Potential Role of Propolis in the Prevention and Treatment of Metabolic Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Types of Propolis and Chemical Composition
4. Preclinical Studies Investigating the Effects of Propolis in Metabolic Diseases
5. Clinical Studies Investigating the Effects of Propolis in Metabolic Diseases
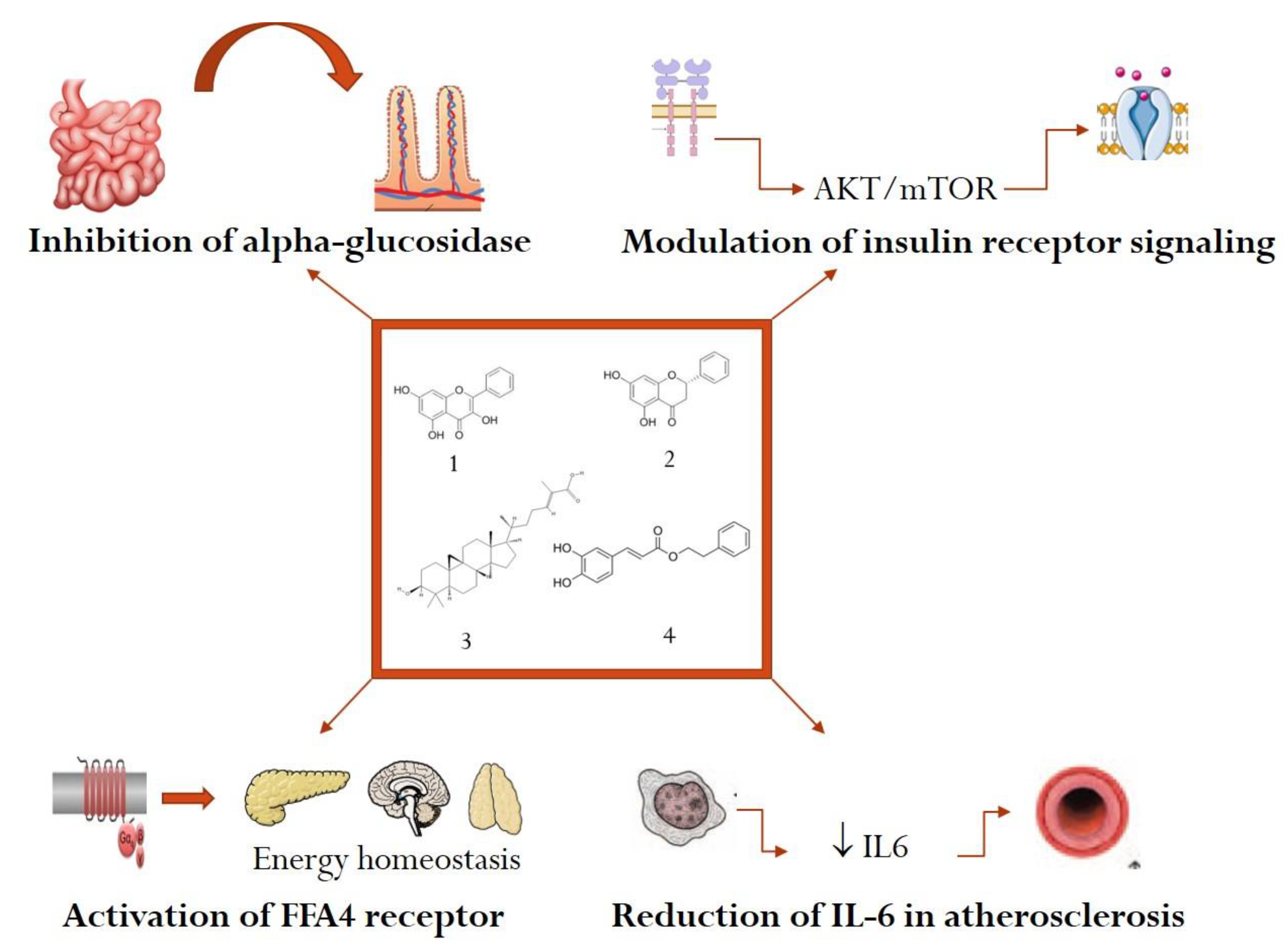
6. Mechanistic Studies with Active Constituents from Propolis in Metabolic Diseases
6.1. Inhibition of Alpha-Amylase and Alpha-Glucosidase in Diabetes Mellitus
6.2. Modulation of Insulin Receptor Signaling in Diabetes Mellitus
6.3. Anti-Inflammatory Mechanisms in Dyslipidemia and Atherosclerosis
6.4. Antioxidant Mechanisms in Dyslipidemia
6.5. Activation of FFA4 Receptor with Positive Effects in Obesity
7. Safety Profile of Propolis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | aspartate aminotransferase |
| APOE2 | apolipoprotein E2 |
| FFA4 | free fatty acid receptor 4 |
| FGF | fibroblast growth factor |
| HDL | high-density lipoprotein |
| HOMA-R | homeostasis model assessment for insulin resistance |
| IC50 | half-maximal inhibitory concentration |
| IRS | insulin receptor substrate |
| LDL | low-density lipoprotein |
| LVH | left ventricular hypertrophy |
| MMP-9 | matrix metalloproteinase 9 |
| mTOR | mammalian target of rapamycin |
| PPAR | peroxysome proliferator activated receptor |
| SREBP | sterol regulatory element binding protein |
| STZ | streptozotocin |
| VCAM | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
References
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the Propolis Chemical Composition between 2013 and 2018: A Review. eFood 2019, 1, 24. [Google Scholar] [CrossRef]
- Pereira, R.L.R.; Salatino, M.L.F.; Salatino, A. Production of propolis and geopropolis by stingless bees. MOJ Food Process. Technol. 2020, 8, 1–3. [Google Scholar] [CrossRef]
- Santos, L.M.; Da Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.C.; Umsza-Guez, M.A.; Barbosa, J.D.V.; Portela, R.D.; Machado, B.A.S. Propolis: Types, composition, biological activities, and veterinary product patent prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Bankova, V. Recent trends and important developments in propolis research. Evid. Based Complement. Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef]
- Alday, M.N.-N.E. Advances in Pharmacological Activities and Chemical Composition of Propolis Produced in Americas. In Beekeeping and Bee Conservation—Advances in Research; IntechOpen: London, UK, 2016. [Google Scholar]
- Talhouk, R.; Salloum, R.; Homaidan, F. Inflammatory Diseases. In Medicinal Plants; Apple Academic Press: Palm Bay, FL, USA, 2012; pp. 495–525. [Google Scholar]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Shahinozzaman, M.; Obanda, D.N.; Tawata, S. Chemical composition and pharmacological properties of Macaranga—Type Pacific propolis: A review. Phytother. Res. 2021, 35, 207–222. [Google Scholar] [CrossRef]
- Derevici, A.; Popesco, A.; Popesco, N. Recherches sur Certaines Propriétés Biologiques de la Propolis. Apidologie 1964, 7, 191–200. [Google Scholar] [CrossRef][Green Version]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Sarıçoban, C.; Yerlikaya, S. As a Protective Material: Propolis. J. Agroaliment. Process. Technol. 2016, 22, 56–63. [Google Scholar]
- Khalil, M.L. Biological activity of bee propolis in health and disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Sun, S.; He, J.; Liu, M.; Yin, G.; Zhang, X. A Great Concern Regarding the Authenticity Identification and Quality Control of Chinese Propolis and Brazilian Green Propolis. J. Food Nutr. Res. 2019, 7, 725–735. [Google Scholar] [CrossRef]
