Recent 1D and 2D TD–NMR Pulse Sequences for Plant Science
Abstract
1. Introduction
2. One-Dimensional (1D) Pulse Sequences
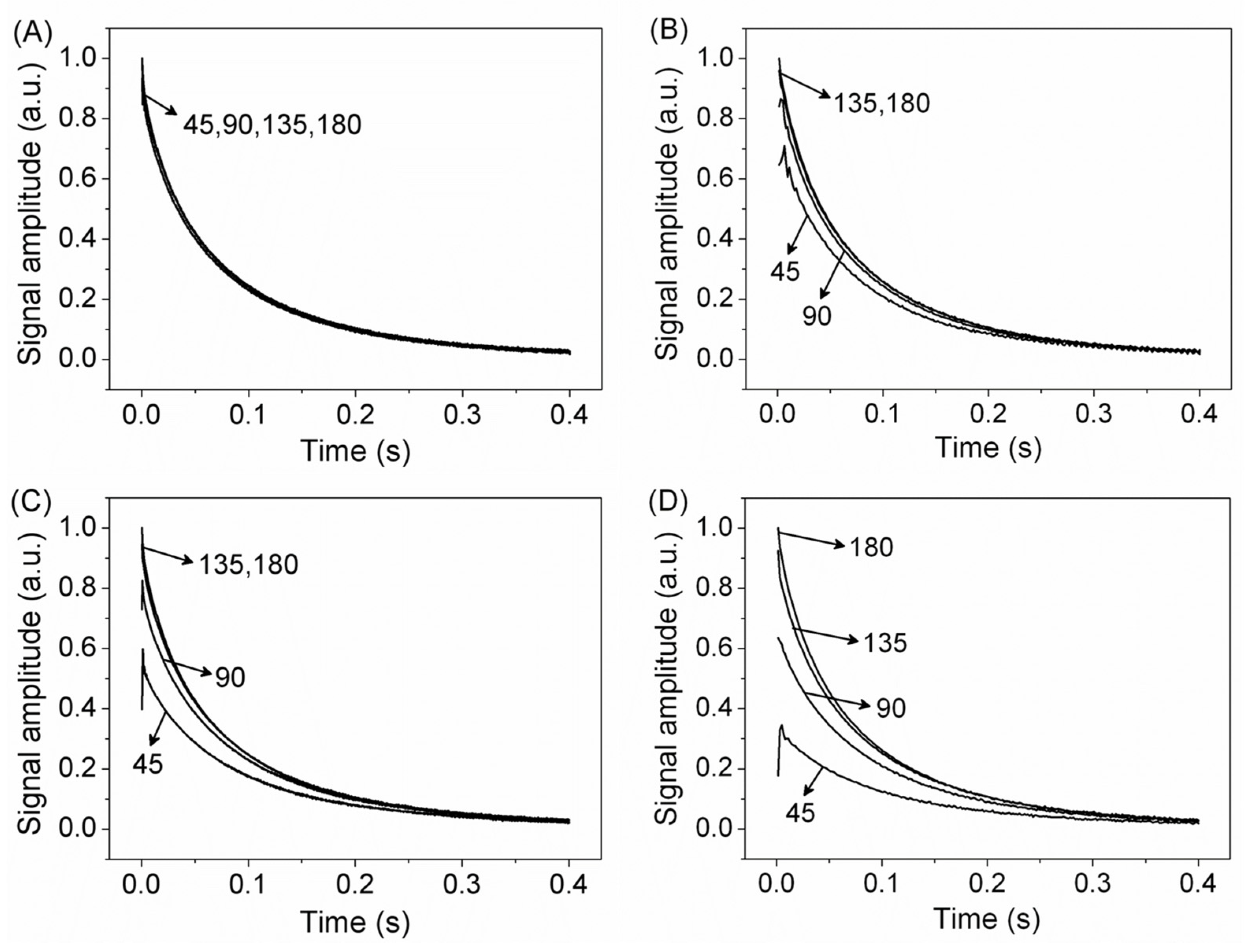
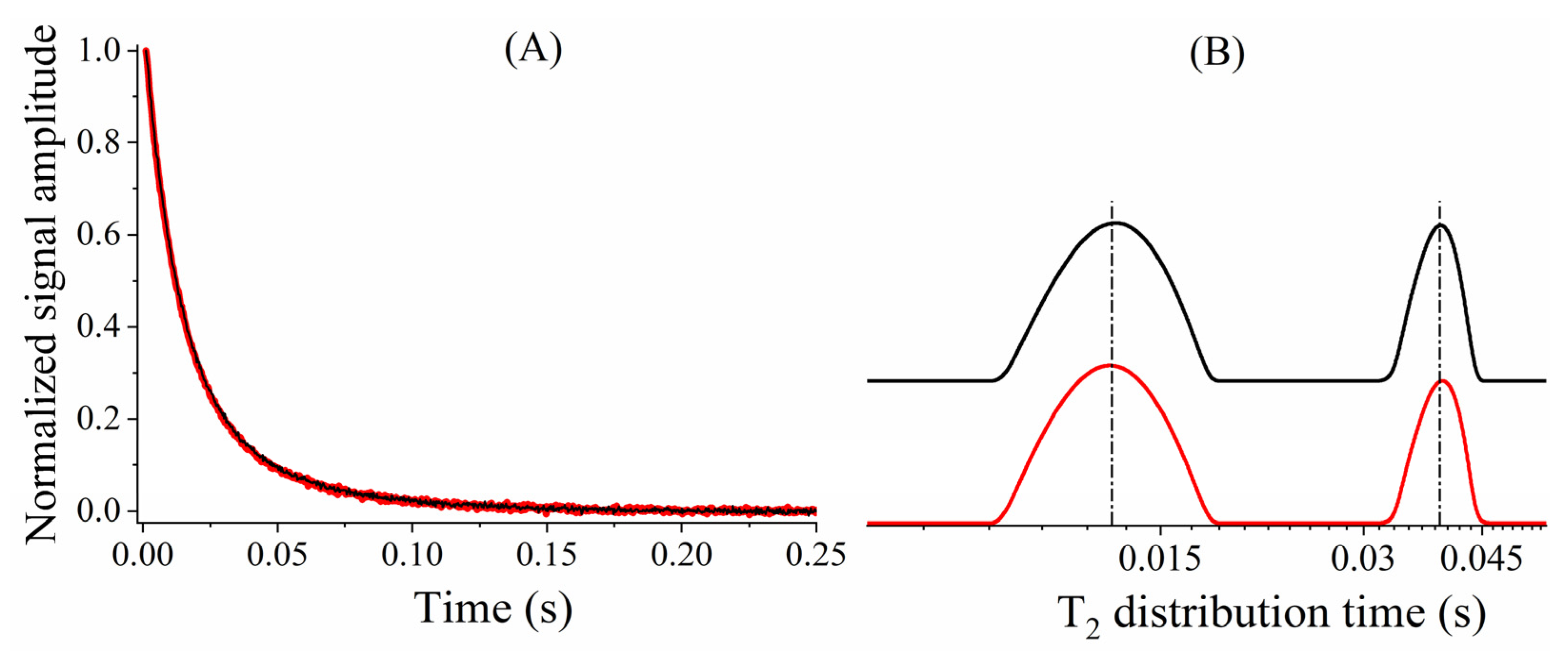
