Multivariate Analysis of Morpho-Physiological Traits Reveals Differential Drought Tolerance Potential of Bread Wheat Genotypes at the Seedling Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatment
2.2. Measurement of Seedling Traits
2.3. Statistical Procedure and Computer Analysis of Data Using Machine Learning Algorithms
3. Results
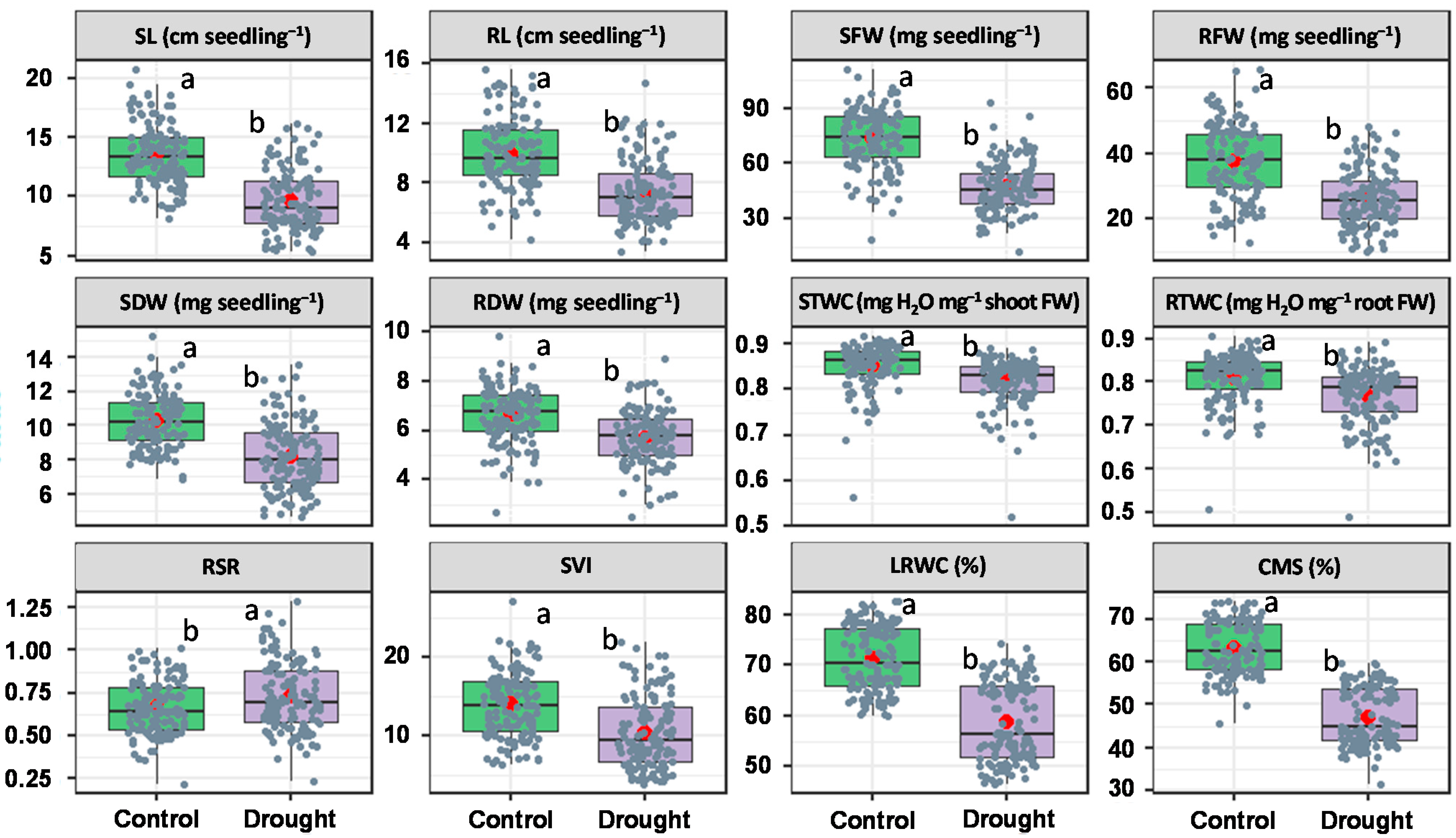
3.1. Mean Variability in Seedling Traits
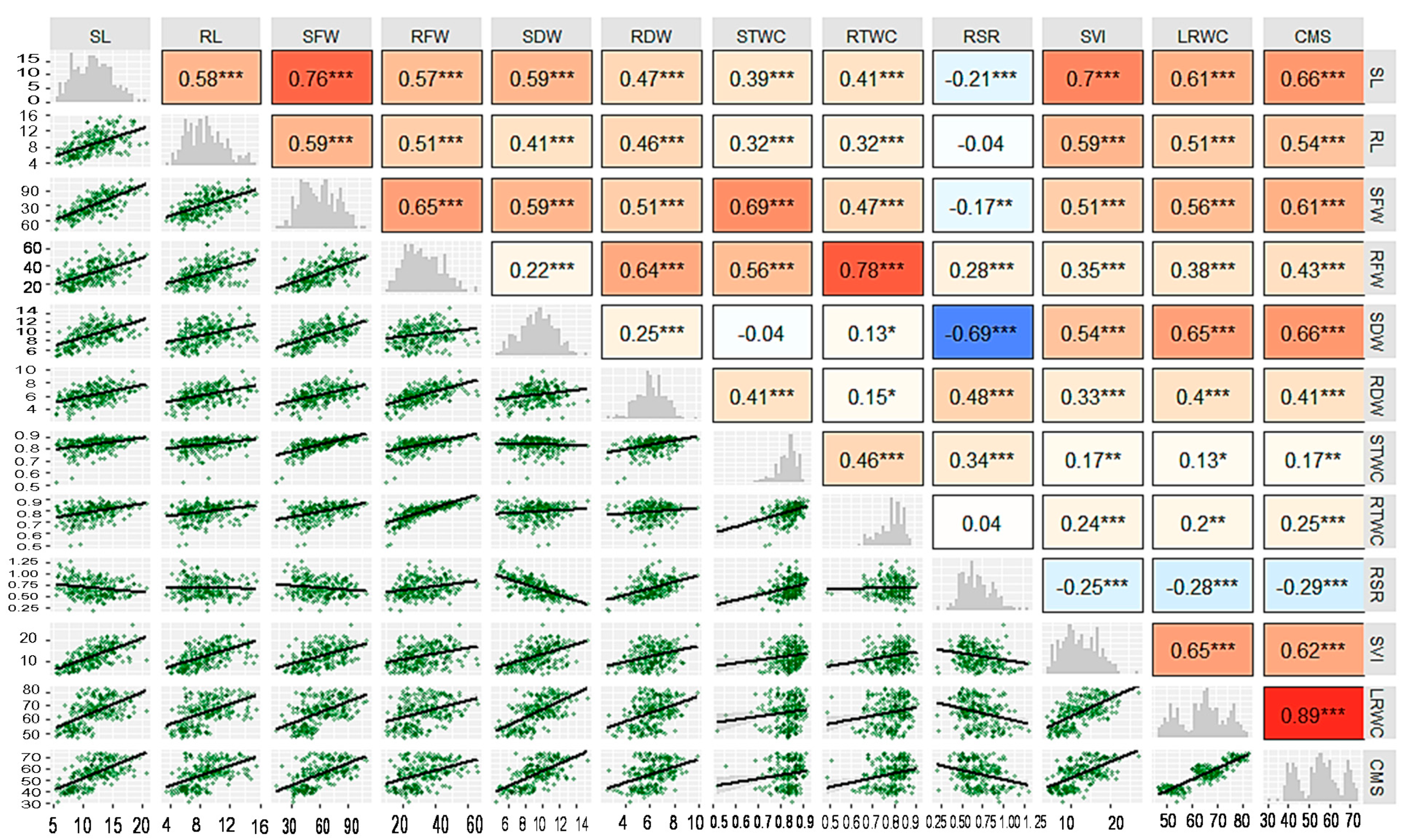
3.2. Correlation Analysis
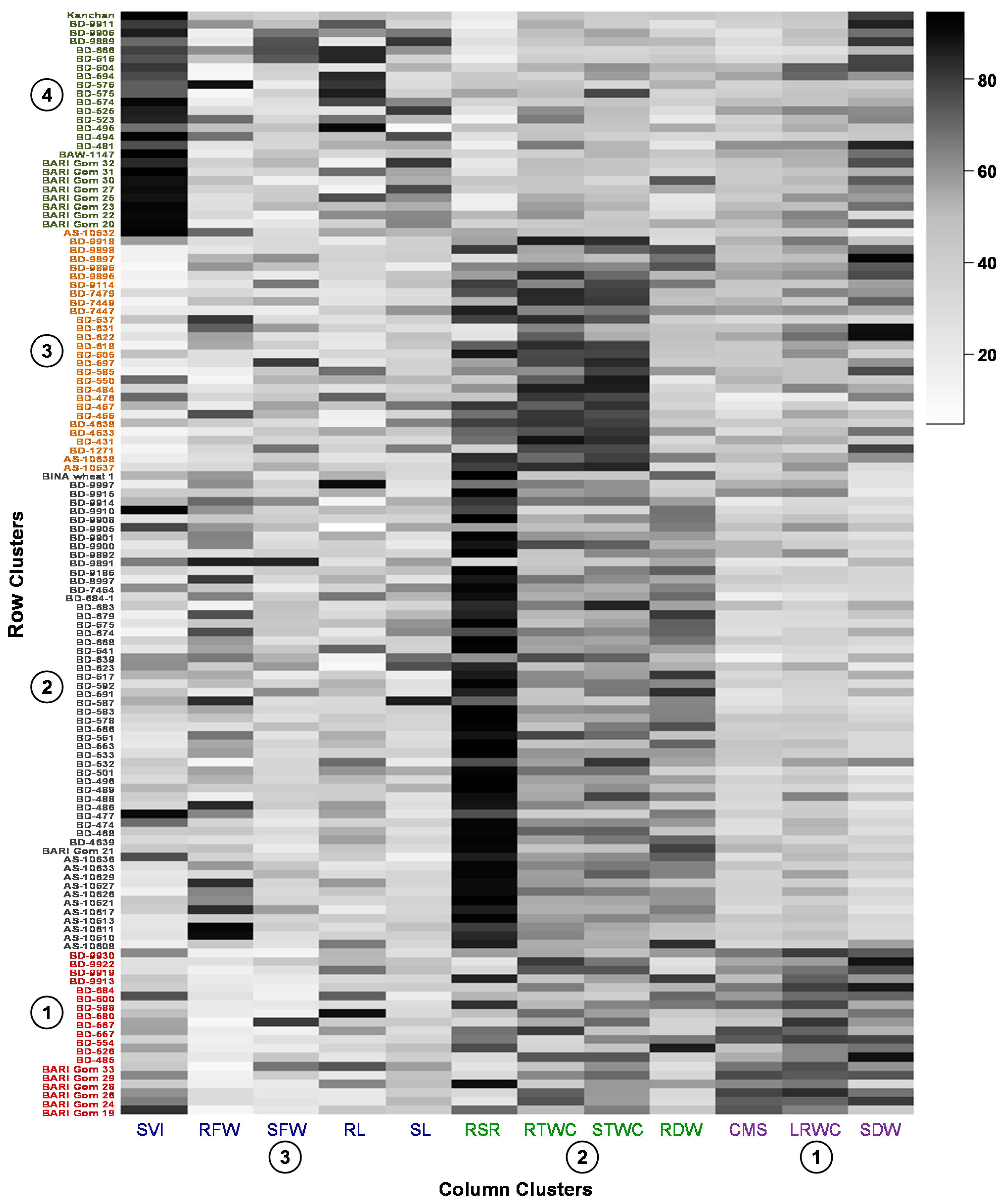
3.3. Hierarchical Clustering and Co-Clustering of Genotypes and Traits
3.4. Variability of the Genotypes in the Extracted Clusters
3.5. Principal Component Analysis
3.6. Linear Discriminant Analysis
3.7. Verification of Cluster Grouping by LDA
3.8. Mahalanobis Distance Matrix
3.9. Co-Cluster Based Selection of Genotypes
4. Discussion
4.1. Trait Variability under PEG-Induced Drought Stress
4.2. Association between Traits, Genotypes and Drought Tolerance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBS | Bangladesh bureau of statistics |
| CRD | Completely randomized design |
| CMS | Cell membrane stability |
| EMS | Ethyl methanesulfonate |
| FAO | Food and agriculture organization |
| IQR | Interquartile range |
| LD | Linear discriminant |
| LDA | Linear discriminant analysis |
| LRWC | Leaf relative water content |
| MoEF | Ministry of environment and forest |
| PC | Principal component |
| PCA | Principal component analysis |