- Alenezi, S.S.; Natto, M.J.; Igoli, J.O.; Gray, A.I.; Fearnley, J.; Fearnley, H.; De Koning, H.P.; Watson, D.G. Novel flavanones with anti-trypanosomal activity isolated from Zambian and Tanzanian propolis samples. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Hosoya, T.; Yoshizumi, K.; Sato, H.; Kumazawa, S. Phytochemical and anti-inflammatory properties of Senegalese propolis and isolated compounds. Fitoterapia 2021, 151, 104861. [Google Scholar] [CrossRef] [PubMed]
- Mukaide, K.; Honda, S.; Vongsak, B.; Kumazawa, S. Prenylflavonoids from propolis collected in Chiang Mai, Thailand. Phytochem. Lett. 2021, 43, 88–93. [Google Scholar] [CrossRef]
- Stojanović, S.; Najman, S.J.; Bogdanova-Popov, B.; Najman, S.S. Propolis: Chemical Composition, Biological and Pharmacological Activity—A Review. Acta Med. Median. 2020, 59, 108–113. [Google Scholar] [CrossRef]
- Athikomkulchai, S.; Awale, S.; Ruangrungsi, N.; Ruchirawat, S.; Kadota, S. Chemical constituents of Thai propolis. Fitoterapia 2013, 88, 96–100. [Google Scholar] [CrossRef]
- Fu, S.-H.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Analysis of flavonoids in propolis by capillary electrophoresis. J. Food Drug Anal. 2005, 13, 4. [Google Scholar] [CrossRef]
- de Mendonça, I.C.G.; Porto, I.C.C.D.M.; Nascimento, T.G.D.; De Souza, N.S.; Oliveira, J.M.D.S.; Arruda, R.E.D.S.; Mousinho, K.C.; dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef]
- Hamasaka, T.; Kumazawa, S.; Fujimoto, T.; Nakayama, T. Antioxidant Activity and Constituents of Propolis Collected in Various Areas of Japan. Food Sci. Technol. Res. 2004, 10, 86–92. [Google Scholar] [CrossRef]
- Kahramanoğlu, I.; Okatan, V.; Wan, C. Biochemical Composition of Propolis and Its Efficacy in Maintaining Postharvest Storability of Fresh Fruits and Vegetables. J. Food Qual. 2020, 2020, 8869624. [Google Scholar] [CrossRef]
- Miguel, M.G. Chemical and biological properties of propolis from the western countries of the Mediterranean basin and Portugal. Int. J. Pharm. Pharm. Sci. 2013, 5, 403–409. [Google Scholar]
- Noureddine, H.; Hage-Sleiman, R.; Wehbi, B.; Fayyad-Kazan, H.; Hayar, S.; Traboulssi, M.; Alyamani, O.A.; Faour, W.H.; ElMakhour, Y. Chemical characterization and cytotoxic activity evaluation of Lebanese propolis. Biomed. Pharmacother. 2017, 95, 298–307. [Google Scholar] [CrossRef]
- Frozza, C.O.D.S.; Garcia, C.S.C.; Gambato, G.; de Souza, M.D.O.; Salvador, M.; Moura, S.; Padilha, F.F.; Seixas, F.K.; Collares, T.; Borsuk, S.; et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food Chem. Toxicol. 2013, 52, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; Pasin, F.D.R.; Tsvetkova, I. Bioactive Constituents of Brazilian Red Propolis. Evid. Based Complement. Altern. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Wali, A.F.; Mushtaq, A.; Rehman, M.U.; Akbar, S.; Masoodi, M.H. Bee Propolis (Bee’s Glue): A Phytochemistry Review. J. Crit. Rev. 2017, 4, 9–13. [Google Scholar] [CrossRef]
- Afrouzan, H.; Tahghighi, A.; Zakeri, S.; Es-Haghi, A. Chemical Composition and Antimicrobial Activities of Iranian Propolis. Iran. Biomed. J. 2017, 22, 50–65. [Google Scholar] [PubMed]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef]
- Anđelković, B.; Vujisić, L.; Vučković, I.; Tešević, V.; Vajs, V.; Gođevac, D. Metabolomics study of Populus type propolis. J. Pharm. Biomed. Anal. 2017, 135, 217–226. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.-M.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Kucharska, A.Z.; Sokół-Łętowska, A.; Mertas, A.; Czuba, Z.P.; Król, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid. Based Complement. Altern. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Fernández, M.C.; Hernández, I.M.; Jaramillo, C.G.J.; González, V.H.; Porto, R.M.D.O.; Delange, D.M.; Fidalgo, L.M.; Piccinelli, A.L.; Campone, L.; et al. Chemical profile and anti-leishmanial activity of three Ecuadorian propolis samples from Quito, Guayaquil and Cotacachi regions. Fitoterapia 2017, 120, 177–183. [Google Scholar] [CrossRef]
