The Effect of Granular Activated Carbon and Biochar on the Availability of Cu and Zn to Hordeum sativum Distichum in Contaminated Soil
Abstract
1. Introduction
2. Objects and Methods
2.1. Study Objects
2.2. Analytical Methods
2.3. Statistical Analysis
3. Results and Discussion
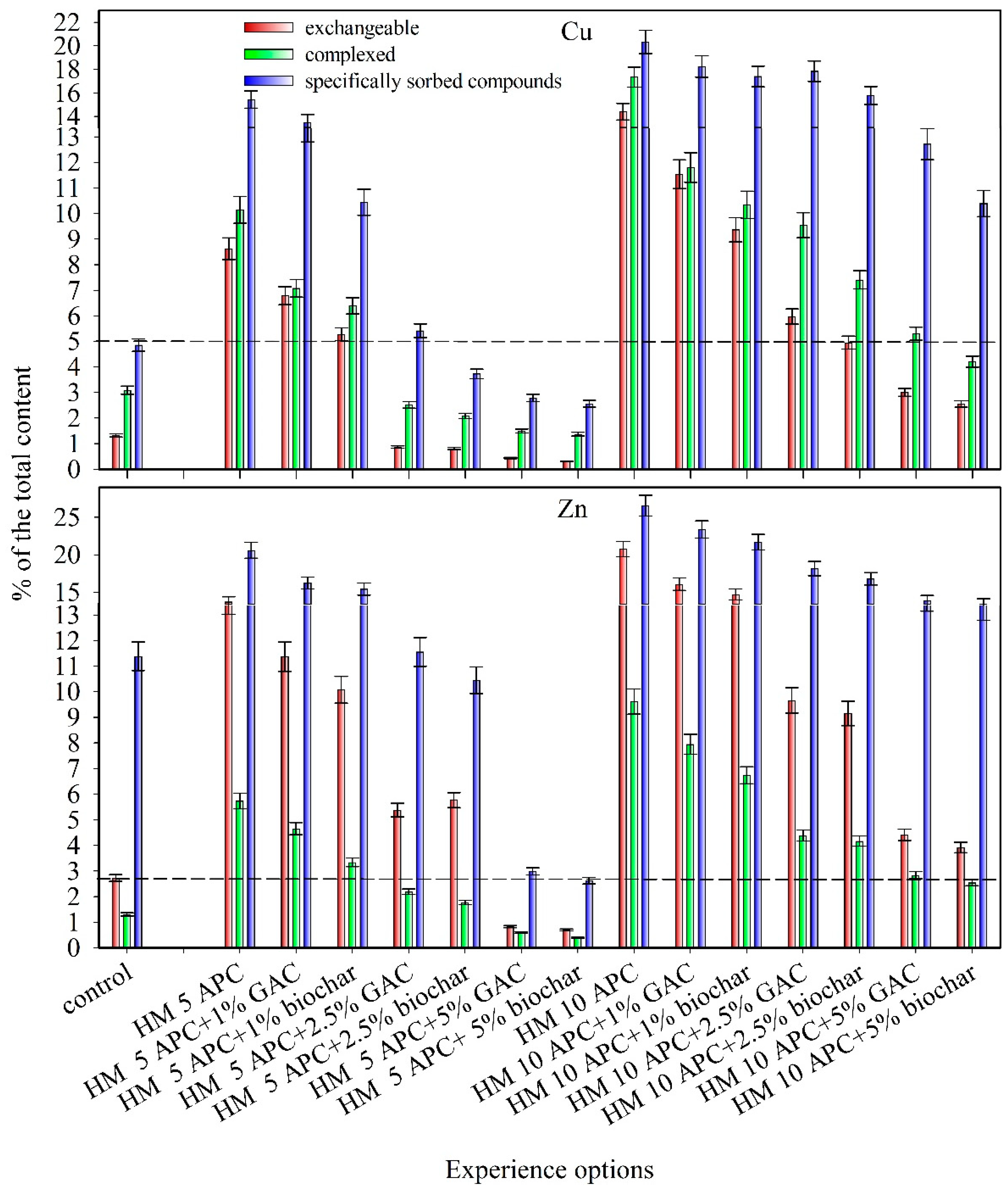
3.1. Cu and Zn Speciation and Bioavailability in Soil
3.2. Cu and Zn Accumulation in Spring Barley
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 6730305. [Google Scholar] [CrossRef]
- Chaplygin, V.A.; Minkina, T.M.; Mandzhieva, S.S.; Nazarenko, O.G.; Zimulina, I.V.; Bauer, T.V.; Litvinov, Y.A.; Rajput, V. Heavy metals in agricultural crops of Rostov region through the example of soft wheat (Triticum aestivum). IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012204. [Google Scholar] [CrossRef]
- Minkina, T.M.; Motuzova, G.V.; Mandzhieva, S.S.; Nazarenko, O.G. Ecological resistance of the soil–plant system to contamination by heavy metals. J. Geochem. Explor. 2012, 123, 33–40. [Google Scholar] [CrossRef]
- Bezuglova, O.S.; Gorbov, S.N.; Tischenko, S.A.; Shimko, A.E. Use of brown coal as a detoxifier of soils contaminated with heavy metals. J. Geochem. Explor. 2018, 184, 232–238. [Google Scholar] [CrossRef]
- Koptsik, G.N. Problems and prospects concerning the phytoremediation of heavy metal polluted soils: A review. Eurasian Soil Sci. 2014, 47, 923–939. [Google Scholar] [CrossRef]
- Pukalchik, M.; Mercl, F.; Terekhova, V.; Tlustoš, P. Biochar, wood ash and humic substances mitigating trace elements stress in contaminated sandy loam soil: Evidence from an integrative approach. Chemosphere 2018, 203, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Gorovtsov, A.; Rajput, V.; Minkina, T.; Mandzhieva, S.; Sushkova, S.; Kornienko, I.; Grigoryeva, T.V.; Chokheli, V.; Aleshukina, I.; Zinchenko, V.; et al. The role of biochar-microbe interaction in alleviating heavy metal toxicity in Hordeum vulgare L. grown in highly polluted soils. Appl. Geochem. 2019. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Ouyang, L. Priming of pyrogenic C (biochar) mineralization by dissolved organic matter and vice versa. Soil Biol. Biochem. 2019, 130, 105–112. [Google Scholar] [CrossRef]
- Vasilyeva, G.K.; Strijakova, E.R.; Shea, P.J. Use of activated carbon for soil bioremediation. In Soil and Water Pollution Monitoring, Protection and Remediation, Dordrecht; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; pp. 309–322. [Google Scholar]
- Bădescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Riedel, G.S.; Riedel, G.; Kwon, S.; Landis, R.; Brown, S.S.; Menzie, C.A.; Ghosh, U. Activated Carbon Mitigates Mercury and Methylmercury Bioavailability in Contaminated Sediments. Environ. Sci. Technol. 2013, 47, 13001–13010. [Google Scholar] [CrossRef]
