Factors Influencing Somatic Embryo Maturation in Sugi (Japanese Cedar, Cryptomeria japonica (Thunb. ex L.f.) D. Don)
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Basal Salts (BS) in Medium on Somatic Embryo Maturation Efficiency
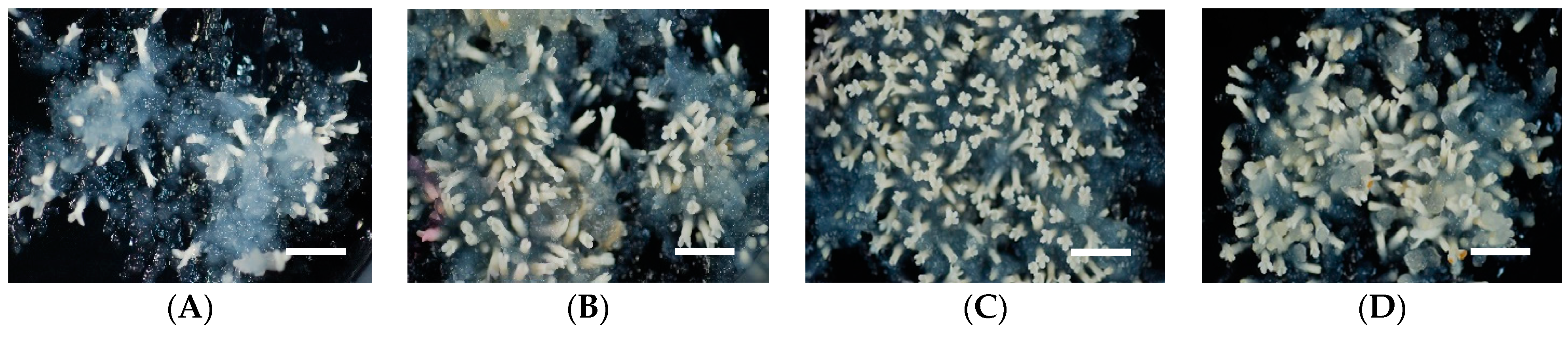
2.2. Effect of Polyethylene Glycol (PEG) Concentration in Medium on Somatic Embryo Maturation Efficiency
2.3. Effect of Abscisic Acid (ABA) Concentration in Medium on Somatic Embryo Maturation Efficiency
2.4. Effect of Additional Supplementation of Potasium Chloride (KCl) in Medium on Somatic Embryo Maturation Efficiency
2.5. Effect of Amino Acid (AA) Concentration in Medium on Somatic Embryo Maturation Efficiency
2.6. Effect of Proliferation Culture Medium (PCM) on Somatic Embryo Maturation Efficiency
2.7. Optimal Factors Giving the Best Results Achieved in Our Six Independent Experiments
3. Materials and Methods
3.1. Plant Material and Culture Conditions
3.2. Maintenance and Proliferation of Embryogenic Cells (ECs)
3.3. Maturation of Somatic Embryos
3.3.1. Effect of Basal Salts (BS) in Medium on Somatic Embryo Maturation Efficiency
3.3.2. Effect of Polyethylene Glycol (PEG) Concentration in Medium on Somatic Embryo Maturation Efficiency
3.3.3. Effect of Abscisic Acid (ABA) Concentration in Medium on Somatic Embryo Maturation Efficiency
3.3.4. Effect of Additional Supplementation of Potassium Chloride (KCl) to the Medium on Somatic Embryo Maturation Efficiency
3.3.5. Effect of Amino Acid (AA) Concentration in Medium on Somatic Embryo Maturation Efficiency
3.3.6. Effect of Proliferation Culture Medium (PCM) on Somatic Embryo Maturation Efficiency
3.4. Data Analysis
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, J.S.; Barret, J.D.; Bonga, J.M. Application of somatic embryogenesis in high-value clonal forestry: Deployment, genetic control, and stability of cryopreserves clones. Cell. Dev. Biol. Plant 1998, 34, 231–239. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Trontin, J.F.; Becwar, M.R.; Devillard, C.; Park, Y.S.; Lelu-Walter, M.A. Recent progress in somatic embryogenesis of four Pinus spp. Tree For. Sci. Biotechol. 2007, 1, 11–25. [Google Scholar]
- Bonga, J.M.; Klimaszewska, K.; von Aderkas, P. Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Organ Cult. 2010, 100, 241–254. [Google Scholar] [CrossRef]
- Maruyama, E.T.; Hosoi, Y. Post-maturation treatments improves and synchronizes somatic embryo germination of three species of Japanese pines. Plant Cell Tissue Organ Cult. 2012, 110, 45–52. [Google Scholar] [CrossRef]
- Maruyama, E.T.; Hosoi, Y. Progress in somatic embryogenesis of Japanese pines. Front. Plant Sci. 2019, 10, 31. [Google Scholar] [CrossRef]
- Forestry Agency. Statistical Handbook of Forest and Forestry; Forestry Agency, Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2014; pp. 8–9. (In Japanese)
- Saito, M.; Teranishi, H. A breeding strategy of male sterile Cryptomeria japonica D. Don cultivars. Jpn. J. Palynol. 2014, 60, 27–35, (In Japanese with English abstract). [Google Scholar]
- Moriguchi, Y.; Ueno, S.; Higuchi, Y.; Miyajima, D.; Itoo, S.; Futamura, N.; Shinohara, K.; Tsumura, Y. Establishment of a microsatellite panel covering the sugi (Cryptomeria japonica) genome, and its application for localization of a male-sterile gene (ms-2). Mol. Breed. 2014, 33, 315–325. [Google Scholar] [CrossRef]
- Maruyama, E.T.; Ueno, S.; Hirayama, S.; Kaneeda, T.; Moriguchi, Y. Somatic embryogenesis and plant regeneration from sugi (Japanese Cedar, Cryptomeria japonica D. Don, Cupressaceae) seed families by marker assisted selection for the male sterility allele ms1. Plants 2020, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Tautorus, T.E.; Fowke, L.C.; Dunstan, D.I. Somatic embryogenesis in conifers. Can. J. Bot. 1991, 69, 1873–1899. [Google Scholar] [CrossRef]