- Oliveira, A.; França, H.; Kuster, R.; Teixeira, L.; Rocha, L. Chemical composition and antibacterial activity of Brazilian propolis essential oil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 121–130. [Google Scholar] [CrossRef]
- Papachroni, D.; Graikou, K.; Kosalec, I.; Damianakos, H.; Ingram, V.; Chinou, I. Phytochemical Analysis and Biological Evaluation of Selected African Propolis Samples from Cameroon and Congo. Nat. Prod. Commun. 2015, 10, 67–70. [Google Scholar] [CrossRef]
- Popova, M.P.; Graikou, K.; Chinou, I.; Bankova, V.S. GC-MS Profiling of Diterpene Compounds in Mediterranean Propolis from Greece. J. Agric. Food Chem. 2010, 58, 3167–3176. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Adgaba, N.; Bayaqoob, N.I.M.; Al-Khazim, A.; Simoneit, B.R.T.; El-Mubarak, A.H.; Al-Mutlaq, K.F. Characteristics and chemical compositions of propolis from Ethiopia. SpringerPlus 2014, 3, 253. [Google Scholar] [CrossRef]
- Soltani, E.-K.; Cerezuela, R.; Charef, N.; Mezaache-Aichour, S.; Esteban, M.A.; Zerroug, M.M. Algerian propolis extracts: Chemical composition, bactericidal activity and in vitro effects on gilthead seabream innate immune responses. Fish Shellfish Immunol. 2017, 62, 57–67. [Google Scholar] [CrossRef]
- Eroglu, N.; Akkus, S.; Yaman, M.; Asci, B.; Silici, S. Amino Acid and Vitamin Content of Propolis Collected by Native Caucasican Honeybees. J. Apic. Sci. 2016, 60, 101–110. [Google Scholar] [CrossRef]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285–292. [Google Scholar]
- Cardinault, N.; Cayeux, M.-O.; Du Sert, P.P. La propolis: Origine, composition et propriétés. Phytothérapie 2012, 10, 298–304. [Google Scholar] [CrossRef]
- Al Mărghitaş, L.; Dezmirean, D.S.; Bobiş, O. Important Developments in Romanian Propolis Research. Evid. Based Complement. Altern. Med. 2013, 2013, 159392. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-type Propolis: Chemical Composition, Botanical Origin and Biological Activity. Nat. Prod. Commun. 2015, 10, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Saftić, L.; Peršurić, Ž.; Fornal, E.; Pavlešić, T.; Pavelić, S.K. Targeted and untargeted LC-MS polyphenolic profiling and chemometric analysis of propolis from different regions of Croatia. J. Pharm. Biomed. Anal. 2019, 165, 162–172. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evid. Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Kumazawa, S.; Goto, H.; Hamasaka, T.; Fukumoto, S.; Fujimoto, T.; Nakayama, T. A New Prenylated Flavonoid from Propolis Collected in Okinawa, Japan. Biosci. Biotechnol. Biochem. 2004, 68, 260–262. [Google Scholar] [CrossRef]
- Devequi-Nunes, D.; Machado, B.A.S.; Barreto, G.D.A.; Silva, J.R.; Da Silva, D.F.; Da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced type 2 diabetes in rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Ahmad, A.; Eleazu, C.O.; Mohamed, M. Oxidative Stress, NF-κB-Mediated Inflammation and Apoptosis in the Testes of Streptozotocin–Induced Diabetic Rats: Combined Protective Effects of Malaysian Propolis and Metformin. Antioxidants 2019, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- El Menyiy, N.; Al-Wali, N.; El Ghouizi, A.; El-Guendouz, S.; Salom, K.; Lyoussi, B. Potential therapeutic effect of Moroccan propolis in hyperglycemia, dyslipidemia, and hepatorenal dysfunction in diabetic rats. Iran. J. Basic Med. Sci. 2019, 22, 1331–1339. [Google Scholar]
- Rivera-Yañez, N.; Rodriguez-Canales, M.; Nieto-Yañez, O.; Jiménez-Estrada, M.; Ibarra-Barajas, M.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. Hypoglycaemic and Antioxidant Effects of Propolis of Chihuahua in a Model of Experimental Diabetes. Evid. Based Complement. Altern. Med. 2018, 2018, 4360356. [Google Scholar] [CrossRef] [PubMed]