| PEG | Polyethylene glycol |
| PPED | Photoperiod with photosynthetic photon flux density |
| Q1 | First quartile (25th Percentile) |
| Q3 | Third quartile (75th Percentile) |
| RDW | Root dry weight |
| RFW | Root fresh weight |
| RH | Relative humidity |
| RHCOC | Robust hierarchical co-cluster |
| RL | Root length |
| RSR | Root-shoot weight ratio |
| RTWC | Root tissue water content |
| RWC | Relative water content |
| SDW | Shoot dry weight |
| SFW | Shoot fresh weight |
| SL | Shoot length |
| STI | Stress tolerance index |
| STWC | Shoot tissue water content |
| SVI | Seedling vigor index |
References
- Atak, M.; Mavi, K.; Uremis, İ. Bio-herbicidal effects of oregano and rosemary essential oils on germination and seedling growth of bread wheat cultivars and weeds. Rom. Biotechnol. Lett. 2016, 21, 11149–11159. [Google Scholar]
- FAO. FAOSTAT, Food and Agriculture Organization, Rome. 2021. Available online: http://www.fao.org/faostat/en/#home (accessed on 15 February 2021).
- BBS. Yearbook of Agricultural Statistics of Bangladesh—2019; Bangladesh Bureau of Statistics, Ministry of Planning, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2019; pp. 78–79.
- Hossain, A.; Silva, J.A.T. Wheat production in Bangladesh: Its future in the light of global warming. AoB Plants 2013, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Hossain, M.Z.; Sikder, M.B. Farmers’ adaptation strategies to drought and their determinants in barind tract, Bangladesh. SAARC J. Agric. 2019, 17, 161–174. [Google Scholar] [CrossRef]