- Bandara, T.; Franks, A.; Xu, J.; Bolan, N.; Wang, H.; Tang, C. Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit. Rev. Environ. Sci. Technol. 2020, 50, 903–978. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, N.; Purakayastha, T.J. Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci. Total Environ. 2017, 577, 376–385. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Sima, J.; Zhao, L.; Mašek, O.; Cao, X. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, M.; Fan, X.; Song, J.; Peng, P.; Li, K.; Jia, W.; Song, H. Influence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Cimò, G.; Kucerik, J.; Berns, A.E.; Schaumann, G.E.; Alonzo, G.; Conte, P. Effect of Heating Time and Temperature on the Chemical Characteristics of Biochar from Poultry Manure. J. Agric. Food Chem. 2014, 62, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Zeng, W.; Yuan, X.; Li, B.; Li, T.; Zu, Y.; Jiang, M.; Li, Y. Field experiment on the effects of sepiolite and biochar on the remediation of Cd- and Pb-polluted farmlands around a Pb–Zn mine in Yunnan Province, China. Environ. Sci. Pollut. Res. 2019, 26, 7743–7751. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci. Total Environ. 2014, 468–469, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Houben, D.; Evrard, L.; Sonnet, P. Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Peng, T.; Li, G.; Wang, S.; Duan, L.; Mulder, J.; Cornelissen, G.; Cheng, Z.; Yang, S.; Hou, D. Sulfur-modified rice husk biochar: A green method for the remediation of mercury contaminated soil. Sci. Total Environ. 2018, 621, 819–826. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of Biochar: Microchemical Properties; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009. [Google Scholar]
- Rizhiya, E.Y.; Buchkina, N.P.; Mukhina, I.M.; Belinets, A.S.; Balashov, E.V. Effect of biochar on the properties of loamy sand Spodosol soil samples with different fertility levels: A laboratory experiment. Eurasian Soil Sci. 2015, 48, 192–200. [Google Scholar] [CrossRef]
- Bauer, T.; Pinskii, D.; Minkina, T.; Nevidomskaya, D.; Mandzhieva, S.; Burachevskaya, M.; Chaplygin, V.; Popileshko, Y. Time effect on the stabilization of technogenic copper compounds in solid phases of Haplic Chernozem. Sci. Total Environ. 2018, 626, 1100–1107. [Google Scholar] [CrossRef]
- Bezuglova, O.S.; Yudina, N.V. Interrelationship between the physical properties and the humus content of chernozems in the south of European Russia. Eurasian Soil Sci. 2006, 39, 187–194. [Google Scholar] [CrossRef]
- Zamulina, I.; Pinsky, D.; Burachevskaya, M.; Bauer, T.; Pshenichnaya, A. The effect of granular activated carbon on the physical properties of soils at copper contamination. E3s Web Conf. 2020, 175, 09003. [Google Scholar] [CrossRef]