- Jain, S.M.; Gupta, P.K.; Newton, R.J. (Eds.) Somatic Embryogenesis in Woody Plants; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; Volume 3, pp. 317–355. [Google Scholar]
- Klimaszewska, K.; Hargreaves, C.; Lelu-Walter, M.A.; Trontin, J.F. Advances in conifer somatic embryogenesis since year 2000. In In Vitro Embryogenesis in Higher Plants, Methods in Molecular Biology; Germana, M., Lambardi, M., Eds.; Humana Press: New York, NY, USA, 2016; Volume 1359, pp. 131–166. [Google Scholar]
- Maruyama, E.; Tanaka, T.; Hosoi, Y.; Ishii, K.; Morohoshi, N. Embryogenic cell culture, protoplast regeneration, cryopreservation, biolistic gene transfer and plant regeneration in Japanese cedar (Cryptomeria japonica D. Don). Plant Biotechnol. 2000, 17, 281–296. [Google Scholar] [CrossRef]
- Maruyama, E.T.; Hosoi, Y.; Futamura, N.; Saito, M. Initiation of embryogenic cultures from immature seeds of pollen-free sugi (Cryptomeria japonica). Kanto Shinrin Kenkyu 2014, 65, 107–110, (In Japanese with English abstract). [Google Scholar]
- Maruyama, E.T.; Hosoi, Y.; Ueno, S.; Onishi, N.; Onishi, N.; Totsuka, S.; Iwai, J.; Moriguchi, Y. Somatic embryogenic cell induction from seed of pollen-free sugi (Cryptomeria japonica) produced at the Niigata prefecture. Kanto Shinrin Kenkyu 2017, 68, 41–44, (In Japanese with English abstract). [Google Scholar]
- Maruyama, E.T.; Miyazawa, S.; Ueno, S.; Onishi, N.; Totsuka, S.; Iwai, J.; Moriguchi, Y. Differences among families on embryogenic cell induction from seed of pollen-free sugi (Cryptomeria japonica) produced at the Niigata prefecture. Kanto Shinrin Kenkyu 2018, 69, 1–2, (In Japanese with English abstract). [Google Scholar]
- Maruyama, E.T.; Kaneeda, T.; Ueno, S.; Hirayama, S.; Itoh, Y.; Bamba, Y.; Moriguchi, Y. Embryogenic cell induction from immature seeds derived from polycross of sugi (Cryptomeria japonica). Kanto Shinrin Kenkyu 2020, 71, 49–52, (In Japanese with English abstract). [Google Scholar]
- Maruyama, E.T.; Ueno, S.; Hosoi, Y.; Miyazawa, S.-I.; Mori, H.; Kaneeda, T.; Bamba, Y.; Itoh, Y.; Hirayama, S.; Kawakami, K.; et al. Somatic embryogenesis initiation in sugi (Japanese Cedar, Cryptomeria japonica D. Don): Responses from male-fertile, male-sterile, and polycoss-pollinated-derived seed explants. Plants 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Igasaki, T.; Sato, T.; Akashi, N.; Mohri, T.; Maruyama, E.; Kinoshita, I.; Walter, C.; Shinohara, K. Somatic embryogenesis and plant regeneration from immature zygotic embryos of Cryptomeria japonica D. Don. Plant Cell Rep. 2003, 22, 239–243. [Google Scholar] [CrossRef]
- Igasaki, T.; Akashi, N.; Ujino-Ihara, T.; Matsubayashi, Y.; Sakagami, Y.; Shinohara, K. Phytosulfokine stimulates somatic embryogenesis in Cryptomeria japonica. Plant Cell Physiol. 2003, 44, 1412–1416. [Google Scholar] [CrossRef]
- Nakagawa, R.; Ogita, S.; Kubo, T.; Funada, R. Effect of polyamines and L-ornithine on the development of proembryogenic masses of Cryptomeria japonica. Plant Cell Tissue Organ Cult. 2006, 85, 229–234. [Google Scholar] [CrossRef]
- Maruyama, E.; Hosoi, Y. Polyethylene glycol enhance somatic embryo production in Japanese cedar (Cryptomeria japonica D. Don). Propag. Ornam. Plants 2007, 7, 57–61. [Google Scholar]
- Maruyama, E.T.; Hosoi, Y.; Miyazawa, S.; Ueno, S.; Onishi, N.; Totsuka, S.; Iwai, J.; Moriguchi, Y. Pollen-free plant regeneration from embryogenic cells derived from sugi (Cryptomeria japonica). Kanto Shinrin Kenkyu 2019, 70, 37–40, (In Japanese with English abstract). [Google Scholar]
- Maruyama, E.T.; Ishii, K.; Hosoi, Y. Cryopreservation of embryogenic cells of sugi (Cryptomeria japonica). In Cryopreservation of Plant Cells and Organs; Takao, N., Hirai, D., Matsumoto, T., Tanaka, D., Eds.; National Institute of Agrobiological Sciences: Tsukuba, Japan, 2016; pp. 145–146. (In Japanese) [Google Scholar]