- Singh, N.P.; Pal, P.K.; Vaishali, S.K. Morpho-physiological characterization of Indian wheat genotypes and their evaluation under drought condition. Afr. J. biotechnol. 2014, 13, 20. [Google Scholar]
- Olivares-Villegas, J.J.; Reynolds, M.P.; McDonald, G.K. Drought adaptive attributes in the Seri/Babax hexaploid wheat population. Funct. Plant Biol. 2007, 34, 189–203. [Google Scholar] [CrossRef]
- Schauberger, B.; Archontoulis, S.; Arneth, A.; Balkovic, J.; Ciais, P.; Deryng, D.; Elliott, J.; Folberth, C.; Khabarov, N.; Müller, C.; et al. Consistent negative response of US crops to high temperatures in observations and crop models. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. 2020, 155, 211–234. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; Van Der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Cook, B.I.; Ault, T.R.; Smerdon, J.E. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Sci. Adv. 2015, 1, e1400082. [Google Scholar] [CrossRef] [Green Version]
- Schwalm, C.R.; Anderegg, W.R.L.; Michalak, A.M.; Fisher, J.B.; Biondi, F.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Wolf, A.; et al. Global patterns of drought recovery. Nature 2017, 548, 202–205. [Google Scholar] [CrossRef]
- MoEF. Bangladesh Climate Change Strategy and Action Plan 2009; Ministry of Environment and Forests, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2009; p. xvii + 76.