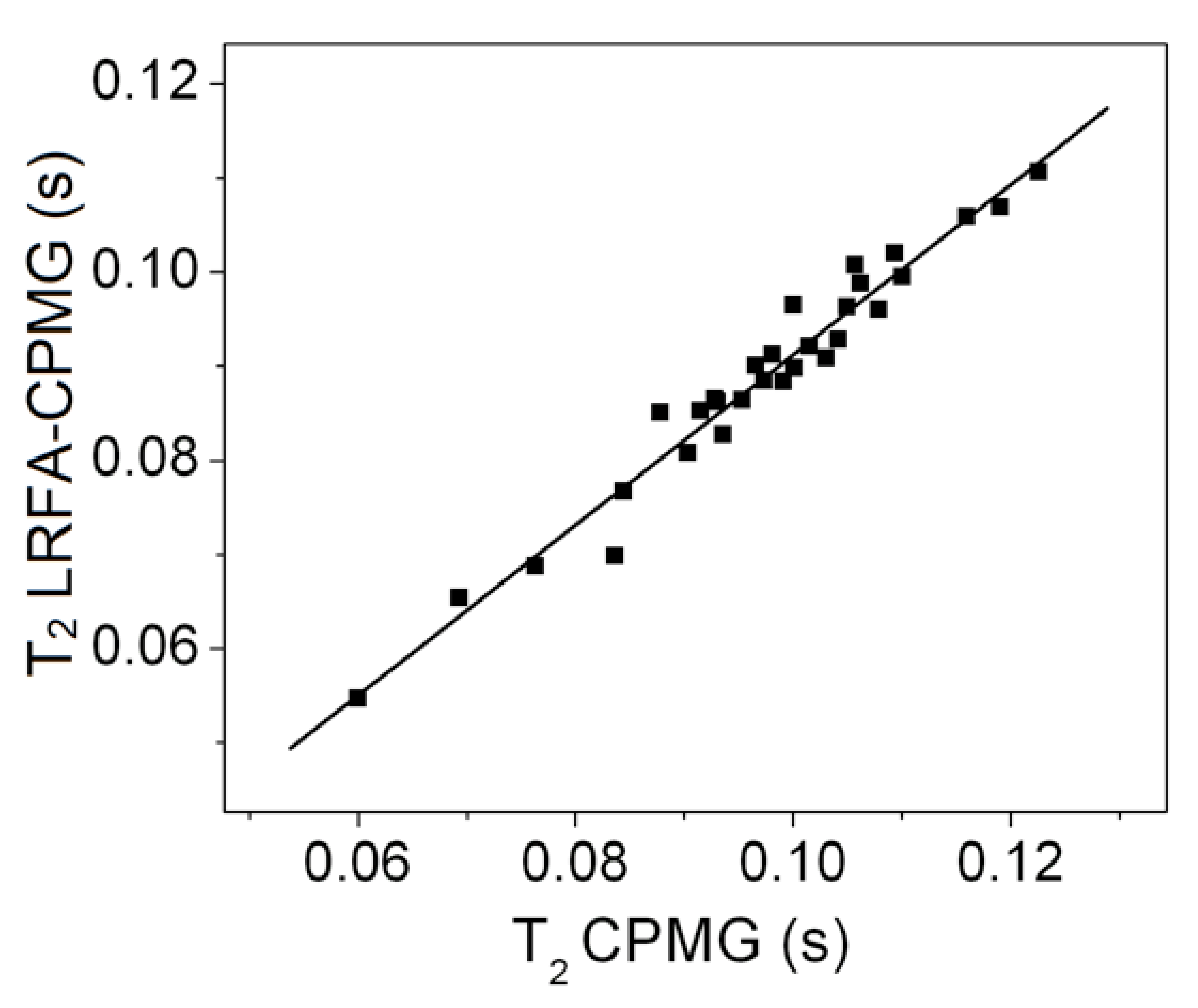
2.1. CPMG with Low Refocusing Pulses