- Taniguchi, T.; Konagaya, K.; Nanasato, Y. Somatic embryogenesis in artificially pollinated seed families of 2nd generation plus trees and cryopreservation of embryogenic tissue in Cryptomeria japonica D. Don (sugi). Plant Biotechnol. 2020, 37, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Ohmiya, Y.; Kurita, M.; Tsubomura, M.; Kondo, T. Regeneration of transgenic Cryptomeria japonica D. Don after Agrobacterium tumefaciens-mediated transformation of embryogenic tissue. Plant Cell Rep. 2008, 27, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Konagaya, K.; Kurita, M.; Taniguchi, T. High-efficiency Agrobacterium-mediated transformation of Cryptomeria japonica D. Don by co-cultivation on filter paper wicks followed by meropenem treatment to eliminate Agrobacterium. Plant Biotechnol. 2013, 30, 523–528. [Google Scholar] [CrossRef][Green Version]
- Carlsson, J. Nitrogen Uptake and Assimilation during Norway Spruce Somatic Embryogenesis: Investigating the Role of Glutamine. Doctoral Thesis, Swedish University of Agricultural Sciences, Umeå, Sweden, 2018. [Google Scholar]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryogenesis in Sawara cypress (Chamaecyparis pisifera Sieb. et Zucc.) for stable and efficient plant regeneration, propagation and protoplast culture. J. For. Res. 2002, 7, 23–34. [Google Scholar] [CrossRef]
- Bonga, J.M. The effect of various culture media on the formation of embryo-like structures in cultures derived from explants taken from mature Larix decidua. Plant Cell Tissue Organ Cult. 2004, 77, 43–48. [Google Scholar] [CrossRef]
- Salaj, T.; Fráterová, L.; Cárach, M.; Salaj, J. The effect of culture medium formulation on Pinus nigra somatic embryogenesis. Dendrobiology 2014, 71, 119–128. [Google Scholar] [CrossRef][Green Version]
- Dahrendorf, J.; Clapham, D.; Egertsdotter, U. Analisys of nitrogen utilization capability during the proliferation and maturation phases of Norway spruce (Picea abies (L.) H. Karst) somatic embryogenesis. Forests 2018, 9, 288. [Google Scholar] [CrossRef]
- Ammirato, P.V. Embryogenesis. In Hand Book of Plant Cell Culture, Techniques for Propagation and Breeding; Evans, D.A., Sharp, W.R., Ammirato, P.V., Yamada, Y., Eds.; Macmillan Publishing Co.: New York, NY, USA, 1983; Volume 1, pp. 82–123. [Google Scholar]
- Becwar, M.R.; Nagmani, R.; Wann, S.R. Initiation of embryogenic cultures and somatic embryo development in loblolly pine (Pinus taeda). Can. J. For. Res. 1990, 20, 810–817. [Google Scholar] [CrossRef]
- Smith, D.R. Growth Medium. U.S. Patent 5,565,455, 15 October 1996. [Google Scholar]
- Lelu, M.A.; Bastien, C.; Drugeault, A.; Gouez, M.L.; Klimaszewska, K. Somatic embryogenesis and plantlet development in Pinus sylvestris and Pinus pinaster on medium with and without growth regulators. Physiol. Plant. 1999, 105, 719–728. [Google Scholar] [CrossRef]
- Bozhkov, P.V.; Mikhlina, S.B.; Shiryaeva, G.A.; Lebedenko, L.A. Influence of nitrogen balance of culture medium on Norway spruce (Picea abies (L.) Karst) somatic polyembryogenesis: High frequency establishment of embryonal-suspensor mass lines from mature zygotic embryos. J. Plant. Physiol. 1993, 142, 735–741. [Google Scholar] [CrossRef]
- Carlsson, J.; Svennerstam, H.; Moritz, T.; Egertsdotter, U.; Ganeteg, U. Nitrogen uptake and assimilation in proliferating embryogenic cultures of Norway spruce–Investigating the specific role of glutamine. PLoS ONE 2017, 12, e0181785. [Google Scholar] [CrossRef]
- Khlifi, S.; Tremblay, F. Maturation of black spruce somatic embryos. Part I. Effect of L-glutamine on the number and germinability of somatic embryos. Plant Cell Tissue Organ Cult. 1995, 41, 23–32. [Google Scholar]