- Vongsak, B.; Kongkiatpaiboon, S.; Jaisamut, S.; Machana, S.; Pattarapanich, C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Rev. Bras. Farmacogn. 2015, 25, 445–450. [Google Scholar] [CrossRef]
- Popova, M.; Lyoussi, B.; Aazza, S.; Antunes, D.; Bankova, V.; Miguel, G. Antioxidant and α-Glucosidase Inhibitory Properties and Chemical Profiles of Moroccan Propolis. Nat. Prod. Commun. 2015, 10, 1961–1964. [Google Scholar] [CrossRef]
- Ibrahim, R.B.; Amin, A.; Mustafa, I.O.; Onanuga, I.O.; Folarin, R.O.; Balogun, W.G. Hepatoprotective and Pancreatoprotective Properties of the Ethanolic Extract of Nigerian Propolis. J. Intercult. Ethnopharmacol. 2015, 4, 102–108. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, M.; Shou, Q.; Li, Y.; Hu, F. Biological Activities of Chinese Propolis and Brazilian Propolis on Streptozotocin-Induced Type 1 Diabetes Mellitus in Rats. Evid. Based Complement. Altern. Med. 2011, 2011, 468529. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.M.; Abo-Salem, O.M.; Aly, H.A.; Mansour, A.M. Potential antidiabetic and hypolipidemic effects of propolis extract in streptozotocin-induced diabetic rats. Pak. J. Pharm. Sci. 2009, 22, 168–174. [Google Scholar]
- Zamami, Y.; Takatori, S.; Koyama, T.; Goda, M.; Iwatani, Y.; Doi, S.; Kawasaki, H. Effect of propolis on insulin resistance in fructose-drinking rats. J. Pharm. Soc. Jpn. 2007, 127, 2065–2073. [Google Scholar] [CrossRef][Green Version]
- Fuliang, H.; Hepburn, H.; Xuan, H.; Chen, M.; Daya, S.; Radloff, S. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol. Res. 2005, 51, 147–152. [Google Scholar] [CrossRef]
- Matsui, T.; Ebuchi, S.; Fujise, T.; Abesundara, K.J.M.; Doi, S.; Yamada, H.; Matsumoto, K. Strong Antihyperglycemic Effects of Water-Soluble Fraction of Brazilian Propolis and Its Bioactive Constituent, 3,4,5-Tri-O-caffeoylquinic Acid. Biol. Pharm. Bull. 2004, 27, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Jurčević, I.L.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Junaković, E.P.; Terzić, S.; Jutrić, D. Effect of Propolis on Diet-Induced Hyperlipidemia and Atherogenic Indices in Mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef]
- Azab, A.E.; Lashkham, N.M.; Albasha, M.O. Haemato-Protective and Hypolipidemic Effects of Aqueous Extract of Libyan Propolis Against Sodium Nitrite Induced Haematotoxicity and Hyperlipidemia in Guinea Pigs. Am. J. Biosci. Bioeng. 2015, 3, 22–32. [Google Scholar] [CrossRef][Green Version]
- Albokhadaim, I. Influence of dietary supplementation of propolis on hematology, biochemistry and lipid profile of rats fed high cholesterol diet. J. Adv. Vet. Anim. Res. 2015, 2, 56–63. [Google Scholar] [CrossRef]
- Silva, D.; Miranda, A.; D’Angelo, L.R.B.; Rosa, B.; Soares, E.; Ramalho, J.G.D.C.; Boriollo, M.F.G.; Garcia, J.A.D. Propolis and swimming in the prevention of atherogenesis and left ventricular hypertrophy in hypercholesterolemic mice. Braz. J. Biol. 2015, 75, 414–422. [Google Scholar] [CrossRef]
- Fang, Y.; Sang, H.; Yuan, N.; Sun, H.; Yao, S.; Wang, J.; Qin, S. Ethanolic extract of propolis inhibits atherosclerosis in ApoE-knockout mice. Lipids Heath. Dis. 2013, 12, 123. [Google Scholar] [CrossRef]
- Daleprane, J.B.; Freitas, V.D.S.; Pacheco, A.; Rudnicki, M.; Faine, L.A.; Dörr, F.A.; Ikegaki, M.; Salazar, L.A.; Ong, T.P.; Abdalla, D.S.P. Anti-atherogenic and anti-angiogenic activities of polyphenols from propolis. J. Nutr. Biochem. 2012, 23, 557–566. [Google Scholar] [CrossRef]
- Nader, M.A.; El-Agamy, D.S.; Suddek, G.M. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch. Pharmacal Res. 2010, 33, 637–643. [Google Scholar] [CrossRef]