- Hussain, S.; Jamil, M.; Napar, A.A.; Rahman, R.; Bano, A.; Afzal, F.; Kazi, A.G.; Mujeeb-Kazi, A. Heat stress in wheat and interdisciplinary approaches for yield maximization. In Plant-Environment Interaction: Responses and Approaches to Mitigate Stress; Azooz, M.M., Ahmad, P., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Tuberosa, R.; Salvi, S. Genomics-based approaches to improve drought tolerance of crops. Trends Plant Sci. 2006, 11, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Muscoloa, A.; Sidaria, M.; Anastasib, U.; Santonocetoa, C.; Maggioc, A. Effect of PEG induced drought stress on seed germination of four lentil genotypes. J. Plant Interact. 2014, 9, 354–363. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, R.; Ashraf, M.Y.; Ashraf, M.; Waraich, E.A. Sunflower (Helianthus annuus L.) response to drought stress at germination and seedling growth stages. Pak. J. Bot. 2009, 41, 647–654. [Google Scholar]
- Meneses, C.H.S.G.; Bruno, R.L.A.; Fernandes, P.D.; Pereira, W.E.; Lima, L.H.G.M.; Lima, M.M.A.; Vidal, M.S. Germination of cotton cultivar seeds under water stress induced by polyethyleneglycol-6000. Sci. Agric. 2011, 68, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Djibril, S.; Mohamed, O.K.; Diaga, D.; Diegane, D.; Abaya, B.F.; Maurice, S.; Alain, B. Growth and development of date palm (Phoenix dactylifera L.) seedlings under drought and salinity stresses. Afr. J. Biotechnol. 2005, 4, 968–972. [Google Scholar]
- Jatoi, S.A.; Latif, M.M.; Arif, M.; Ahson, M.; Khan, A.; Siddiqui, S.U. Comparative assessment of wheat landraces against polyethylene glycol simulated drought stress. Sci. Technol. Dev. 2014, 33, 1–6. [Google Scholar]
- Saha, R.R.; Hannan, A.; Nessa, A.; Malek, M.A.; Islam, M.R. Selection of drought tolerant wheat genotypes by osmotic stress imposed at germination and early seedling stage. SAARC J. Agric. 2017, 15, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Hellal, F.A.; El-Shabrawi, H.M.; Abd El-Hady, M.; Khatab, I.A.; El-Sayed, S.A.A.; Abdelly, C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J. Genet. Eng. Biotechnol. 2018, 16, 203–212. [Google Scholar] [CrossRef]
- Meher; Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef]
- Bilgili, D.; Atak, M.; Mavi, K. Effects of Peg-Induced Drought Stress on Germination and Seedling Performance of Bread Wheat Genotypes. YYU J. Agric. Sci. 2019, 29, 765–771. [Google Scholar]
- Faisal, S.; Mujtaba, S.M.; Asma Mahboob, W. Polyethylene Glycol Mediated Osmotic Stress Impacts on Growth and Biochemical Aspects of Wheat (Triticum aestivum L.). J. Crop Sci. Biotechnol. 2019, 22, 213–223. [Google Scholar] [CrossRef]
- Hannan, A.; Hassan, L.; Hoque, M.N.; Arif, M.T.; Robin, A.H.K. Increasing new root length reflects survival mechanism of rice (Oryza sativa L.) genotypes under PEG induced osmotic stress. Plant Breed. Biotechnol. 2020, 8, 46–57. [Google Scholar] [CrossRef]
- Ghosh, S.; Shahed, M.A.; Robin, A.H.K. Polyethylene glycol induced osmotic stress affects germination and seedling establishment of wheat genotypes. Plant Breed. Biotechnol. 2020, 8, 174–185. [Google Scholar] [CrossRef]
- Mujtaba, S.; Faisal, S.; Khan, M.; Mumtaz, S.; Khanzada, B. Physiological studies on six wheat (Triticum aestivum L.) genotypes for drought stress tolerance at seedling stage. Agric. Res. Technol. Open Access J. 2016, 1, 1–6. [Google Scholar]
- Ahmed, H.G.M.-D.; Zeng, Y.; Yang, X.; Anwaar, H.A.; Mansha, M.Z.; Hanif, C.M.S.; Ikram, K.; Ullah, A.; Alghanem, S.M.S. Conferring drought-tolerant wheat genotypes through morpho-physiological and chlorophyll indices at seedling stage. Saudi J. Biol. Sci. 2020, 27, 2116–2123. [Google Scholar] [CrossRef]