- GOST-5852-79. Reagents. Copper (II) Acetate Monohydrate; Standards Publishing House: Moscow, Russia, 1979. [Google Scholar]
- GN-2.1.7.2511-09. Approximate Permissible Concentrations (APC) of Chemicals in Soil: Hygiene Standards; Rospotrebnadzor: Moscow, Russia, 2009. [Google Scholar]
- Huang, S.-h. Fractional distribution and risk assessment of heavy metal contaminated soil in vicinity of a lead/zinc mine. Trans. Nonferr. Met. Soc. China 2014, 24, 3324–3331. [Google Scholar] [CrossRef]
- Minkina, T.M.; Mandzhieva, S.S.; Burachevskaya, M.V.; Bauer, T.V.; Sushkova, S.N. Method of determining loosely bound compounds of heavy metals in the soil. MethodsX 2018, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Vitkovskaya, S.E. Methods for Assessing the Effectiveness and Environmental Safety and Chemical Ameliorants; AFI: St. Petersburg, FL, USA, 2017; ISBN 978-5-905200-35-9. [Google Scholar]
- GOST-R-58595. Soils. Sampling; M. Standartinform: Moscow, Russia, 2019. [Google Scholar]
- GOST-6217-74. Crushed Active Wood Coal; Standards Publishing House: Moscow, Russia, 1974. [Google Scholar]
- Minkina, T.; Rajput, V.; Fedorenko, G.; Fedorenko, A.; Mandzhieva, S.; Sushkova, S.; Morin, T.; Yao, J. Anatomical and ultrastructural responses of Hordeum sativum to the soil spiked by copper. Environ. Geochem. Health 2020, 42, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Stevenson, F.J. Infrared spectra of Cu2+ Pb2+ and Ca2+ complexes of soil humic substances. Geoderma 1982, 27, 195–208. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Comparative study for adsorption of methylene blue dye on biochar derived from orange peel and banana biomass in aqueous solutions. Environ. Monit. Assess. 2019, 191, 735. [Google Scholar] [CrossRef] [PubMed]
- GOST-R-52917. Solid Mineral Fuel. Methods for Determining Moisture in an Analytical Sample; M. Standartinform: Moscow, Russia, 2008. [Google Scholar]
- Rajput, V.D.; Minkina, T.; Fedorenko, A.; Fedorenko, G.; Mandzhieva, S.; Sushkova, S.; Chernikova, N.; Duplii, N.; Azarov, A.; Usatov, A. Interaction of CuO Nanoparticles with Hordeum Sativum Distichum in an Aquatic Medium and in the Soil. In Recent Advances in Geo-Environmental Engineering, Geomechanics and Geotechnics, and Geohazards; Springer International Publishing: Cham, Switzerland, 2019; pp. 25–27. [Google Scholar]
- White, P.A.; Claxton, L.D. Mutagens in contaminated soil: A review. Mutat. Res./Rev. Mutat. Res. 2004, 567, 227–345. [Google Scholar] [CrossRef] [PubMed]
- Anokhina, T.O.; Kochetkov, V.V.; Zelenkova, N.F.; Balakshina, V.V.; Boronin, A.M. Biodegradation of Phenanthrene by Pseudomonas Bacteria Bearing Rhizospheric Plasmids in Model Plant–Microbial Associations. Appl. Biochem. Microbiol. 2004, 40, 568–572. [Google Scholar] [CrossRef]