- Ichi, I.; Hori, H.; Takashima, Y.; Adachi, N.; Kataoka, R.; Okihara, K.; Hashimoto, K.; Kojo, S. The Beneficial Effect of Propolis on Fat Accumulation and Lipid Metabolism in Rats Fed a High-Fat Diet. J. Food Sci. 2009, 74, H127–H131. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Hyodo, T.; Aoyama, H.; Miyata, R.; Kumazawa, S.; Tsuda, T. Artepillin C, a Key Component of Brazilian Propolis, Induces Thermogenesis in Inguinal White Adipose Tissue of Mice through a Creatine-Metabolism-Related Thermogenic Pathway. J. Agric. Food Chem. 2019, 68, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Koya-Miyata, S.; Arai, N.; Mizote, A.; Taniguchi, Y.; Ushio, S.; Iwaki, K.; Fukuda, S. Propolis Prevents Diet-Induced Hyperlipidemia and Mitigates Weight Gain in Diet-Induced Obesity in Mice. Biol. Pharm. Bull. 2009, 32, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Afsharpour, F.; Javadi, M.; Hashemipour, S.; Koushan, Y.; Haghighian, H.K. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Complement. Ther. Med. 2019, 43, 283–288. [Google Scholar] [CrossRef]
- Zakerkish, M.; Jenabi, M.; Zaeemzadeh, N.; Hemmati, A.A.; Neisi, N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 7289. [Google Scholar] [CrossRef]
- Samadi, N.; Mozaffari-Khosravi, H.; Rahmanian, M.; Askarishahi, M. Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: A randomized, double-blind clinical trial. J. Integr. Med. 2017, 15, 124–134. [Google Scholar] [CrossRef]
- Zhao, L.; Pu, L.; Wei, J.; Li, J.; Wu, J.; Xin, Z.; Gao, W.; Guo, C. Brazilian Green Propolis Improves Antioxidant Function in Patients with Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2016, 13, 498. [Google Scholar] [CrossRef]
- Fukuda, T.; Fukui, M.; Tanaka, M.; Senmaru, T.; Iwase, H.; Yamazaki, M.; Aoi, W.; Inui, T.; Nakamura, N.; Marunaka, Y. Effect of Brazilian green propolis in patients with type 2 diabetes: A double-blind randomized placebo-controlled study. Biomed. Rep. 2015, 3, 355–360. [Google Scholar] [CrossRef]
- Natsir, R.; Usman, A.N.; Ardyansyah, B.D.; Fendi, F. Propolis and honey trigona decrease leptin levels of central obesity patients. Enfermería Clínica 2020, 30, 96–99. [Google Scholar] [CrossRef]
- Mujica, V.; Orrego, R.; Fuentealba, R.; Leiva, E.; Zúñiga-Hernández, J. Propolis as an Adjuvant in the Healing of Human Diabetic Foot Wounds Receiving Care in the Diagnostic and Treatment Centre from the Regional Hospital of Talca. J. Diabetes Res. 2019, 2019, 2507578. [Google Scholar] [CrossRef] [PubMed]
- Henshaw, F.R.; Bolton, T.; Nube, V.; Hood, A.; Veldhoen, D.; Pfrunder, L.; McKew, G.L.; Macleod, C.; McLennan, S.V.; Twigg, S.M. Topical application of the bee hive protectant propolis is well tolerated and improves human diabetic foot ulcer healing in a prospective feasibility study. J. Diabetes Complicat. 2014, 28, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S. Molecular Mechanism in α-Glucosidase and Glucoamylase. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Pujirahayu, N.; Bhattacharjya, D.K.; Suzuki, T.; Katayama, T. α-Glucosidase Inhibitory Activity of Cycloartane-Type Triterpenes Isolated from Indonesian Stingless Bee Propolis and Their Structure-Activity Relationship. Pharmaceuticals 2019, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, G.; Kong, L.; Peng, W.; Zhang, H. Galangin and Pinocembrin from Propolis Ameliorate Insulin Resistance in HepG2 Cells via Regulating Akt/mTOR Signaling. Evid. Based Complement. Altern. Med. 2018, 2018, 7971842. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Chang, Y.; Li, Y.; Zhou, Y.; Qin, J.; Sun, Z.; Li, H. Caffeic Acid Phenethyl Ester (Propolis Extract) Ameliorates Insulin Resistance by Inhibiting JNK and NF-κB Inflammatory Pathways in Diabetic Mice and HepG2 Cell Models. J. Agric. Food Chem. 2017, 65, 9041–9053. [Google Scholar] [CrossRef]