- Pullman, G.; Olson, K.; Fischer, T.; Egertsdotter, U.; Frampton, J.; Bucalo, K. Fraser fir somatic embryogenesis: High frequency initiation, maintenance, embryo development, germination and cryopreservation. New For. 2016, 47, 453–480. [Google Scholar] [CrossRef]
- Pullman, G.S.; Zeng, H.; Copeland-Kamp, B.; Crockett, J.; Lucrezi, J.; May, S.W.; Bucalo, K. Conifer somatic embryogenesis: Improvements by supplementation of medium with oxidation-reduction agents. Tree Physiol. 2015, 35, 209–224. [Google Scholar] [CrossRef]
- Pullman, G.S.; Bucalo, K. Pine somatic embryogenesis: Analyses of seed tissue and medium to improve protocol development. New For. 2014, 45, 353–377. [Google Scholar] [CrossRef]
- Li, X.Y.; Huang, F.H.; Murphy, J.B.; Gbur, E.E., Jr. Polyethylene glycol and maltose enhance somatic embryo maturation in loblolly pine (Pinus taeda L.). Cell. Dev. Biol. Plant 1998, 34, 22–26. [Google Scholar] [CrossRef]
- Salaj, T.; Matúŝová, R.; Salaj, J. The effect of carbohydrates and polyethylene glycol on somatic embryo maturation in hybrid fir Abies alba x Abies numidica. Acta Biol. Crac. 2004, 46, 159–167. [Google Scholar]
- Maruyama, E.; Ishii, K.; Hosoi, Y. Efficient plant regeneration of Hinoki cypress (Chamaecyparis obtusa Sieb. et Zucc.) via somatic embryogenesis. J. For. Res. 2005, 10, 73–77. [Google Scholar] [CrossRef]
- Krajñáková, J.; Häggman, H.; Gömöry, D. Effect of sucrose concentration, polyethylene glycol and activated charcoal on maturation and regeneration of Abies cephalonica somatic embryos. Plant Cell Tissue Organ Cult. 2009, 96, 251–262. [Google Scholar] [CrossRef]
- Shoji, M.; Sato, H.; Nakagawa, R.; Funada, R.; Kubo, T.; Ogita, S. Influence of osmotic pressure on somatic embryo in Pinus densiflora. J. For. Res. 2006, 11, 449–453. [Google Scholar] [CrossRef]
- Businge, E.; Bygdell, J.; Wingsle, G.; Moritz, T.; Egertsdotter, U. The effect of carbohydrates and osmoticum on storage reserve accumulation and germination of Norway spruce somatic embryos. Physiol. Plant 2013, 149, 273–285. [Google Scholar] [CrossRef]
- Maruyama, T.E.; Hosoi, Y. Protocol for somatic embryogenesis in Japanese black pine (Pinus thunbergii Parl.) and Japanese red pine (Pinus densiflora Sieb. et Zucc.). In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants Volume 1, 2nd ed.; Jain, S.M., Gupta, P.K., Eds.; Springer: Cham, Switzerland, 2018; pp. 229–241. [Google Scholar]
- Kermode, A. Role of abscisic acid in seed dormancy. J. Plant Growth Regul. 2005, 24, 319–344. [Google Scholar] [CrossRef]
- Kong, L.; von Aderkas, P. Genotype effects on ABA consumption and somatic embryo maturation in interior spruce (Picea glauca x engelmanni). J. Exp. Bot. 2007, 58, 1525–1531. [Google Scholar] [CrossRef]
- Rai, M.K.; Shekhawat, N.S.; Gupta, A.K.; Phulwaria, M.; Ram, K.; Jaiswal, U. The role of abscisic acid in plant tissue culture: A review of recent progress. Plant Cell Tissue Organ Cult. 2011, 106, 179–190. [Google Scholar] [CrossRef]
- Roberts, D.R.; Sutton, B.C.S.; Flinn, B.S. Synchronous and high frequency germination of interior spruce somatic embryos following partial drying at high relative humidity. Can. J. Bot. 1990, 68, 1086–1090. [Google Scholar] [CrossRef]
- Dunstan, D.I.; Bethune, T.D.; Abrams, S.R. Racemic abscisic acid and abscisyl alcohol promote maturation of white spruce (Picea glauca) somatic embryos. Plant Sci. 1991, 76, 219–228. [Google Scholar] [CrossRef]
- Montalbán, I.A.; Moncaleán, P. Pinus radiata (D. Don) somatic embryogenesis. In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants, 2nd ed.; Jain, S.M., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 1–11. [Google Scholar]