- Chachar, Z.; Chachar, N.; Chachar, Q.; Mujtaba, S.; Chachar, G.; Chachar, S. Identification of drought tolerant wheat genotypes under water deficit conditions. Int. J. Res. Granthaalayah 2016, 4, 206–214. [Google Scholar] [CrossRef]
- Bilal, M.; Rashid, R.; Rehman, S.; Iqbal, F.; Ahmed, J.; Abid, M.; Ahmed, Z.; Hayat, A. Evaluation of wheat genotypes for drought tolerance. J. Green Physiol. Genet. Genom. 2015, 1, 11–21. [Google Scholar]
- Noorka, I.R.; Batool, A.; Rauf, S.; Teixeira da Silva, J.; Ashraf, E. Estimation of heterosis in wheat (Triticum aestivum L.) under contrasting water regimes. Int. J. Plant Breed. 2013, 7, 55–60. [Google Scholar]
- Almeselmani, M.; Abdullah, F.; Hareri, F.; Naaesan, M.; Ammar, M.A.; Kanbar, O.Z.; Saud, A.A. Effect of drought on different physiological characters and yield component in different varieties of Syrian durum wheat. J. Agric. Sci. 2011, 3, 127. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.-D.; Sajjad, M.; Li, M.; Azmat, M.A.; Rizwan, M.; Maqsood, R.H.; Khan, S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability 2019, 11, 2584. [Google Scholar] [CrossRef] [Green Version]
- Arjenaki, F.G.; Jabbari, R.; Morshedi, A. Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. Int. J. Agric. Crop Sci. 2012, 4, 726–729. [Google Scholar]
- Ahmadizadeh, M. Physiological and agro-morphological response to drought stress. Middle-East J. Sci. Res. 2013, 13, 998–1009. [Google Scholar]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalili, M.; Pour-Aboughadareh, A.; Naghavi, M.R. Assessment of drought tolerance in barley: Integrated selection criterion and drought tolerance indices. Environ. Exp. Biol. 2016, 14, 33–41. [Google Scholar] [CrossRef]
- Böhm, K.; Smidt, E.; Tintner, J. Application of Multivariate Data Analyses in Waste Management. In Multivariate Analysis in Management, Engineering and the Sciences; de Freitas, L.V., de Freitas, A.P.B.R., Eds.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.N.; Badsha, M.B.; Mollah, M.N.H. Robust Hierarchical Co-clustering to Explore Toxicogenomic Biomarkers and Their Regulatory Doses of Chemical Compounds. bioRxiv 2020, 094946. [Google Scholar] [CrossRef]
- Aslam, M.; Maqbool, M.A.; Zaman, Q.U.; Shahid, M.; Akhtar, M.A.; Rana, A.S. Comparison of different tolerance indices and PCA biplot analysis for assessment of salinity tolerance in lentil (Lens culinaris) genotypes. Int. J. Agric. Biol. 2017, 19, 470–478. [Google Scholar] [CrossRef]
- Islam, M.R.; Hamid, A.; Khaliq, Q.A.; Ahmed, J.U.; Haque, M.M.; Karim, M.A. Genetic variability in flooding tolerance of mungbean (Vigna radiata L. Wilczek) genotypes. Euphytica 2007, 156, 247–255. [Google Scholar] [CrossRef]
- Jacomini, E.; Bertani, A.; Mapelli, S. Accumulation of polyethylene glycol 6000 and its effects on water content and carbohydrate level in water-stressed tomato plants. Can. J. Bot. 1988, 66, 970–973. [Google Scholar] [CrossRef]
- Mickky, B.M. Could sodium benzoate enhance broad bean salinity tolerance? II. Germination parameters, carbohydrates, proteins, nucleic acids and hydrolytic enzymes. J. Chem. Biol. Phys. Sci. 2016, 6, 351–367. [Google Scholar]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long-term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops to Temperature Water Stress, Shanhua, Taiwan, 13‒16 August 1992; Food and Agriculture Organization: Shanhua, Taiwan, 1992; pp. 257–270. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics, A Biometrical Approach, 3rd ed.; McGraw Hill, Inc. Book Co.: New York, NY, USA, 1997; pp. 352–358. [Google Scholar]
- McGill, R.; Tukey, J.W.; Larsen, W.A. Variations of box plots. Am. Stat. 1978, 32, 12–16. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Emerson, J.W.; Green, W.A.; Schloerke, B.; Crowley, J.; Cook, D.; Hofmann, H.; Wickham, H. The Generalized Pairs Plot. J. Comput. Graph. Stat. 2012, 22, 79–91. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Khan, A.; Khaliq, I.; Ahmad, M.; Ahmed, H.; Khan, A.G.; Farooq, M.S. Comparative performance of spring wheat (Triticum aestivum L.) through heat stress indices. Pak. J. Bot. 2018, 50, 481–488. [Google Scholar]