- Bacchiega, B.C.; Bacchiega, A.B.; Usnayo, M.J.G.; Bedirian, R.; Singh, G.; Pinheiro, G.D.R.C. Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J. Am. Heart Assoc. 2017, 6, e005038. [Google Scholar] [CrossRef]
- Simon, T.; Taleb, S.; Danchin, N.; Laurans, L.; Rousseau, B.; Cattan, S.; Montely, J.-M.; Dubourg, O.; Tedgui, A.; Kotti, S.; et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 2012, 34, 570–577. [Google Scholar] [CrossRef]
- Bachiega, T.F.; Orsatti, C.L.; Pagliarone, A.C.; Sforcin, J.M. The Effects of Propolis and its Isolated Compounds on Cytokine Production by Murine Macrophages. Phytother. Res. 2012, 26, 1308–1313. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.; Kim, N.; Woo, M.; Kim, H.Y. Effect of propolis phenolic compounds on free fatty acid receptor 4 activation. Food Sci. Biotechnol. 2020, 29, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Siheri, W.; Alenezi, S.; Tusiimire, J.; Watson, D.G. The Chemical and Biological Properties of Propolis. In Bee Products—Chemical and Biological Properties; J.B. Metzler: Stuttgart, Germany, 2017; pp. 137–178. [Google Scholar]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid. Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef] [PubMed]
- Aloud, A.A.; Chinnadurai, V.; Govindasamy, C.; AlSaif, M.A.; Al-Numair, K.S. Galangin, a dietary flavonoid, ameliorates hyperglycaemia and lipid abnormalities in rats with streptozotocin-induced hyperglycaemia. Pharm. Biol. 2018, 56, 302–308. [Google Scholar] [CrossRef]
- Charoensin, S.; Punvittayagul, C.; Pompimom, W.; Mevatee, U.; Wongpoomchai, R. Toxicological and clastogenic evaluation of pinocembrin and pinostrobin isolated from Boesenbergia pandurate in Wistar rats. Thai J. Toxicol. 2010, 25, 29. [Google Scholar]
- Walgrave, S.E.; Warshaw, E.M.; Glesne, L.A. Allergic contact dermatitis from propolis. Dermatitis 2005, 16, 209–215. [Google Scholar]
- Hsu, C.-Y.; Chiang, W.-C.; Weng, T.-I.; Chen, W.-J.; Yuan, A. Laryngeal edema and anaphalactic shock after topical propolis use for acute pharyngitis. Am. J. Emerg. Med. 2004, 22, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
| Flavonoids | apigenin, kaempferol, pinobanksin, chrysin, tektochrisin, pinocembrin, galangin, quercetin, myricetin, rutin, rhamnetin, isorhamnetin, luteolin, naringenin, acacetin, baicalein, hesperitin, sakuranetin, formononetin, liquiritigenin, isalpinin, daidzein, genistein, eupatorin, hispidulin, propolins, prokinawan, isosativan, medicarpin, vestitol, nymphaeol, isonymphaeol [1,18,23,24,25,26,27,28,29,30,31,32,33] |
| Phenyl carboxylic acids and derivatives | caffeic acid, caffeic acid phenethyl ester, cichoric acid, cinnamic acid, ferulic acid, p-coumaric acid, benzoic acid, salicylic acid, rosmarinic acid, chlorogenic acid, caffeoylquinic acid, vanillic acid, artepillin C, baccharin, drupanin [18,22,27,29,33,34,35,36,37,38] |
| Terpenoids | geraniol, nerol, bisabolol, guaiol, farnesol, linalool, limonene, eudesmol, terpineol, camphor, squalene, copaene, calarene, calamenene, caryophyllene, patchoulene, elemene, ferruginol, junicedric acid, pimaric acid, abietic acid, isocupressic acid, acetylisocupressic acid, communic acid, imbricatoloic acid, totarol, amyrin, amyrone, lupeol, lupenone, moretenol, ferutinin, teferin, germanicol, agarospirol, lanosterol, erythrodiol, cycloartenol, ambonic acid, mangiferonic acid, ambolic acid [1,6,22,28,31,34,35,37,39,40,41,42,43,44] |
| Amino acids | aspartic acid, glutamic acid, serine, glycine, histidine, arginine, threonine, alanine, proline, tyrosine, valine, methionine, isoleucine, leucine, phenylalanine, lysine, tryptophane, asparagine, cystine [1,45] |