- Lelu-Walter, M.A.; Pãques, L.E. Simplified and improved somatic embryogenesis of hybrid larches (Larix x eurolepis and Larix × marschlinsii). Perspectives for breeding. Ann. For. Sci. 2009, 66, 104. [Google Scholar] [CrossRef]
- Lambardi, M.; Harry, I.S.; Menabeni, D.; Thorpe, T.A. Organogenesis and somatic embryogenesis in Cupressus sempervirens. Plant Cell Tissue Organ Cult. 1995, 40, 179–182. [Google Scholar] [CrossRef]
- Bonga, J.M.; Von Aderkas, P. Rejuvenation of tissues from mature conifers and its implication for propagation in vitro. In Clonal Forestry, I., Genetics and Biotechnology; Ahuja, M.R., Libby, W.J., Eds.; Springer: Berlin, Germany, 1993; pp. 182–199. [Google Scholar]
- Li, X.Y.; Huang, F.H. Induction of somatic embryogenesis in loblolly pine (Pinus taeda L.). Cell. Dev. Biol. Plant 1996, 32, 129–135. [Google Scholar] [CrossRef]
- Li, X.Y.; Huang, F.H.; Gbur, E.E., Jr. Polyethylene glycol-promoted development of somatic embryo in loblolly pine (Pinus taeda L.). Cell. Dev. Biol. Plant 1997, 33, 184–189. [Google Scholar] [CrossRef]
- Carlsson, J.; Egertsdotter, U.; Ganeteg, U.; Svennerstam, H. Nitrogen utilization during germination of somatic embryos of Norway spruce: Revealing the importance of supplied glutamine for nitrogen metabolism. Trees 2019, 33, 383–394. [Google Scholar] [CrossRef]
- Guevin, T.G.; Kirby, E.G. Effects of glutamine and osmoticum on somatic embryo maturation in Norway spruce (Picea abies) (l.) karst. In Somatic Cell Genetics and Molecular Genetics of Trees; Ahuja, M.R., Boerjan, W., Neale, D., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 11–16. [Google Scholar]
- Dal Vesco, L.L.; Guerra, M.P. The effectiveness of nitrogen sources in Feijoa somatic embryogenesis. Plant Cell Tissue Organ Cult. 2001, 64, 19–25. [Google Scholar] [CrossRef]
- Montalbán, I.A.; de Diego, N.; Moncaleán, P. Enhancing initiation and proliferation in radiata pine (Pinus radiata D. Don) somatic embryogenesis through seed family screening, zygotic embryo staging and media adjustments. Acta Physiol. Plant 2012, 34, 451–460. [Google Scholar] [CrossRef]
- Hargreaves, C.L.; Reeves, C.B.; Gough, K.; Montalbán, I.A.; Low, C.; van Ballekom, S.; Dungey, H.S.; Moncaleán, P. Nurse tissue for embryo rescue: Testing new conifer somatic embryogenesis methods in a F1 hybrid pine. Trees 2017, 31, 273–283. [Google Scholar] [CrossRef]
- Rahmouni, S.; El Ansari, Z.N.; Badoc, A.; Martin, P.; El Kbiach, M.L.; Lamarti, A. Effect of amino acids on secondary somatic embryogenesis of Moroccan cork oak (Quercus suber L.) tree. Am. J. Plant Sci. 2020, 11, 626–641. [Google Scholar] [CrossRef]
- Montalbán, I.A.; Castander-Olarieta, A.; Hargreaves, C.; Gough, K.; Reeves, C.; van Ballekom, S.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P. Hybrid pine (Pinus attenuata x Pinus radiata) somatic embryogenesis: What do prefer, mother or nurse? Forests 2021, 34, 45. [Google Scholar]
- Cánovas, F.M.; Avila, C.; Cantón, F.R.; Cañas, R.A.; de la Torre, F. Ammonium assimilation and amino acid metabolism in conifers. J. Exp. Bot. 2007, 58, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Llebres, M.T.; Pascual, M.B.; Debille, S.; Trontin, J.F.; Harvengt, L.; Avila, C.; Canovas, F.M. The role of arginine metabolic pathway during embryogenesis and germination in maritime pine (Pinus pinaster). Tree Physiol. 2017, 38, 471–484. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.F.; Navarro, B.V.; Cerruti, G.V.; Elbl, P.; Minocha, R.; Minocha, S.C.; Wendt dos Santos, A.L.; Floh, E.l.S. Polyamine- and amino acid-related metabolism: The roles of arginine and ornithine are associated with the embryogenic potential. Plant Cell Physiol. 2018, 59, 1084–1098. [Google Scholar] [CrossRef]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryo production and plant regeneration of Japanese black pine (Pinus thunbergii). J. For. Res. 2005, 10, 403–407. [Google Scholar] [CrossRef]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Propagation of Japanese red pine (Pinus densiflora Zieb. et Zucc.) via somatic embryogenesis. Prop. Ornam. Plants 2005, 5, 199–204. [Google Scholar]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryogenesis and plant regeration in Yakutanegoyou, Pinus armandii Franch. var. amamiana (Koidz.) Hatusima, an endemic and endangered species in Japan. Cell. Dev. Biol. Plant 2007, 43, 28–34. [Google Scholar] [CrossRef]