- Robin, A.H.K.; Matthew, C.; Uddin, M.J.; Bayazid, K.N. Salinity-induced reduction in root surface area and changes in major root and shoot traits at the phytomer level in wheat. J. Exp. Bot. 2016, 67, 3719–3729. [Google Scholar] [CrossRef]
- Sani, D.O.; Boureima, M.M. Effect of polyethylene glycol (PEG) 6000 on germination and seedling growth of pearl millet [Pennisetum glaucum (L.) R. Br.] and LD50 for in vitro screening for drought tolerance. Afr. J. Biotechnol. 2015, 13, 3742–3747. [Google Scholar]
- Duman, I. Effects of seed priming with PEG or K3PO4 on germination and seedling growth in lettuce. Pak. J. Biol. Sci. 2006, 11, 923–928. [Google Scholar] [CrossRef]
- Radhouane, L. Response of Tunisian autochthonous pearl millet (Pennisetum glaucum L.) to drought stress induced by polyethylene glycol (PEG) 6000. Afr. J. Biotechnol. 2007, 6, 1102–1105. [Google Scholar]
- Keyvan, S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 2010, 8, 1051–1060. [Google Scholar]
- Pour-Aboughadareh, A.; Ahmadi, J.; Mehrabi, A.A.; Etminan, A.; Moghaddam, M.; Siddique, K.H. Physiological responses to drought stress in wild relatives of wheat: Implications for wheat improvement. Acta Physiol. Plant. 2017, 39, 106–114. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.-D.; LI, M.-J.; Khan, S.H.; Kashif, M. Early selection of bread wheat genotypes using morphological and photosynthetic attributes conferring drought tolerance. J. Integr. Agric. 2019, 18, 2483–2491. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Bassuony, F.M.; Baraka, D.M.; Khali, R.R. Physiological effects of nicotinamide and ascorbic acid on Zea mays plant grown under salinity stress. I. Changes in growth, some relevant metabolic activities and oxidative defense systems. Res. J. Agric. Biol. Sci. 2009, 5, 72–81. [Google Scholar]
- Ali, M.A.; Jabran, K.; Awan, S.I.; Abbas, A.; Ehsanullah, M.; Zulkiffal, T.; Acet, J.F.; Rehman, A. Morpho-physiological diversity and its implications for improving drought tolerance in grain sorghum at different growth stages. Aust. J. Crop Sci. 2011, 5, 311–320. [Google Scholar]
- Ghafoor, G.; Hassan, G.; Ahmad, I.; Khan, S.N.; Suliman, S. Correlation analysis for different parameters of F2 bread wheat population. Pure Appl. Biol. 2013, 2, 28–31. [Google Scholar] [CrossRef]
- Baloch, M.J.; Dunwell, J.; Khakwani, A.A.; Dennet, M.; Jatoi, W.A.; Channa, S.A. Assessment of wheat cultivars for drought tolerance via osmotic stress imposed at early seedling growth stages. J. Agric. Res. 2012, 50, 299–310. [Google Scholar]
- Nouraein, M.; Mohammadi, S.A.; Aharizad, S.; Moghaddam, M.; Sadeghzadeh, B. Evaluation of drought tolerance indices in wheat recombinant inbred line population. Ann. Biol. Res. 2013, 4, 113–122. [Google Scholar]
- El-Mohsen, A.A.A.; El-Shafi, M.A.; Gheith, E.; Suleiman, H. Using Different Statistical Procedures for Evaluating Drought Tolerance Indices of Bread Wheat Genotypes. Adv. Agric. Biol. 2015, 4, 19–30. [Google Scholar]
- Ahmad, N.S.; Kareem, S.H.S.; Mustafa, K.M.; Ahmad, D.A. Early screening of some Kurdistan wheat (Triticum aestivum L.) cultivars under drought stress. J. Agric. Sci. 2017, 9. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hordyńska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechyńska-Hebda, M. Variation among wheat (Triticum aestivum L.) genotypes in response to the drought stress: I—selection approaches. J. Plant Interact. 2019, 14, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, F.; Arzani, A.; Karimi, V. Evaluation of yield-based drought tolerance indices for screening safflower (Carthamus tinctorius L.) genotypes. Agron. J. 2014, 106, 1219–1224. [Google Scholar] [CrossRef] [Green Version]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