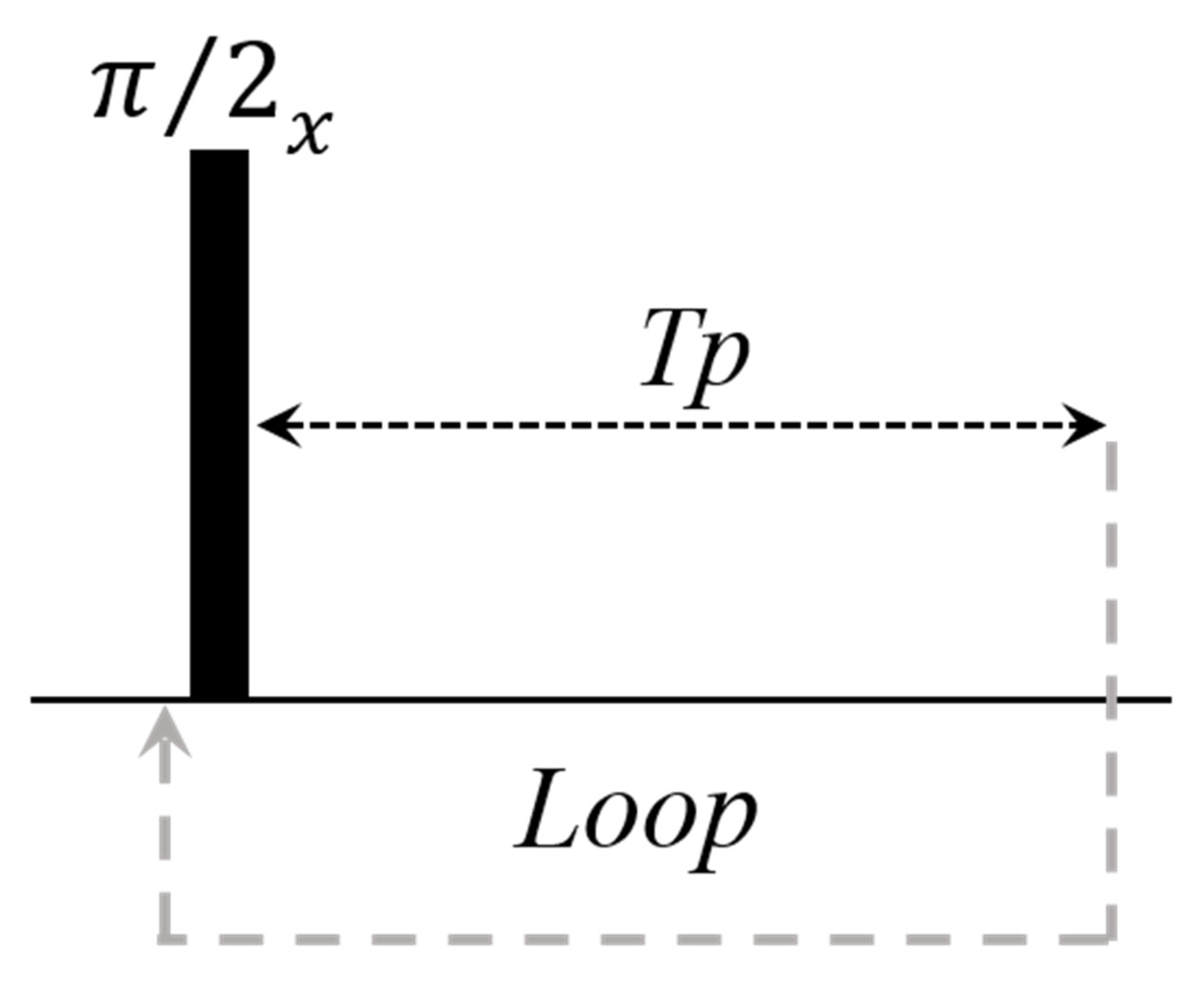
2.2. Continuous Wave Free Precession (CWFP) Sequences