- Anisimov, V.S.; Anisimova, L.N.; Frigidova, L.M.; Dikarev, D.V.; Frigidov, R.A.; Korneev, Y.N.; Sanzharov, A.I.; Arysheva, S.P. Evaluation of the Migration Capacity of Zn in the Soil–Plant System. Eurasian Soil Sci. 2018, 51, 407–417. [Google Scholar] [CrossRef]
- GOST-26929-94. Raw Materials and Food Products. Sample Preparation. Mineralization to Determine the Content of Toxic Elements; Standards Publishing House: Moscow, Russia, 1984. [Google Scholar]
- Minkina, T.M.; Motuzova, G.V.; Nazarenko, O.G.; Kryshchenko, V.S.; Mandzhieva, S.S. Forms of heavy metal compounds in soils of the steppe zone. Eurasian Soil Sci. 2008, 41, 708–716. [Google Scholar] [CrossRef]
- Chaplygin, V.; Minkina, T.; Mandzhieva, S.; Burachevskaya, M.; Sushkova, S.; Poluektov, E.; Antonenko, E.; Kumacheva, V. The effect of technogenic emissions on the heavy metals accumulation by herbaceous plants. Environ. Monit. Assess. 2018, 190, 124. [Google Scholar] [CrossRef] [PubMed]
- Minkina, T.M.; Linnik, V.G.; Nevidomskaya, D.G.; Bauer, T.V.; Mandzhieva, S.S.; Khoroshavin, V.Y. Forms of Cu (II), Zn (II), and Pb (II) compounds in technogenically transformed soils adjacent to the Karabashmed copper smelter. J. Soils Sediments 2018, 18, 2217–2228. [Google Scholar] [CrossRef]
- Smith, T.K. RR Brooks Biological Methods of Prospecting for Minerals. Chichester and New York (Wiley-Interscience). Mineral. Mag 2018, 48, 466–467. [Google Scholar] [CrossRef]
- Mandzhieva, S.S.; Minkina, T.M.; Chaplygin, V.A.; Bauer, T.V.; Burachevskaya, M.V.; Nevidomskaya, D.G.; Sushkova, S.N.; Sherstnev, A.K.; Zamulina, I.V. Protective mechanism of the soil–plant system with respect to heavy metals. J. Soils Sediments 2017, 17, 1291–1300. [Google Scholar] [CrossRef]
- Minkina, T.M.; Nevidomskaya, D.G.; Pol’shina, T.N.; Fedorov, Y.A.; Mandzhieva, S.S.; Chaplygin, V.A.; Bauer, T.V.; Burachevskaya, M.V. Heavy metals in the soil–plant system of the Don River estuarine region and the Taganrog Bay coast. J. Soils Sediments 2017, 17, 1474–1491. [Google Scholar] [CrossRef]
- GN-2.1.7.2042-06. Maximum Permissible Concentrations (MPC) of Chemicals in the Soil: Hygienic Standards; Rospotrebnadzor: Moscow, Russia, 2006. [Google Scholar]
- Pinskii, D.L.; Minkina, T.M.; Bauer, T.V.; Nevidomskaya, D.G.; Mandzhieva, S.S.; Burachevskaya, M.V. Copper Adsorption by Chernozem Soils and Parent Rocks in Southern Russia. Geochem. Int. 2018, 56, 266–275. [Google Scholar] [CrossRef]
- Burachevskaya, M.; Minkina, T.; Mandzhieva, S.; Bauer, T.; Chaplygin, V.; Sushkova, S.; Tsitsuashvili, V.; Popileshko, Y. Chemical partitioning of Zn in soil: Application of two sequential extraction procedures. Geochem. Explor. Environ. Anal. 2019, 19, 93. [Google Scholar] [CrossRef]