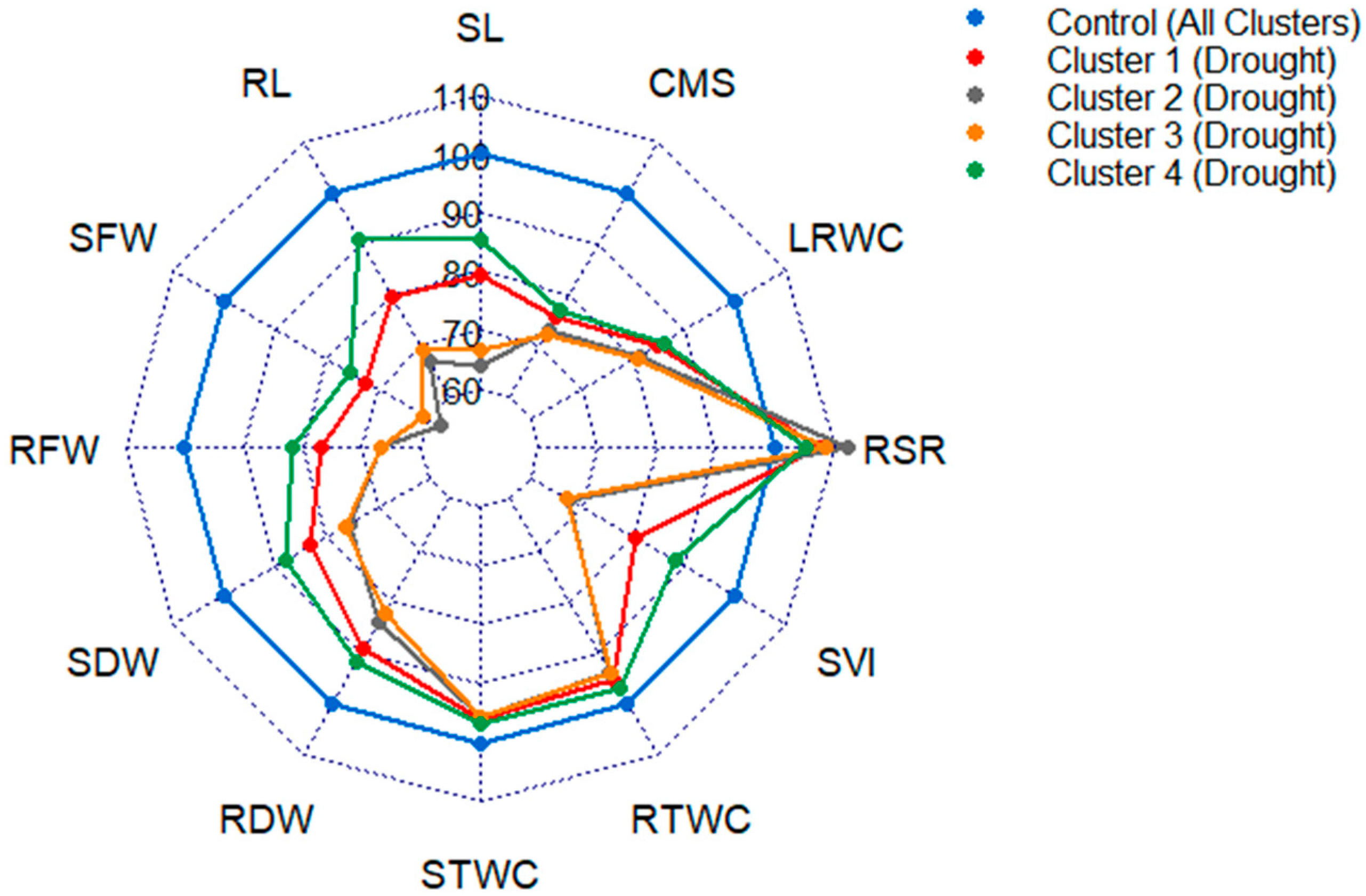

| Seedling Traits | Average STI of Clusters | |||
|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
| Number of genotypes | 19 | 55 | 27 | 26 |
| Shoot length (SL) | 0.60 | 0.69 | 0.60 | 1.07 |
| Root length (RL) | 0.67 | 0.78 | 0.52 | 1.10 |
| Shoot fresh weight (SFW) | 0.44 | 0.67 | 0.63 | 0.87 |
| Root fresh weight (RFW) | 0.32 | 0.99 | 0.58 | 0.82 |
| Shoot dry weight (SDW) | 0.94 | 0.63 | 0.81 | 1.18 |
| Root dry weight (RDW) | 0.71 | 1.06 | 0.69 | 0.85 |
| Shoot tissue water content (STWC) | 0.82 | 1.02 | 0.96 | 0.95 |
| Root tissue water content (RTWC) | 0.82 | 0.99 | 0.96 | 0.98 |
| Root-shoot weight ratio (RSR) | 0.75 | 1.69 | 0.83 | 0.72 |
| Seedling vigor index (SVI) | 0.71 | 0.70 | 0.43 | 1.62 |
| Leaf relative water content (LRWC) | 0.96 | 0.77 | 0.72 | 1.00 |
| Cell membrane stability (CMS) | 0.87 | 0.69 | 0.64 | 0.91 |
| Cluster | Treatment | SL | RL | SFW | RFW | SDW | RDW | STWC | RTWC | RSR | SVI | LRWC | CMS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | Control | 11.91 | 9.21 | 56.50 | 24.86 | 10.86 | 5.76 | 0.79 | 0.76 | 0.54 | 13.21 | 74.78 | 66.93 |
| Drought | 9.44 | 7.32 | 40.79 | 19.10 | 9.03 | 5.14 | 0.76 | 0.73 | 0.58 | 10.60 | 63.36 | 50.73 | |
| % change | (−) 20.7 b | (−) 20.5 b | (−) 27.8 b | (−) 23.2 b | (−) 16.9 b | (−) 10.7 b | (−) 3.8 ab | (−) 4.8 ab | (+) 7.7 ab | (−) 19.7 b | (−) 15.3 b | (−) 24.2 b | |
| Cluster 2 | Control | 13.94 | 10.66 | 78.27 | 44.55 | 9.27 | 7.39 | 0.88 | 0.83 | 0.81 | 13.65 | 69.17 | 61.04 |
| Drought | 8.94 | 7.14 | 45.22 | 29.74 | 7.01 | 6.22 | 0.84 | 0.78 | 0.91 | 9.20 | 56.18 | 44.71 | |
| % change | (−) 35.9 a | (−) 33.0 a | (−) 42.2 a | (−) 33.2 a | (−) 24.4 a | (−) 15.9 a | (−) 4.4 a | (−) 5.9 a | (+) 12.4 a | (−) 32.6 a | (−) 18.8 a | (−) 26.8 a | |
| Cluster 3 | Control | 12.64 | 8.39 | 72.18 | 32.97 | 10.43 | 5.99 | 0.85 | 0.80 | 0.58 | 10.59 | 67.58 | 59.94 |
| Drought | 8.42 | 5.81 | 44.10 | 21.95 | 7.98 | 4.93 | 0.81 | 0.76 | 0.63 | 7.12 | 54.56 | 43.41 | |
| % change | (−) 33.4 a | (−) 30.7 a | (−) 38.9 a | (−) 33.4 a | (−) 23.5 a | (−) 17.7 a | (−) 4.2 ab | (−) 5.8 a | (+) 8.6 ab | (−) 32.8 a | (−) 19.3 a | (−) 27.6 a | |
| Cluster 4 | Control | 15.03 | 10.78 | 77.52 | 36.12 | 11.80 | 6.34 | 0.84 | 0.81 | 0.54 | 18.86 | 76.62 | 68.52 |
| Drought | 12.87 | 9.78 | 58.54 | 29.59 | 10.36 | 5.82 | 0.82 | 0.79 | 0.57 | 16.63 | 65.82 | 52.91 | |
| % change | (−) 14.4 b | (−) 9.2 c | (−) 24.5 b | (−) 18.1 b | (−) 12.2 b | (−) 8.2 b | (−) 3.2 b | (−) 2.8 b | (+) 5.3 b | (−) 11.8 c | (−) 14.1 b | (−) 22.8 b |
| Variable | Principal Components | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Extracted Eigenvalues | 4.24 | 2.95 | 1.39 |
| Explained variance (%) | 35.4 | 24.6 | 11.6 |
| Cumulative variance (%) | 35.4 | 60.0 | 71.6 |
| Seedling traits | Latent vectors | ||
| Shoot length (SL) | 0.793 | −0.015 | −0.147 |
| Root length (RL) | 0.642 | 0.111 | 0.113 |
| Shoot fresh weight (SFW) | 0.744 | 0.221 | −0.359 |
| Root fresh weight (RFW) | 0.522 | 0.685 | −0.071 |
| Shoot dry weight (SDW) | 0.606 | −0.573 | −0.217 |
| Root dry weight (RDW) | 0.436 | 0.482 | 0.590 |
| Shoot tissue water content (STWC) | 0.266 | 0.747 | −0.223 |
| Root tissue water content (RTWC) | 0.370 | 0.514 | −0.517 |
| Root-shoot weight ratio (RSR) | −0.126 | 0.814 | 0.528 |
| Seedling vigor index (SVI) | 0.799 | −0.214 | 0.093 |
| Leaf relative water content (LRWC) | 0.688 | −0.396 | 0.347 |
| Cell membrane stability (CMS) | 0.696 | −0.414 | 0.327 |
| Variable | Linear Discriminants | ||
|---|---|---|---|
| LD1 | LD2 | LD3 | |
| Proportion of trace (%) | 72.1 | 18.4 | 9.5 |