2.2.1. Quantitative Analyses
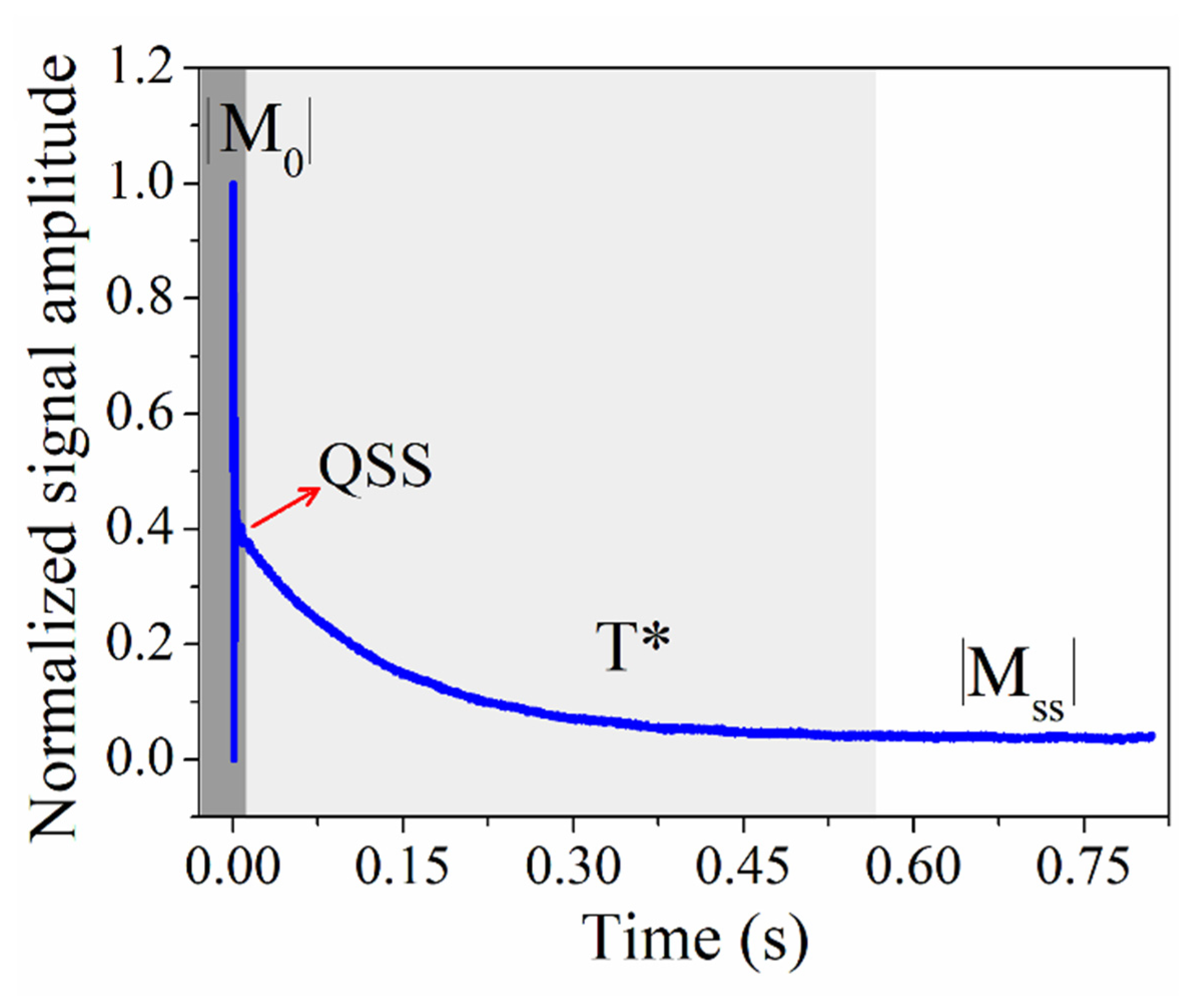
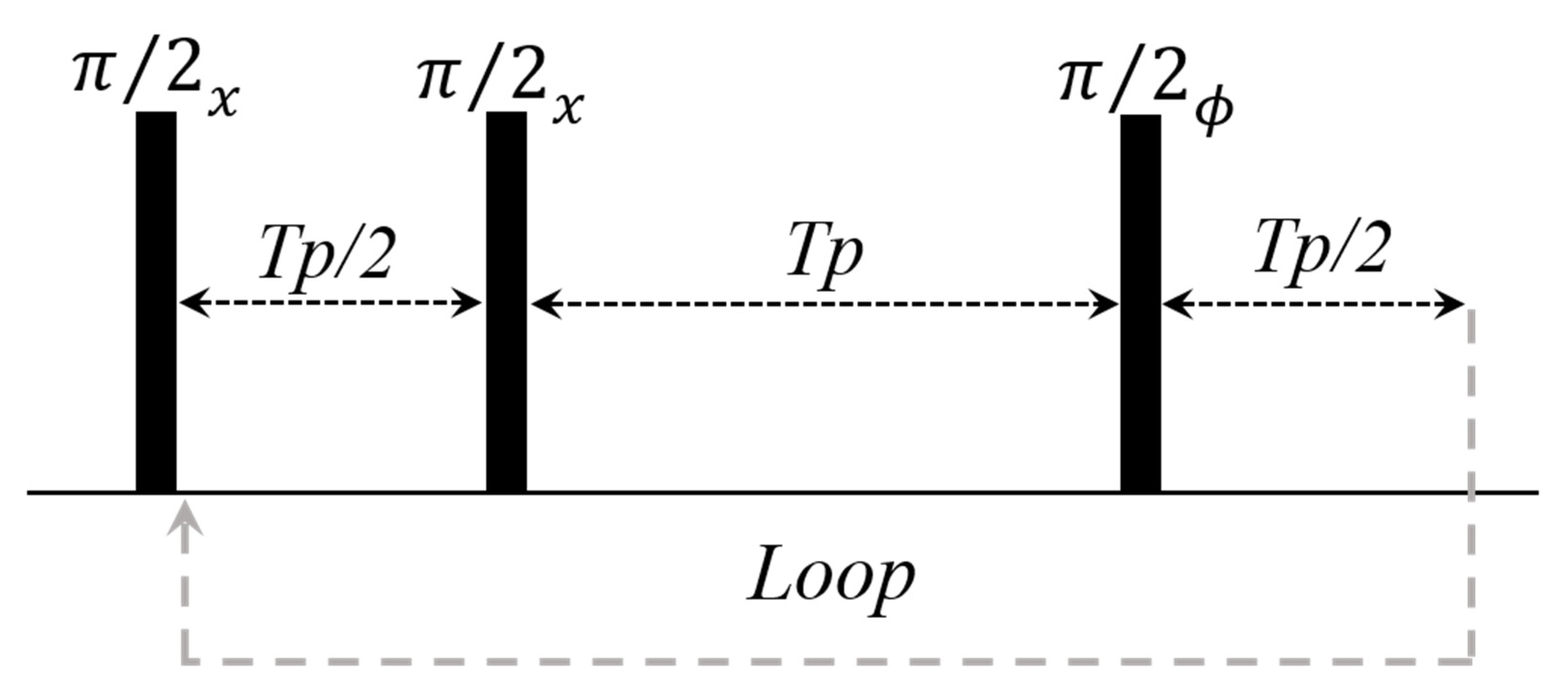