- Maruyama, E.T.; Hosoi, Y.; Katsuki, T. Plant regeneration of Yatsugataketouhi (Picea koyamae) through somatic embryogenesis. Kanto Shinrin Kenkyu 2011, 62, 127–130, (In Japanese with English title). [Google Scholar]
- Hosoi, Y.; Maruyama, T.E. Plant regeneration from embryogenic tissue of Pinus luchuensis Mayr, and endemic species in Ryukyu island, Japan. Plant Biotechnol. 2012, 29, 401–406. [Google Scholar] [CrossRef]
- Breton, D.; Harvengt, L.; Trontin, J.F.; Bouvet, A.; Favre, J.M. High subculture frequency, maltose-based and hormone-free medium sustained early development of somatic embryos in maritime pine. Cell. Dev. Biol. Plant 2005, 41, 494–504. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Stasolla, C.; Yeung, E.C.; Thorpe, T.A. Maturation of somatic embryo in conifers: Morphogenesis, physiology, biochemistry, and molecular biology. Cell. Dev. Biol. Plant 2002, 38, 93–105. [Google Scholar] [CrossRef]
- Izuno, A.; Maruyama, T.E.; Ueno, S.; Ujino-Ihara, T.; Moriguchi, Y. Genotype and transcriptome effects on somatic embryogenesis in Cryptomeria japonica. PLoS ONE 2020, 15, e0244634. [Google Scholar] [CrossRef]
- Attree, S.M.; Fowke, L.C. Embryogeny of gymnosperms: Advances in synthetic seed technology of conifers. Plant Cell Tissue Organ Cult. 1993, 35, 1–35. [Google Scholar] [CrossRef]
- Stasolla, C.; Yeung, E.C. Recent advances in conifer somatic embryogenesis: Improving somatic embryo quality. Plant Cell Tissue Organ Cult. 2003, 74, 15–35. [Google Scholar] [CrossRef]
- Texeira da Silva, J.A.; Malabadi, R.B. Factors affecting somatic embryogenesis in conifers. J. For. Res. 2012, 23, 503–515. [Google Scholar] [CrossRef]
- Nic-Can, G.I.; Avilez-Montalvo, J.R.; Aviles-Montalvo, R.N.; Márquez-López, R.E.; Mellado-Mojica, E.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. The relationship between stress and somatic embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Cham, Switzerland, 2016; pp. 151–170. [Google Scholar]
- Egertsdotter, U. Plant physiological and genetical aspects of the somatic embryogenesis process in conifers. Scand. J. For. Res. 2019, 34, 360–369. [Google Scholar] [CrossRef]
- Elmer, K.H.S. Factors regulating somatic embryogenesis in plants. In Somatic Embryogenesis and Gene Expression; Aslam, J., Srivastava, P.S., Sharma, M.P., Eds.; Narosa Publishing House: New Delhi, India, 2013; pp. 56–81. [Google Scholar]
- Gulzar, B.; Mujib, A.; Malik, M.Q.; Sayeed, R.; Mamgain, J.; Ejaz, B. Genes, proteins and other networks regulating somatic embryogenesis in plants. J. Gen. Eng. Biotechnol. 2020, 18, 31. [Google Scholar] [CrossRef]
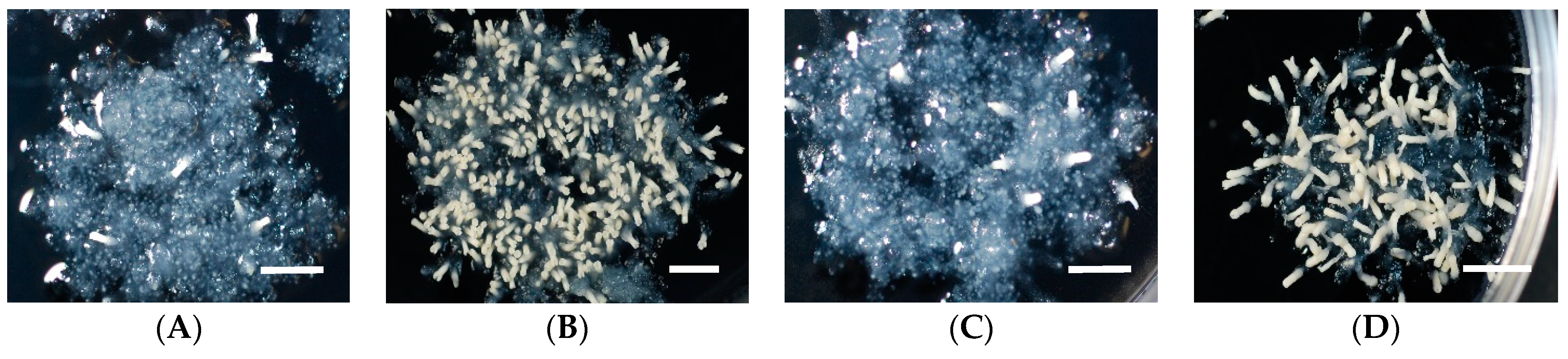

| ECL | Cotyledonary Somatic Embryos per Plate by BS Formulations of Maturation Medium | ||||
|---|---|---|---|---|---|
| B5 | EM | MS | WP | Average per Cell Line | |
| S-18 | 34.7 ± 7.4 | 335.3 ± 136.1 | 5.0 ± 1.0 | 134.7 ± 20.0 | 127.4 ± 147.3 a |
| S-73 | 7.7 ± 5.0 | 223.0 ± 56.2 | 2.3 ± 1.5 | 87.3 ± 39.7 | 80.1 ± 97.6 ab |
| S-100 | 11.0 ± 9.0 | 235.0 ± 145.2 | 2.7 ± 2.5 | 6.3 ± 5.9 | 63.8 ± 120.5 ab |
| S-113 | 9.3 ± 11.0 | 130.7 ± 39.0 | 2.3 ± 0.6 | 5.7 ± 4.0 | 37.0 ± 59.2 b |
| S-352 | 11.3 ± 9.5 | 153.3 ± 83.7 | 3.0 ± 1.0 | 10.0 ± 7.8 | 44.4 ± 75.0 ab |