| Seedling traits | Coefficients | ||
| Root dry weight (RDW) | 1.340 | 0.589 | 0.582 |
| Shoot dry weight (SDW) | −0.729 | −0.836 | 0.048 |
| Shoot tissue water content (STWC) | 0.640 | −0.405 | 1.058 |
| Cell membrane stability (CMS) | −0.538 | 0.294 | −0.333 |
| Root tissue water content (RTWC) | 0.518 | 0.274 | 0.399 |
| Leaf relative water content (LRWC) | −0.513 | 0.065 | −0.438 |
| Shoot length (SL) | −0.433 | −0.406 | −0.058 |
| Seedling vigor index (SVI) | −0.316 | −1.008 | 0.423 |
| Root length (RL) | −0.255 | −0.388 | −0.304 |
| Shoot fresh weight (SFW) | −0.165 | 0.346 | −0.417 |
| Root fresh weight (RFW) | 0.134 | −0.171 | 0.042 |
| Root-shoot weight ratio (RSR) | −0.112 | −1.238 | −1.631 |
| Clusters | True Groups | Total No. Observed | |||
|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | ||
| Cluster 1 | 18 | 0 | 3 | 1 | 22 |
| Cluster 2 | 0 | 47 | 0 | 0 | 47 |
| Cluster 3 | 1 | 8 | 24 | 0 | 33 |
| Cluster 4 | 0 | 0 | 0 | 25 | 25 |
| Total number | 19 | 55 | 27 | 26 | 127 |
| Number corrects | 18 | 47 | 24 | 25 | 114 |
| % correctness | 94.7 | 85.5 | 88.9 | 96.2 | 89.8 |
| Clusters | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 |
|---|---|---|---|---|
| Cluster 1 | 0 | 20.29 a | 11.51 a | 10.88 a |
| Cluster 2 | - | 0 | 7.05 a | 21.02 a |
| Cluster 3 | - | - | 0 | 17.23 a |
| Cluster 4 | - | - | - | 0 |
| Co-Cluster Combinations | Best Performers | |
|---|---|---|
| RC-1 | CC-1 | BD-684 followed by BD-526, BARI Gom 26, BARI Gom 29 and BARI Gom 24 |
| CC-2 | BD-9913 followed by BD-588, BARI Gom 28, BD-526 and BARI Gom 19 | |
| CC-3 | BARI Gom 33 followed by BD-567, BD-580, BD-600 and BARI Gom 19 | |
| RC-2 | CC-1 | BD-553 followed by BD-9905, BD-623, BD-9915 and BD-9892 |
| CC-2 | BD-637 followed by BD-9910, AS-10617, BD-4639 and BD-488 | |
| CC-3 | BD-9891 followed by BD-587, BD-9910, BD-639 and BD-477 | |
| RC-3 | CC-1 | BD-622 followed by BD-631, BD-9895, BD-9897 and BD-9896 |
| CC-2 | AS-10638 followed by BD-7479, BD-622, BD-9114 and BD-466 | |
| CC-3 | BD-476 followed by BD-467, BD-550, BD-623 and BD-1271 | |
| RC-4 | CC-1 | BD-574 followed by BD-604, BD-9911, BD-525 and BARI Gom 20 |
| CC-2 | BD-525 followed by BD-604, BARI Gom 30, BD-495 and BARI Gom 27 | |
| CC-3 | BD-666 followed by BD-494, BD-616, BD-523 and AS-10632 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohi-Ud-Din, M.; Hossain, M.A.; Rohman, M.M.; Uddin, M.N.; Haque, M.S.; Ahmed, J.U.; Hossain, A.; Hassan, M.M.; Mostofa, M.G. Multivariate Analysis of Morpho-Physiological Traits Reveals Differential Drought Tolerance Potential of Bread Wheat Genotypes at the Seedling Stage. Plants 2021, 10, 879. https://doi.org/10.3390/plants10050879
Mohi-Ud-Din M, Hossain MA, Rohman MM, Uddin MN, Haque MS, Ahmed JU, Hossain A, Hassan MM, Mostofa MG. Multivariate Analysis of Morpho-Physiological Traits Reveals Differential Drought Tolerance Potential of Bread Wheat Genotypes at the Seedling Stage. Plants. 2021; 10(5):879. https://doi.org/10.3390/plants10050879
Chicago/Turabian StyleMohi-Ud-Din, Mohammed, Md. Alamgir Hossain, Md. Motiar Rohman, Md. Nesar Uddin, Md. Sabibul Haque, Jalal Uddin Ahmed, Akbar Hossain, Mohamed M. Hassan, and Mohammad Golam Mostofa. 2021. "Multivariate Analysis of Morpho-Physiological Traits Reveals Differential Drought Tolerance Potential of Bread Wheat Genotypes at the Seedling Stage" Plants 10, no. 5: 879. https://doi.org/10.3390/plants10050879
APA StyleMohi-Ud-Din, M., Hossain, M. A., Rohman, M. M., Uddin, M. N., Haque, M. S., Ahmed, J. U., Hossain, A., Hassan, M. M., & Mostofa, M. G. (2021). Multivariate Analysis of Morpho-Physiological Traits Reveals Differential Drought Tolerance Potential of Bread Wheat Genotypes at the Seedling Stage. Plants, 10(5), 879. https://doi.org/10.3390/plants10050879