2.2.2. CWFP Sequence to Measure T1 and T2 in a Single Experiment
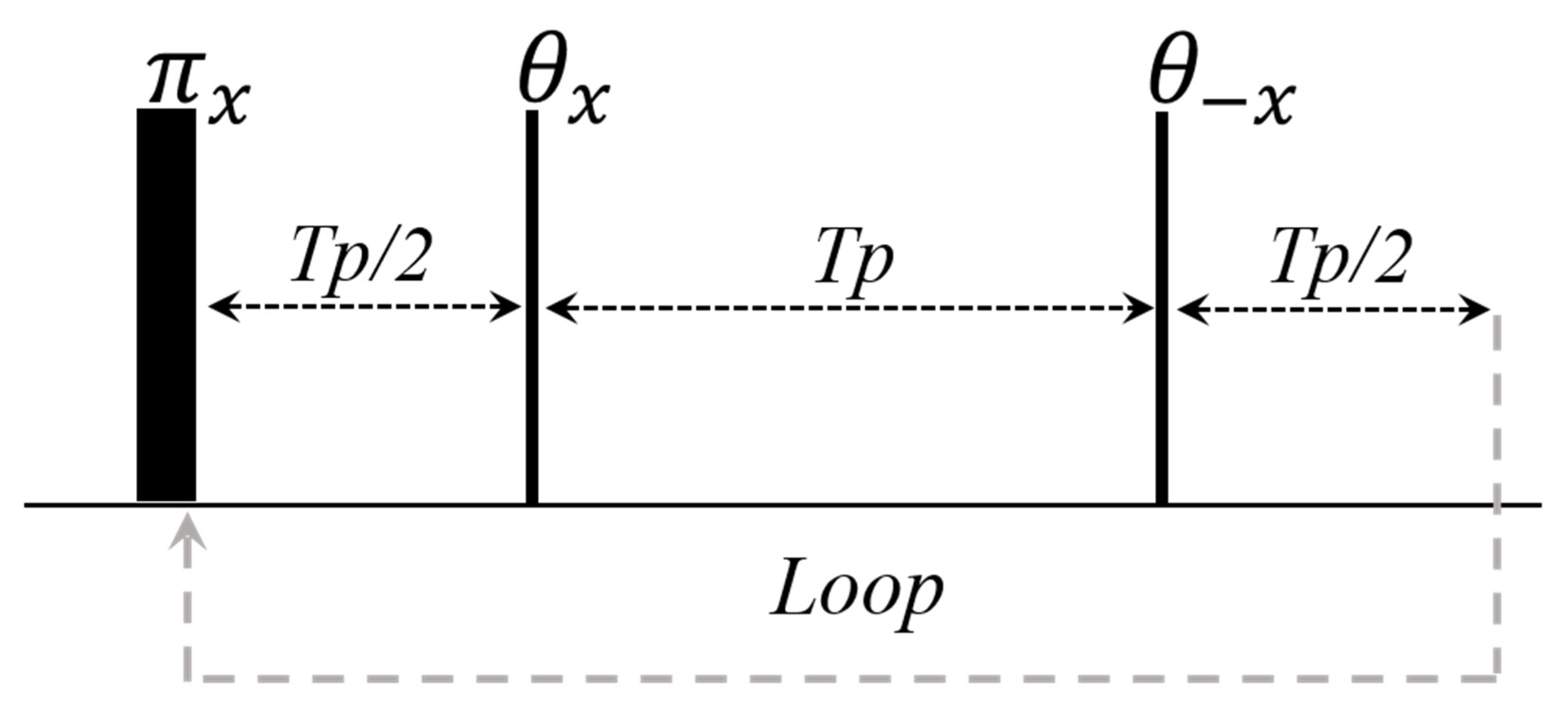