| Average per medium | 14.8 ± 12.7 c | 215.5 ± 113.4 a | 3.1 ± 1.6 d | 48.8 ± 57.5 b | |
| Variables | df | Residual df | Deviances | p Value | Significance | Deviance Explained (%) |
|---|---|---|---|---|---|---|
| Null | 59 | 925.01 | ||||
| ECL | 4 | 55 | 76.45 | <0.001 | *** | 8.3 |
| BS | 3 | 52 | 701.02 | <0.001 | *** | 75.8 |
| Plate | 2 | 50 | 6.02 | <0.05 | * | 0.7 |
| ECL:BS | 12 | 38 | 76.14 | <0.001 | *** | 8.2 |
| ECL | Cotyledonary Somatic Embryos per Plate by PEG Concentrations | ||||
|---|---|---|---|---|---|
| PEG 10% | PEG 15% | PEG 17.5% | PEG 20% | Average per Cell Line | |
| S-18 | 222.3 ± 95.6 | 544.0 ± 200.3 | 640.3 ± 61.0 | 36.7 ± 7.8 | 360.8 ± 272.0 a |
| S-100 | 160.3 ± 45.6 | 322.3 ± 109.3 | 532.0 ± 120.0 | 20.0 ± 17.8 | 258.7 ± 211.8 a |
| S-352 | 210.0 ± 100.5 | 395.7 ± 209.9 | 446.3 ± 145.4 | 51.7 ± 19.6 | 275.9 ± 201.2 a |
| T-1151 | 222.3 ± 127.6 | 461.3 ± 77.6 | 812.7 ± 196.2 | 58.7 ± 18.2 | 388.8 ± 314.4 a |
| T-158 | 157.7 ± 73.0 | 317.0 ± 106.2 | 686.0 ± 172.9 | 43.7 ± 25.8 | 301.1 ± 269.7 a |
| Average per PEG concentration | 194.5 ± 84.0 b | 408.1 ± 155.4 a | 623.5 ± 180.2 a | 42.1 ± 21.0 c | |
| Variables | df | Residual df | Deviances | p Value | Significance | Deviance Explained (%) |
|---|---|---|---|---|---|---|
| Null | 59 | 515.742 | ||||
| ECL | 4 | 55 | 13.13289 | < 0.05 | * | 2.5 |
| PEG concentration | 3 | 52 | 415.1126 | < 0.001 | *** | 80.5 |
| Plate | 2 | 50 | 0.261557 | 0.877 | ns | 0.1 |
| ECL:PEG concentration | 12 | 38 | 14.18647 | 0.289 | ns | 2.8 |
| ECL | Cotyledonary Somatic Embryos per Plate by ABA Concentrations | ||||
|---|---|---|---|---|---|
| ABA 0 µM | ABA 50 µM | ABA 100 µM | ABA 200 µM | Average per Cell Line | |
| S-18 | 19.3 ± 10.5 | 186.3 ± 161.1 | 385.0 ± 117.4 | 163.7 ± 49.9 | 188.6 ± 161.9 a |
| S-64 | 2.0 ± 1.7 | 273.0 ± 206.9 | 366.0 ± 128.3 | 22.3 ± 8.6 | 165.8 ± 194.4 b |
| S-85 | 2.3 ± 2.5 | 237.7 ± 18.0 | 256.7 ± 64.0 | 344.7 ± 129.6 | 210.3 ± 146.2 b |
| S-100 | 8.7 ± 7.8 | 354.0 ± 62.4 | 377.3 ± 119.4 | 166.0 ± 117.5 | 226.5 ± 174.4 ab |
| S-113 | 1.7 ± 2.1 | 37.3 ± 18.9 | 228.7 ± 113.1 | 77.7 ± 12.0 | 86.3 ± 102.8 b |
| S-352 | 3.3 ± 0.6 | 50.0 ± 25.5 | 331.7 ± 59.5 | 54.3 ± 27.2 | 109.8 ± 138.7 b |
| T-1151 | 8.0 ± 4.6 | 271.7 ± 98.3 | 408.3 ± 205.9 | 139.0 ± 13.5 | 206.8 ± 183.7 ab |
| Average per ABA concentration | 6.5 ± 7.5 c | 201.4 ± 145.2 ab | 336.2 ± 122.3 a | 138.2 ± 116.8 b | |
| Variables | df | Residual df | Deviances | p Value | Significance | Deviance Explained (%) |
|---|---|---|---|---|---|---|
| Null | 83 | 1078.277 | ||||
| ECL | 6 | 77 | 66.60139 | <0.001 | *** | 6.2 |
| ABA concentration | 3 | 74 | 775.4969 | <0.001 | *** | 71.9 |
| Plate | 2 | 72 | 6.15035 | <0.05 | * | 0.6 |
| ECL:ABA concentration | 18 | 54 | 134.1964 | <0.001 | *** | 12.4 |
| ECL | Cotyledonary Somatic Embryos per Plate by Additional KCl Supplementation | ||
|---|---|---|---|
| KCl (–) | KCl (+) | Average per Cell Line | |
| S-18 | 381.0 ± 151.0 | 420.8 ± 72.4 | 400.9 ± 113.6 ab |
| S-85 | 294.4 ± 111.7 | 214.4 ± 43.0 | 254.4 ± 90.2 ab |
| S-100 | 325.2 ± 73.3 | 309.2 ± 68.7 | 317.2 ± 67.5 ab |
| S-182 | 197.4 ± 62.7 | 208.2 ± 91.0 | 202.8 ± 73.9 b |
| S-352 | 304.2 ± 204.7 | 336.2 ± 105.0 | 320.2 ± 154.3 ab |
| T-1151 | 459.8 ± 289.8 | 362.6 ± 263.8 | 411.2 ± 266.2 a |
| T-158 | 385.4 ± 126.6 | 391.8 ± 144.8 | 388.6 ± 128.3 ab |
| Average per KCl supplementation | 335.3 ± 167.6 a | 320.5 ± 142.7 a | |
| Variables | df | Residual df | Deviances | p Value | Significance | Deviance Explained (%) |
|---|---|---|---|---|---|---|
| Null | 69 | 100.8604 | ||||
| ECL | 6 | 63 | 25.15085 | <0.001 | *** | 24.9 |
| KCl supplementation | 1 | 62 | 0.257053 | 0.612 | ns | 0.3 |
| Plate | 4 | 58 | 0.92416 | 0.921 | ns | 0.9 |