- Bauer, T.; Pinskii, D.; Minkina, T.; Mandzhieva, S.; Burachevskaya, M.; Kalinitchenko, V.; Barakhov, A. Stabilization dynamics of easily and poorly soluble Zn compounds in the soil. Geochem. Explor. Environ. Anal. 2019, 19, 184–192. [Google Scholar] [CrossRef]
- Mertens, J.; Smolders, E. Zinc. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 465–493. [Google Scholar]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef]
- Sanitary Regulations and Norms. Hygienic Requirements for Safety and Nutritional Value of Food Products; Publishing House of the Siberian Cedar Foundation: Novosibirsk, Russia, 2002. [Google Scholar]
- Otabbong, E.; Sadovnikova, L.; Lakimenko, O.; Nilsson, I.; Persson, J. Sewage sludge: Soil conditioner and nutrient source II. Availability of Cu, Zn, Pb and Cd to barley in a pot experiment. Acta Agric. Scand. Sect. B Soil Plant Sci. 1997, 47, 65–70. [Google Scholar] [CrossRef]
- Napoli, M.; Cecchi, S.; Grassi, C.; Baldi, A.; Zanchi, C.A.; Orlandini, S. Phytoextraction of copper from a contaminated soil using arable and vegetable crops. Chemosphere 2019, 219, 122–129. [Google Scholar] [CrossRef]
- Rajput, V.; Chaplygin, V.; Gorovtsov, A.; Fedorenko, A.; Azarov, A.; Chernikova, N.; Barakhov, A.; Minkina, T.; Maksimov, A.; Mandzhieva, S.; et al. Assessing the toxicity and accumulation of bulk- and nano-CuO in Hordeum sativum L. Environ. Geochem. Health 2020. [Google Scholar] [CrossRef]
- Sridhar, B.B.M.; Han, F.X.; Diehl, S.V.; Monts, D.L.; Su, Y. Effects of Zn and Cd accumulation on structural and physiological characteristics of barley plants. Braz. J. Plant Physiol. 2007, 19, 15–22. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Rajput, V.D.; Gorovtsov, A.V.; Fedorenko, G.M.; Minkina, T.M.; Fedorenko, A.G.; Lysenko, V.S.; Sushkova, S.S.; Mandzhieva, S.S.; Elinson, M.A. The influence of application of biochar and metal-tolerant bacteria in polluted soil on morpho-physiological and anatomical parameters of spring barley. Environ. Geochem. Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of Nutrient-Enriched Biochar as a Soil Amendment during Maize Growth: Exploring Practical Alternatives to Recycle Agricultural Residuals and to Reduce Chemical Fertilizer Demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Rivier, P.-A.; Rasse, D.; Joner, E.J. Biochar Affects Heavy Metal Uptake in Plants through Interactions in the Rhizosphere. Appl. Sci. 2020, 10, 5105. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.-H.; Kim, H.-U.; Owens, G.; Kim, K.-R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Börjesson, G.; Kätterer, T. Soil fertility effects of repeated application of sewage sludge in two 30-year-old field experiments. Nutr. Cycl. Agroecosyst. 2018, 112, 369–385. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.V.; Ginder-Vogel, M.; Voegelin, A.; Cambell, K. Biogeochemical Redox Processes and their Impact on Contaminant Dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Pollution |