| Aliphatic hydrocarbons and aliphatic acids | eicosine, 1-octadecene, eicosane, heneicosane, docosane, tricosane, tetracosane, pentacosane, hexacosane, heptacosane, octacosane, nonacosane, triacontane, behenic acid, cerotic acid, lauric acid, linoleic acid, lignoceric acid, montanic acid, nonanoic acid, palmitic acid, oleic acid, stearic acid, behenic acid, decanoic acid, dodecanoic acid, tetradecanoic acid, heptadecanoic acid, tetracosanoic acid, eicosanoic acid, hexacosanoic acid [1,34,43,46] |
| Sugars and sugar alcohols | xylose, galactose, mannose, glucuronic acid, lactose, maltose, melibiose, d-ribofuranose, d-fructose, d-gulose, talose, sucrose, d-glucose, erytritol, xylitol, inositol, d-glucitol [1,33] |
| Vitamins | B1, B2, B3, B5, B6, C, E [1,4,45] |
| Minerals | Sr, Ba, Cd, Sn, Pb, Ti, Ag, Co, Mo, Al, Si, V, Ni, Mn, Cr, Na, Mg, Cu, Ca, Zn, Fe, K [1,33] |
| Alkaloids | demecolcine, papaverine, thebaine, morpholine, norlobeline, pagicerine, oreophilin [4,34,44] |
| No. | Experimental Model/Dose of Propolis (In Vivo) | Findings | Reference |
|---|---|---|---|
| Antidiabetic effect | |||
| 1. | d-glucose induced diabetes in rats/100–200 mg/kg | Reduction of fasting blood glucose; reduction of insulin resistance; reduction of body weight | Laaroussi et al., 2020 [55] |
| 2. | Streptozotocin induced diabetes in rats/300 mg/kg | Reduction of fasting blood glucose | Nna et al., 2019 [56] |
| 3. | Streptozotocin induced diabetes in rats/50–100 mg/kg | Reduction of blood glucose; reduction of serum creatinine and urea | El Menyiy et al., 2019 [57] |
| 4. | Streptozotocin induced diabetes in mice/300 mg/kg | Reduction of blood glucose | Rivera-Yanez et al., 2018 [58] |
| 5. | In vitro assessment of alpha-glucosidase | Inhibition of alpha-glucosidase with IC50 of 70.79 ± 6.44 µg/mL | Vongsak et al., 2015 [59] |
| 6. | In vitro assessment of alpha-glucosidase and α-amylase | Inhibition of alpha-glucosidase with IC50 of 0.01 ± 0.01 mg/mL; inhibition of alpha-amylase with IC50 of 0.09 ± 0.01 mg/mL | Popova et al., 2015 [60] |
| 7. | Alloxan induced diabetes in rats/200–300 mg/kg EO | Reduction of blood glucose; conservation of normal pancreatic cell architecture | Babatunde et al., 2015 [61] |
| 8. | Streptozotocin induced diabetes in rats/100 mg/kg | Reduction of fasting blood glucose; reduction of glycated hemoglobin; restoration of STZ-altered hepatorenal functions | Zhu et al., 2013 [62] |
| 9. | Streptozotocin induced diabetes in rats/200 mg/kg | Reduction of serum glucose; reduction of oxidative stress parameters | El Sayed et al., 2009 [63] |
| 10. | Fructose induced diabetes in rats/100–300 mg/kg | Reduction of plasma level of insulin and HOMA-R index of insulin resistance | Zamami et al., 2007 [64] |
| 11. | Alloxan induced diabetes in rats/1 mL/100 g | Reduction of blood glucose; reduction of fructosamine, malonaldehyde and nitric oxide | Fuliang et al., 2005 [65] |
| 12. | In vitro assessment of maltase and α-amylase | Inhibition of maltase with IC50 of 1.0 mg/mL; inhibition of alpha-amylase with IC50 of 4.7 mg/mL | Matsui et al., 2004 [66] |
| Antihyperlipidemic effect | |||
| 13. | High-fat diet mice/50 mg/kg | Reduction of total cholesterol and triglycerides; reduction of atherogenic index of plasma | Orsolic et al., 2019 [67] |
| 14. | Sodium nitrite induced hyperlipidemia in guinea pigs/200 mg/kg | Reduction of cholesterol, triglycerides; reduction of atherogenic index of plasma | Azab et al., 2015 [68] |
| 15. | High-fat diet rats/1–2% w/w | Reduction of cholesterol, triacylgycerol and ALT | Albokhadaim, 2015 [69] |
| 16. | LDL r-/- mice/70 µL/animal | Increase of plasmatic HDL; prevention of LVH and arterial atherogenesis | Silva et al., 2015 [70] |
| 17. | ApoE-knockout mice/160 mg/kg | Reduction of total cholesterol, triglycerides, and non-HDL; decrease atherosclerotic lesion development in aortic root | Fang et al., 2013 [71] |