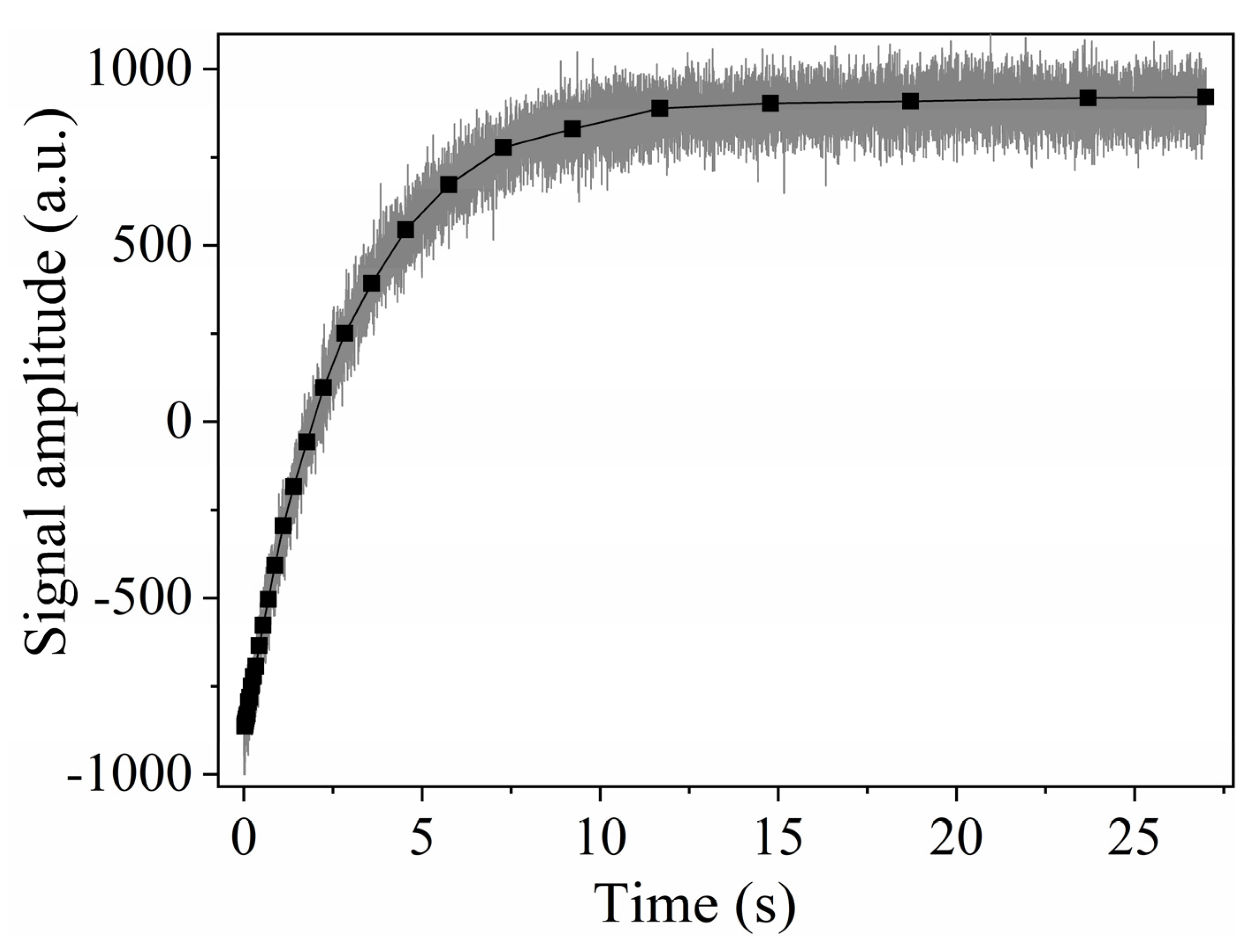
2.2.3. CWFP Sequence to Measure T1 in a Single