|---|---|
| Control | Soil without pollution |
| Cu | 660 mg/kg for Cu (5 APC *) |
| Cu + 1% sorbent ** | 660 mg/kg for Cu (5 APC) |
| Cu + 2.5% sorbent | 660 mg/kg for Cu (5 APC) |
| Cu + 5% sorbent | 660 mg/kg for Cu (5 APC) |
| Zn | 1100 mg/kg Zn (5 APC) |
| Zn + 1% sorbent | 1100 mg/kg Zn (5 APC) |
| Zn + 2.5% sorbent | 1100 mg/kg Zn (5 APC) |
| Zn + 5% sorbent | 1100 mg/kg Zn (5 APC) |
| Cu | 1320 mg/kg Cu (10 APC) |
| Soil + 1% sorbent | 1320 mg/kg Cu (10 APC) |
| Soil + 2.5% sorbent | 1320 mg/kg Cu (10 APC) |
| Soil + 5% sorbent | 1320 mg/kg Cu (10 APC) |
| Zn | 2200 mg/kg Zn (10 APC) |
| Zn + 1% sorbent | 2200 mg/kg Zn (10 APC) |
| Zn + 2.5% sorbent | 2200 mg/kg Zn (10 APC) |
| Zn + 5% sorbent | 2200 mg/kg Zn (10 APC) |
| Sorbents | Content of Elements and Ash, % | Atomic Ratio of Elements | Water Content, % | pH | Specific Surface Area, m2 g−1 | Volume of Pores, cm3 g−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | H | O | Ash | H/C | O/C | (N + O)/C | Total | Micro | Meso | Macro | ||||
| GAC* | 72.5 | 2.0 | 3.2 | 4.3 | 18.0 | 0.53 | 0.05 | 0.07 | 3.2 | 9.3 | 766 | 0.82 | 0.49 | 0.17 | 0.16 |
| Biochar | 73.7 | 2.3 | 2.7 | 6.1 | 15.2 | 0.44 | 0.06 | 0.09 | 3.6 | 9.1 | 624 | 0.85 | 0.60 | 0.04 | 0.21 |
| Treatment | Loosely Bound Compounds | Total Content | ||
|---|---|---|---|---|
| Exchangeable | Complexed | Specifically Sorbed | ||
| Cu | ||||
| Control | 0.6 ± 0.1 * | 1.4 ± 0.1 | 2.2 ± 0.2 | 45.3 |
| Cu 5 APC ** | 59.7 ± 5.4 | 70.2 ± 6.3 | 106.8 ± 9.6 | 692.0 |
| Cu 5 APC +1% GAC *** | 46.2 ± 4.2 | 48.1 ± 4.3 | 91.7 ± 8.3 | 680.0 |
| Cu 5 APC +1% biochar | 36.5 ± 3.3 | 44.2 ± 4.0 | 72.1 ± 6.5 | 691.0 |
| Cu 5 APC +2.5% GAC | 5.9 ± 0.5 | 17.2 ± 1.5 | 37.1 ± 3.3 | 684.0 |
| Cu 5 APC +2.5% biochar | 5.6 ± 0.5 | 14.5 ± 1.3 | 25.9 ± 2.3 | 696.0 |
| Cu 5 APC +5% GAC | 3.0 ± 0.3 | 10.3 ± 0.9 | 19.3 ± 1.7 | 691.0 |
| Cu 5 APC +5% biochar | 2.1 ± 0.2 | 9.3 ± 0.8 | 17.4 ± 1.6 | 678.0 |
| Cu 10 APC | 197.5 ± 17.8 | 237.8 ± 21.4 | 278.8 ± 25.1 | 1371.0 |
| Cu 10 APC +1% GAC | 158.1 ± 14.2 | 161.7 ± 14.6 | 250.0 ± 22.5 | 1370.0 |
| Cu 10 APC +1% biochar | 129.2 ± 11.6 | 142.7 ± 12.8 | 240.1 ± 21.6 | 1380.0 |
| Cu 10 APC +2.5% GAC | 82.0 ± 7.4 | 131.0 ± 11.8 | 245.0 ± 22.0 | 1373.0 |
| Cu 10 APC +2.5% biochar | 67.8 ± 6.1 | 101.4 ± 9.1 | 216.1 ± 19.4 | 1369.0 |
| Cu 10 APC +5% GAC | 41.0 ± 3.7 | 72.5 ± 6.5 | 173.8 ± 15.6 | 1367.0 |
| Cu 10 APC +5% biochar | 35.1 ± 3.2 | 57.8 ± 5.2 | 142.9 ± 12.9 | 1375.0 |
| MPC 3.0 **** [62] | APC 132.0 ** [41] | |||
| Zn | ||||
| Control | 2.3 ± 0.2 | 1.1 ± 0.1 | 9.6 ± 0.9 | 84.3 |
| Zn 5 APC | 162.1 ± 14.6 | 67.8 ± 6.1 | 243.5 ± 21.9 | 1182.0 |
| Zn 5 APC +1% GAC | 134.1 ± 12.1 | 55.0 ± 4.9 | 191.6 ± 17.2 | 1180.0 |
| Zn 5 APC +1% biochar | 119.2 ± 10.7 | 39.5 ± 3.6 | 182.5 ± 16.4 | 1183.0 |
