3 | 9 | 9 | 2.3 | |
| 3× | 168 | 9 | 9 | 2.3 | 18 ** | 18 ** | 4.6 ** |
| 224 | 9 | 9 | 2.3 | 18 | 18 | 4.6 | |
| 280 | 9 | 9 | 2.3 | 18 | 18 | 4.6 | |
| Spring 2017 | Summer 2017 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NO3-N | NH4-N | Mn | Zn | B | NO3-N | NH4-N | Mn | Zn | B | |
| Model Variables 1 | Significance Level 2 | |||||||||
| D | * | * | NS | NS | * | * | NS | * | * | *** |
| N | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| M | NS | NS | * | NS | NS | NS | NS | NS | NS | NS |
| Spring 2018 | Summer 2018 | |||||||||
| D | NS | * | * | NS | *** | * | NS | * | NS | * |
| N | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| M | NS | NS | *** | NS | NS | NS | * | NS | NS | NS |
| D × M | * | NS | NS | NS | NS | * | NS | ** | ** | NS |
| N × R × M | NS | NS | NS | NS | * | NS | NS | NS | NS | NS |
| Soil Nutrient Concentration (mg kg−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spring 2018 | |||||||||
| Treatments 1 | NO3-N | NH4-N | P | K | Ca | Mg | B | Cu | Fe |
| Spring 2018 | |||||||||
| 0× | 1.9 ± 1.4 | 3.85 ± 0.6 | 28 ± 4 | 28 ± 3 | 356 ± 38a | 28 ± 4a | 0.4 ± 0.0ab | 13 ± 1 | 69 ± 12 |
| 1× | 2.7 ± 1.5 | 3.72 ± 0.9 | 47 ± 8 | 27 ± 2 | 184 ± 22ab | 21 ± 2b | 0.4 ± 0.1a | 12 ± 2 | 44 ± 7 |
| 2× | 2.6 ± 1.8 | 5.45 ± 1.1 | 33 ± 5 | 26 ± 2 | 109 ± 18ab | 11 ± 1b | 0.3 ± 0.0b | 11 ± 1 | 46 ± 7 |
| 3× | 4.1 ± 1.7 | 5.40 ± 0.5 | 26 ± 6 | 18 ± 3 | 186 ± 73b | 7 ± 2b | 0.3 ± 0.0ab | 11 ± 1 | 62 ± 8 |
| Sign. 2 | NS | NS | NS | NS | ** | ** | * | NS | NS |
| Summer 2018 | |||||||||
| 0× | 1.6 ± 0.4 | 3.3 ± 0.4b | 21 ± 4 | 15 ± 1 | 325 ± 45a | 13 ± 3a | 0.2 ± 0.1 | 9 ± 2 | 59 ± 1 |
| 1× | 1.7 ± 0.6 | 3.6 ± 0.5b | 26 ± 4 | 18 ± 2 | 224 ± 37ab | 13 ± 2a | 0.3 ± 0.0 | 7 ± 1 | 35 ± 1 |
| 2× | 1.4 ± 0.3 | 5.4 ± 0.8b | 27 ± 3 | 22 ± 4 | 208 ± 40ab | 17 ± 7a | 0.2 ± 0.0 | 9 ± 1 | 55 ± 6 |
| 3× | 1.2 ± 0.2 | 7.6 ± 1.1a | 48 ± 13 | 12 ± 2 | 93 ± 27b | 4 ± 3b | 0.3 ± 0.1 | 11 ± 2 | 42 ± 1 |
| Sign. 2 | NS | *** | NS | NS | ** | *** | NS | NS | NS |
| 2017 | 2018 | 2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | Spring | Summer | |||||
| Leaf Area Index | ||||||||||
| Treatments 1 | Volk | Swc | Volk | Swc | Volk | Swc | Volk | Swc | Swc | Swc |
| 0× | 2.6 | 3.4 | 2.6 | 2.4 | nd 2 | 3.6ab | nd | 3.7a | 3.4a | 3.5a |
| 1× | 3.0 | 3.7 | 2.5 | 2.6 | nd | 3.7ab | nd | 3.9a | 3.6a | 3.9a |
| 2× | 2.5 | 3.7 | 2.4 | 2.8 | nd | 3.9a | nd | 3.9a | 3.1a | 4.0a |
| 3× | 2.7 | 3.5 | 2.2 | 2.3 | nd | 3.3b | nd | 3.1b | 2.3b | 2.6b |
| Sign. 3 | NS | NS | NS | NS | ̶ | * | ̶ | *** | *** | *** |
| Model variables 4 | ANOVA | |||||||||
| R | *** | NS | NS | NS | NS | NS | ||||
| M | NS | NS | * | *** | *** | *** | ||||
| N | NS | NS | NS | NS | NS | NS | ||||
| 2017 | 2018 | 2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | Spring | Summer | |||||
| Tree Canopy Volume (m3) | ||||||||||
| Treatments 1 | Volk | Swc | Volk | Swc | Volk | Swc | Volk | Swc | Swc | Swc |
| 0× | 23.0b | 18.4 | 20.2 | 13.6 | ̶ 2 | 14.4 | ̶ | 14.7 | 17.6b | 18.7b |
| 1× | 27.0a | 18.4 | 23.8 | 13.6 | ̶ | 14.3 | ̶ | 17.5 | 19.3ab | 20.2ab |
| 2× | 25.1ab | 19.1 | 22.1 | 14.4 | ̶ | 15.6 | ̶ | 17.8 | 20.7a | 22.6a |
| 3× | 26.3ab | 18.9 | 22.9 | 14.2 | ̶ | 15.0 | ̶ | 16.6 | 19.1ab | 19.5ab |
| Significance 3 | * | NS | NS | NS | NS | NS | NS | NS | * | * |
| Model variables 4 | ANOVA | |||||||||
| R | *** | *** | NS | NS | NS | NS | ||||
| M | NS | NS | NS | NS | * | * | ||||
| N | NS | NS | NS | NS | NS | NS | ||||
| R×N | * | * | NS | NS | NS | NS | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, A.A.; Morgan, K.T.; Kadyampakeni, D.M.; Mahmoud, K.A. The Effect of Foliar and Ground-Applied Essential Nutrients on Huanglongbing-Affected Mature Citrus Trees. Plants 2021, 10, 925. https://doi.org/10.3390/plants10050925
Atta AA, Morgan KT, Kadyampakeni DM, Mahmoud KA. The Effect of Foliar and Ground-Applied Essential Nutrients on Huanglongbing-Affected Mature Citrus Trees. Plants. 2021; 10(5):925. https://doi.org/10.3390/plants10050925
Chicago/Turabian StyleAtta, Alisheikh A., Kelly T. Morgan, Davie M. Kadyampakeni, and Kamal A. Mahmoud. 2021. "The Effect of Foliar and Ground-Applied Essential Nutrients on Huanglongbing-Affected Mature Citrus Trees" Plants 10, no. 5: 925. https://doi.org/10.3390/plants10050925
APA StyleAtta, A. A., Morgan, K. T., Kadyampakeni, D. M., & Mahmoud, K. A. (2021). The Effect of Foliar and Ground-Applied Essential Nutrients on Huanglongbing-Affected Mature Citrus Trees. Plants, 10(5), 925. https://doi.org/10.3390/plants10050925