| 18. | LDL r-/- mice/250 mg/kg | Normalisation of lipid profile/downregulation of VCAM, FGF, VEGF and MMP-9 gene expression | Daleprane et al., 2012 [72] |
| 19. | High-fat diet rabbits/75 mg/kg | Reduction of total cholesterol, LDL and triglycerides | Nader et al., 2010 [73] |
| 20. | High-fat diet rats/0.05–0.5% w/w | Reduction of cholesterol and triglycerides/increase of PPARα protein level in the liver | Ichi et al., 2009 [74] |
| Anti-obesity effect | |||
| 21. | Obese C57BL/6J mice | Increased thermogenesis in white adipose tissue (WAT); activation of creatine metabolism pathways | Nishikawa et al., 2020 [75] |
| 22. | Obese C57BL/6J mice/5–50 mg/kg | Reduction of body weight gain; down-regulation of fatty acid synthase and SREBP mRNA expression | Koya-Miyata et al., 2009 [76] |
| Disease | Type of Clinical Study | Number of Patients | Treatment/Dose | Results | Reference |
|---|---|---|---|---|---|
| Diabetes mellitus | Randomized, placebo-controlled study | 62 patients with type 2 diabetes | 1500 mg/day propolis, orally for 8 weeks | Reduction of HbA1C; increase of TAC blood levels and activity of GPx and SOD | Afsharpour et al., 2019 [77] |
| Diabetes mellitus | Randomized, placebo-controlled study | 50 patients with type 2 diabetes | 1000 mg/day propolis, orally for 90 days | Reduction of HbA1C, HOMA-IR, hs-CRP | Zakerkish et al., 2019 [78] |
| Diabetes mellitus | Randomized, placebo-controlled study | 66 patients with type 2 diabetes | 900 mg/day propolis, orally for 12 weeks | Significant reduction of FBG and HbA1C; decrease of total cholesterol | Samadi et al., 2017 [79] |
| Diabetes mellitus | Randomized controlled study | 32 patients with type 2 diabetes | 900 mg/day propolis, orally for 18 weeks | Reduction of carbonyls, LDH activity and TNFα | Zhao et al., 2016 [80] |
| Diabetes mellitus | Randomized, placebo-controlled study | 80 patients with type 2 diabetes | 226.8 mg/day propolis, orally for 8 weeks | Prevention of eGFR worsening; limited impact on HOMA-IR | Fukuda et al., 2015 [81] |
| Obesity | Randomized, placebo-controlled study | 30 patients with central obesity | 60 mg/day propolis, orally for 2 weeks | Reduction of leptin level | Natsir et al., 2020 [82] |
| Diabetic foot ulcer | Randomized controlled study | 31 patients with diabetic foot wounds | Cutaneously applied propolis | Reduction of wound area, accelerated healing; reduced TNFα | Mujica et al., 2019 [83] |
| Diabetic foot ulcer | Prospective, controlled study | 24 patients with diabetic food ulcer | Cutaneously applied propolis | 41% reduction of ulcer area; accelerated wound healing | Henshaw et al., 2014 [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balica, G.; Vostinaru, O.; Stefanescu, C.; Mogosan, C.; Iaru, I.; Cristina, A.; Pop, C.E. Potential Role of Propolis in the Prevention and Treatment of Metabolic Diseases. Plants 2021, 10, 883. https://doi.org/10.3390/plants10050883
Balica G, Vostinaru O, Stefanescu C, Mogosan C, Iaru I, Cristina A, Pop CE. Potential Role of Propolis in the Prevention and Treatment of Metabolic Diseases. Plants. 2021; 10(5):883. https://doi.org/10.3390/plants10050883
Chicago/Turabian StyleBalica, Georgeta, Oliviu Vostinaru, Cristina Stefanescu, Cristina Mogosan, Irina Iaru, Anamaria Cristina, and Carmen Elena Pop. 2021. "Potential Role of Propolis in the Prevention and Treatment of Metabolic Diseases" Plants 10, no. 5: 883. https://doi.org/10.3390/plants10050883
APA StyleBalica, G., Vostinaru, O., Stefanescu, C., Mogosan, C., Iaru, I., Cristina, A., & Pop, C. E. (2021). Potential Role of Propolis in the Prevention and Treatment of Metabolic Diseases. Plants, 10(5), 883. https://doi.org/10.3390/plants10050883