| Zn 5 APC +2.5% GAC | 63.5 ± 5.7 | 25.8 ± 2.3 | 136.3 ± 12.3 | 1179.0 |
| Zn 5 APC +2.5% biochar | 67.9 ± 6.1 | 20.9 ± 1.9 | 123.1 ± 11.1 | 1178.0 |
| Zn 5 APC +5% GAC | 9.7 ± 0.9 | 6.9 ± 0.6 | 35.1 ± 3.2 | 1175.0 |
| Zn 5 APC +5% biochar | 8.3 ± 0.7 | 4.6 ± 0.4 | 31.1 ± 2.8 | 1185.0 |
| Zn 10 APC | 475.6 ± 42.8 | 220.1 ± 19.8 | 607.7 ± 54.7 | 2289.0 |
| Zn 10 APC +1% GAC | 368.2 ± 33.1 | 181.8 ± 16.4 | 534.7 ± 48.1 | 2288.0 |
| Zn 10 APC +1% biochar | 335.4 ± 30.2 | 153.5 ± 13.8 | 494.4 ± 44.5 | 2280.0 |
| Zn 10 APC +2.5% GAC | 220.3 ± 19.8 | 100.1 ± 9.0 | 414.3 ± 37.3 | 2281.0 |
| Zn 10 APC +2.5% biochar | 208.3 ± 18.7 | 94.9 ± 8.5 | 382.6 ± 34.4 | 2277.0 |
| Zn 10 APC +5% GAC | 100.8 ± 9.1 | 64.9 ± 5.8 | 316.7 ± 28.5 | 2284.0 |
| Zn 10 APC +5% biochar | 89.5 ± 8.1 | 58.2 ± 5.2 | 307.5 ± 27.7 | 2284.0 |
| MPC 23.0 **** [62] | APC 220.0 ** [41] | |||
| Treatment | % from Loosely Bound Compounds | ||
|---|---|---|---|
| Exchangeable | Complexed | Specifically Sorbed | |
| Cu | |||
| Control | 14 | 33 | 53 |
| Cu 5 APC * | 25 | 30 | 45 |
| Cu 5 APC + 1% GAC ** | 25 | 26 | 49 |
| Cu 5 APC + 1% biochar | 24 | 29 | 47 |
| Cu 5 APC + 2.5% GAC | 10 | 29 | 61 |
| Cu 5 APC + 2.5% biochar | 12 | 31 | 57 |
| Cu 5 APC + 5% GAC | 9 | 32 | 59 |
| Cu 5 APC + 5% biochar | 7 | 32 | 61 |
| Cu 10 APC | 28 | 33 | 39 |
| Cu 10 APC + 1% GAC | 28 | 28 | 44 |
| Cu 10 APC + 1% biochar | 25 | 28 | 47 |
| Cu 10 APC + 2.5% GAC | 18 | 29 | 53 |
| Cu 10 APC + 2.5% biochar | 18 | 26 | 56 |
| Cu 10 APC + 5% GAC | 14 | 25 | 61 |
| Cu 10 APC + 5% biochar | 15 | 24 | 61 |
| Zn | |||
| Control | 18 | 8 | 74 |
| Zn 5 APC | 34 | 14 | 52 |
| Zn 5 APC + 1% GAC | 35 | 14 | 51 |
| Zn 5 APC + 1% biochar | 35 | 12 | 53 |
| Zn 5 APC + 2.5% GAC | 28 | 11 | 61 |
| Zn 5 APC + 2.5% biochar | 32 | 10 | 58 |
| Zn 5 APC + 5% GAC | 19 | 13 | 68 |
| Zn 5 APC + 5% biochar | 19 | 10 | 71 |
| Zn 10 APC | 36 | 17 | 47 |
| Zn 10 APC + 1% GAC | 34 | 17 | 49 |
| Zn 10 APC + 1% biochar | 34 | 16 | 50 |
| Zn 10 APC + 2.5% GAC | 30 | 14 | 56 |
| Zn 10 APC + 2.5% biochar | 30 | 14 | 56 |
| Zn 10 APC + 5% GAC | 21 | 13 | 66 |
| Zn 10 APC + 5% biochar | 20 | 12 | 68 |
| Treatment | Cu Content in | AC * Cu | Zn Content in | AC Zn | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Grain | Root | Stem | Grain | |||
| Control | 12.7 ± 1.1 ** | 10.4 ± 0.9 | 7.0 ± 0.6 | 3.0 | 19.20 ± 1.7 | 17.4 ± 1.6 | 10.4 ± 0.9 | 1.5 |
| HM *** 5 APC **** | 243.6 ± 21.9 | 67.1 ± 6.0 | 12.3 ± 1.1 | 1.0 | 348.5 ± 31.4 | 85.8 ± 7.7 | 33.2 ± 3.0 | 0.7 |
| HM 5 APC + 1% GAC ***** | 177.8 ± 16.0 | 52.3 ± 4.7 | 10.0 ± 0.9 | 1.0 | 265.8 ± 23.9 | 64.5 ± 5.8 | 31.1 ± 2.8 | 0.7 |
| HM 5 APC + 1% biochar | 167.2 ± 15.0 | 56.2 ± 5.1 | 9.5 ± 0.9 | 1.1 | 263.6 ± 23.7 | 61.3 ± 5.5 | 30.8 ± 2.8 | 0.8 |