| ECL:KCl supplementation | 6 | 52 | 2.775315 | 0.836 | ns | 2.8 |
| ECL | Cotyledonary Somatic Embryos per Plate by AA Concentrations | ||||
|---|---|---|---|---|---|
| 0× | 1× | 2× | 3× | Average per Cell Line | |
| S-18 | 42.0 ± 33.4 | 330.3 ± 211.8 | 452.7 ± 221.2 | 305.0 ± 155.9 | 282.5 ± 214.7 a |
| S-100 | 3.0 ± 2.6 | 156.3 ± 74.9 | 340.7 ± 53.6 | 274.7 ± 39.5 | 193.7 ± 140.7 b |
| S-113 | 6.7 ± 2.5 | 208.7 ± 69.2 | 115.7 ± 38.6 | 94.3 ± 72.2 | 106.3 ± 87.9 b |
| S-182 | 2.3 ± 2.1 | 233.7 ± 83.3 | 274.0 ± 75.6 | 308.0 ± 152.6 | 204.5 ± 148.8 b |
| S-352 | 2.3 ± 2.5 | 268.7 ± 73.7 | 353.3 ± 50.5 | 302.7 ± 110.7 | 231.8 ± 154.3 b |
| Average per AA concentration | 11.3 ± 20.5 a | 239.5 ± 115.3 b | 307.3 ± 148.7 b | 256.9 ± 129.4 b | |
| Variables | df | Residual df | Deviances | p Value | Significance | Deviance Explained (%) |
|---|---|---|---|---|---|---|
| Null | 59 | 627.6505 | ||||
| ECL | 4 | 55 | 42.45872 | <0.001 | *** | 6.8 |
| AA concentration | 3 | 52 | 442.8232 | <0.001 | *** | 70.6 |
| Plate | 2 | 50 | 0.919119 | 0.632 | ns | 0.1 |
| ECL:AA concentration | 12 | 38 | 73.40887 | <0.001 | *** | 11.7 |
| ECL | Cotyledonary Somatic Embryos per Plate by Proliferation Media | ||||
|---|---|---|---|---|---|
| 3-1 | EM3-1 | EM3-1m | EM10-0 | Average per Cell line | |
| S-18 | 381.6 ± 88.7 | 345.2 ± 133.5 | 269.6 ± 48.4 | 274.2 ± 143.9 | 317.6 ± 112.4 ab |
| S-100 | 268.0 ± 80.3 | 284.0 ± 70.7 | 233.6 ± 69.3 | 252.0 ± 37.7 | 259.4 ± 63.9 b |
| T-1151 | 478.8 ± 115.0 | 458. 4± 89.8 | 493.6 ± 100.1 | 450.0 ± 130.5 | 470.2 ± 102.4 a |
| T-158 | 410.0 ± 293.7 | 402.2 ± 256.8 | 351.4 ± 114.6 | 299.4 ± 94.7 | 365.8 ± 196.9 ab |
| Average per PCM | 384.6 ± 173.3 a | 372.4 ± 157.6 a | 337.1 ± 130.0 a | 318.9 ± 128.3 a | |
| Variables | df | Residual df | Deviances | p Value | Significance | Deviance Explained (%) |
|---|---|---|---|---|---|---|
| Null | 79 | 126.5727 | ||||
| ECL | 3 | 76 | 34.46032 | <0.001 | *** | 27.2 |
| PCM | 3 | 73 | 4.761264 | 0.190 | ns | 3.8 |
| Plate | 4 | 69 | 2.787561 | 0.594 | ns | 2.2 |
| ECL:PCM | 9 | 60 | 3.155583 | 0.958 | ns | 2.5 |
| Factor | Best Result | Reference |
|---|---|---|
| BS in medium | EM | Maruyama et al. 2000 |
| PEG concentration | 175 g L−1 | 169-09125 Wako Pure Chemical, Osaka, Japan |
| ABA concentration | 100 µM | BIA-0125 Apollo Scientific Ltd., Manchester, UK |
| KCl concentration | 0.08 g L−1 | 063-03545 Wako Pure Chemical, Osaka, Japan |
| AA concentration | L (+) Glutamine 2 g L−1 L- Asparagine 1 g L−1 L (+) Arginine 0.5 g L−1 | 078-00525 Wako Pure Chemical, Osaka, Japan 013-04815 Wako Pure Chemical, Osaka, Japan 011-04615 Wako Pure Chemical, Osaka, Japan |
| PCM | 3-1 (EM proliferation medium) | Maruyama et al. 2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, T.E.; Ueno, S.; Mori, H.; Kaneeda, T.; Moriguchi, Y. Factors Influencing Somatic Embryo Maturation in Sugi (Japanese Cedar, Cryptomeria japonica (Thunb. ex L.f.) D. Don). Plants 2021, 10, 874. https://doi.org/10.3390/plants10050874
Maruyama TE, Ueno S, Mori H, Kaneeda T, Moriguchi Y. Factors Influencing Somatic Embryo Maturation in Sugi (Japanese Cedar, Cryptomeria japonica (Thunb. ex L.f.) D. Don). Plants. 2021; 10(5):874. https://doi.org/10.3390/plants10050874
Chicago/Turabian StyleMaruyama, Tsuyoshi E., Saneyoshi Ueno, Hideki Mori, Takumi Kaneeda, and Yoshinari Moriguchi. 2021. "Factors Influencing Somatic Embryo Maturation in Sugi (Japanese Cedar, Cryptomeria japonica (Thunb. ex L.f.) D. Don)" Plants 10, no. 5: 874. https://doi.org/10.3390/plants10050874
APA StyleMaruyama, T. E., Ueno, S., Mori, H., Kaneeda, T., & Moriguchi, Y. (2021). Factors Influencing Somatic Embryo Maturation in Sugi (Japanese Cedar, Cryptomeria japonica (Thunb. ex L.f.) D. Don). Plants, 10(5), 874. https://doi.org/10.3390/plants10050874