| HM 5 APC + 2.5% GAC | 108.8 ± 9.8 | 27.7 ± 2.5 | 8.4 ± 0.8 | 1.8 | 176.2 ± 15.9 | 42.8 ± 3.9 | 25.3 ± 2.3 | 0.8 |
| HM 5 APC + 2.5% biochar | 100.3 ± 9.0 | 25.3 ± 2.3 | 8.2 ± 0.7 | 2.2 | 158.4 ± 14.3 | 35.2 ± 3.2 | 23.6 ± 2.1 | 0.7 |
| HM 5 APC + 5% GAC | 60.5 ± 5.4 | 14.5 ± 1.3 | 7.3 ± 0.7 | 1.9 | 75.3 ± 6.8 | 29.6 ± 2.7 | 16.5 ± 1.5 | 1.5 |
| HM 5 APC + 5% biochar | 59.9 ± 5.4 | 14.2 ± 1.3 | 6.9 ± 0.6 | 2.1 | 63.4 ± 5.7 | 28.8 ± 2.6 | 16.9 ± 1.5 | 1.4 |
| HM 10 APC | 578.2 ± 52.0 | 116.3 ± 10.5 | 27.2 ± 2.4 | 0.8 | 956.8 ± 86.1 | 286.5 ± 25.8 | 98.5 ± 8.9 | 0.7 |
| HM 10 APC + 1% GAC | 365.3 ± 32.9 | 68.0 ± 6.1 | 24.8 ± 2.2 | 0.6 | 627.0 ± 56.4 | 241.5 ± 21.7 | 86.9 ± 7.8 | 0.6 |
| HM 10 APC + 1% biochar | 349.9 ± 31.5 | 65.5 ± 5.9 | 23.9 ± 2.2 | 0.7 | 608.3 ± 54.7 | 239.8 ± 21.6 | 81.4 ± 7.3 | 0.6 |
| HM 10 APC + 2.5% GAC | 177.4 ± 16.0 | 35.5 ± 3.2 | 11.5 ± 1.0 | 0.4 | 325.0 ± 29.3 | 146.8 ± 13.2 | 55.3 ± 5.0 | 0.4 |
| HM 10 APC + 2.5% biochar | 166.9 ± 15.0 | 31.6 ± 2.8 | 11.4 ± 1.0 | 0.4 | 307.0 ± 27.6 | 102.5 ± 9.2 | 49.2 ± 4.4 | 0.4 |
| HM 10 APC + 5% GAC | 73.0 ± 6.6 | 22.4 ± 2.0 | 8.6 ± 0.8 | 0.3 | 204.0 ± 18.4 | 47.4 ± 4.3 | 23.1 ± 2.1 | 0.4 |
| HM 10 APC + 5% biochar | 68.6 ± 6.2 | 20.7 ± 1.9 | 7.1 ± 0.6 | 0.3 | 193.0 ± 17.4 | 43.6 ± 3.9 | 22.5 ± 2.0 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burachevskaya, M.; Mandzhieva, S.; Bauer, T.; Minkina, T.; Rajput, V.; Chaplygin, V.; Fedorenko, A.; Chernikova, N.; Zamulina, I.; Kolesnikov, S.; et al. The Effect of Granular Activated Carbon and Biochar on the Availability of Cu and Zn to Hordeum sativum Distichum in Contaminated Soil. Plants 2021, 10, 841. https://doi.org/10.3390/plants10050841
Burachevskaya M, Mandzhieva S, Bauer T, Minkina T, Rajput V, Chaplygin V, Fedorenko A, Chernikova N, Zamulina I, Kolesnikov S, et al. The Effect of Granular Activated Carbon and Biochar on the Availability of Cu and Zn to Hordeum sativum Distichum in Contaminated Soil. Plants. 2021; 10(5):841. https://doi.org/10.3390/plants10050841
Chicago/Turabian StyleBurachevskaya, Marina, Saglara Mandzhieva, Tatiana Bauer, Tatiana Minkina, Vishnu Rajput, Victor Chaplygin, Aleksey Fedorenko, Natalia Chernikova, Inna Zamulina, Sergey Kolesnikov, and et al. 2021. "The Effect of Granular Activated Carbon and Biochar on the Availability of Cu and Zn to Hordeum sativum Distichum in Contaminated Soil" Plants 10, no. 5: 841. https://doi.org/10.3390/plants10050841
APA StyleBurachevskaya, M., Mandzhieva, S., Bauer, T., Minkina, T., Rajput, V., Chaplygin, V., Fedorenko, A., Chernikova, N., Zamulina, I., Kolesnikov, S., Sushkova, S., & Perelomov, L. (2021). The Effect of Granular Activated Carbon and Biochar on the Availability of Cu and Zn to Hordeum sativum Distichum in Contaminated Soil. Plants, 10(5), 841. https://doi.org/10.3390/plants10050841