Shot Experiment
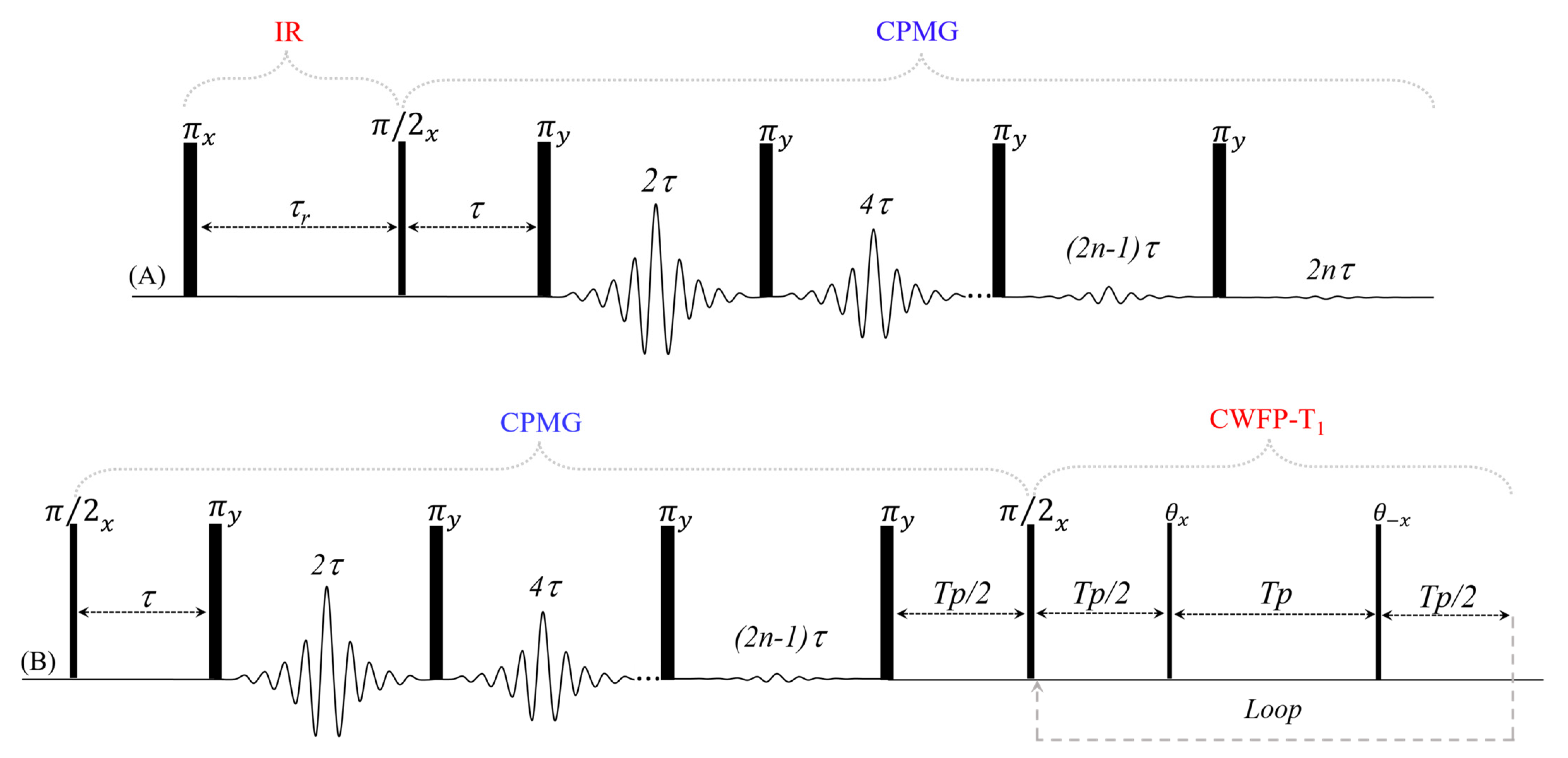
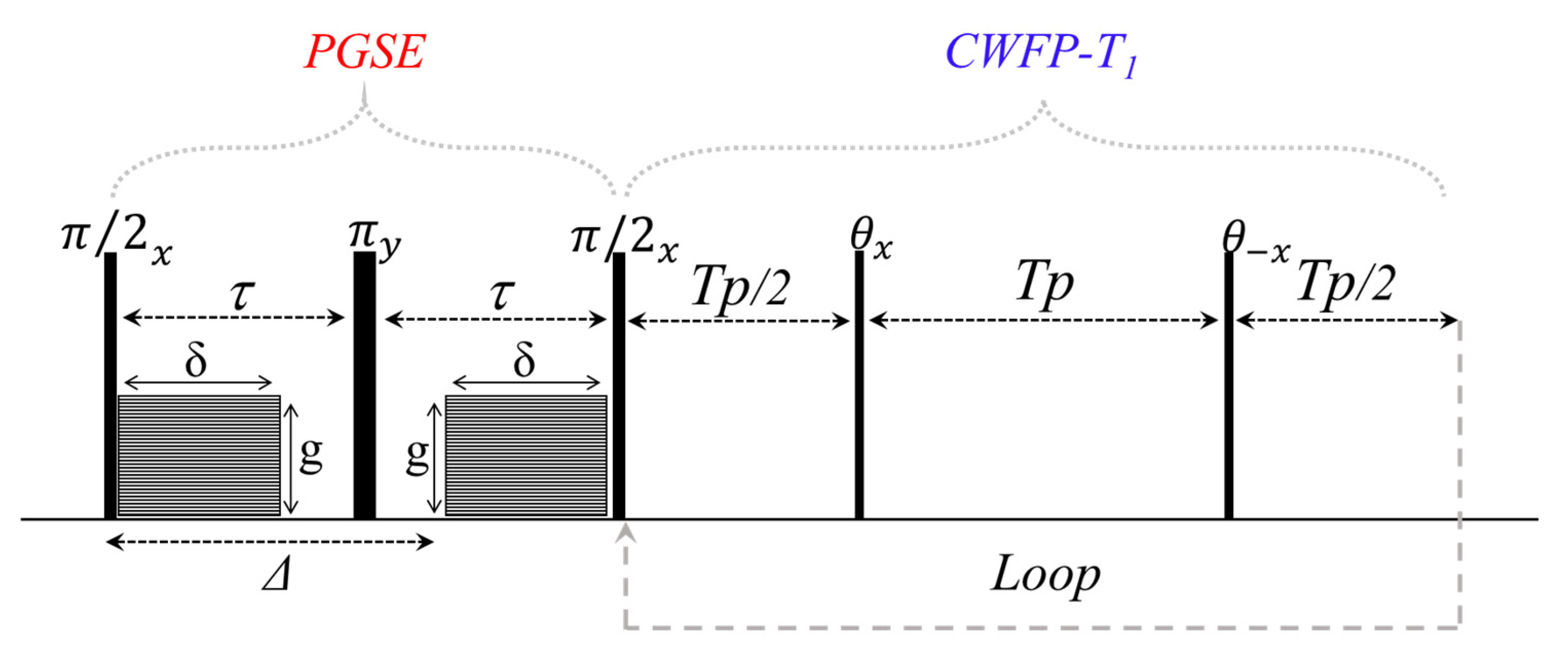
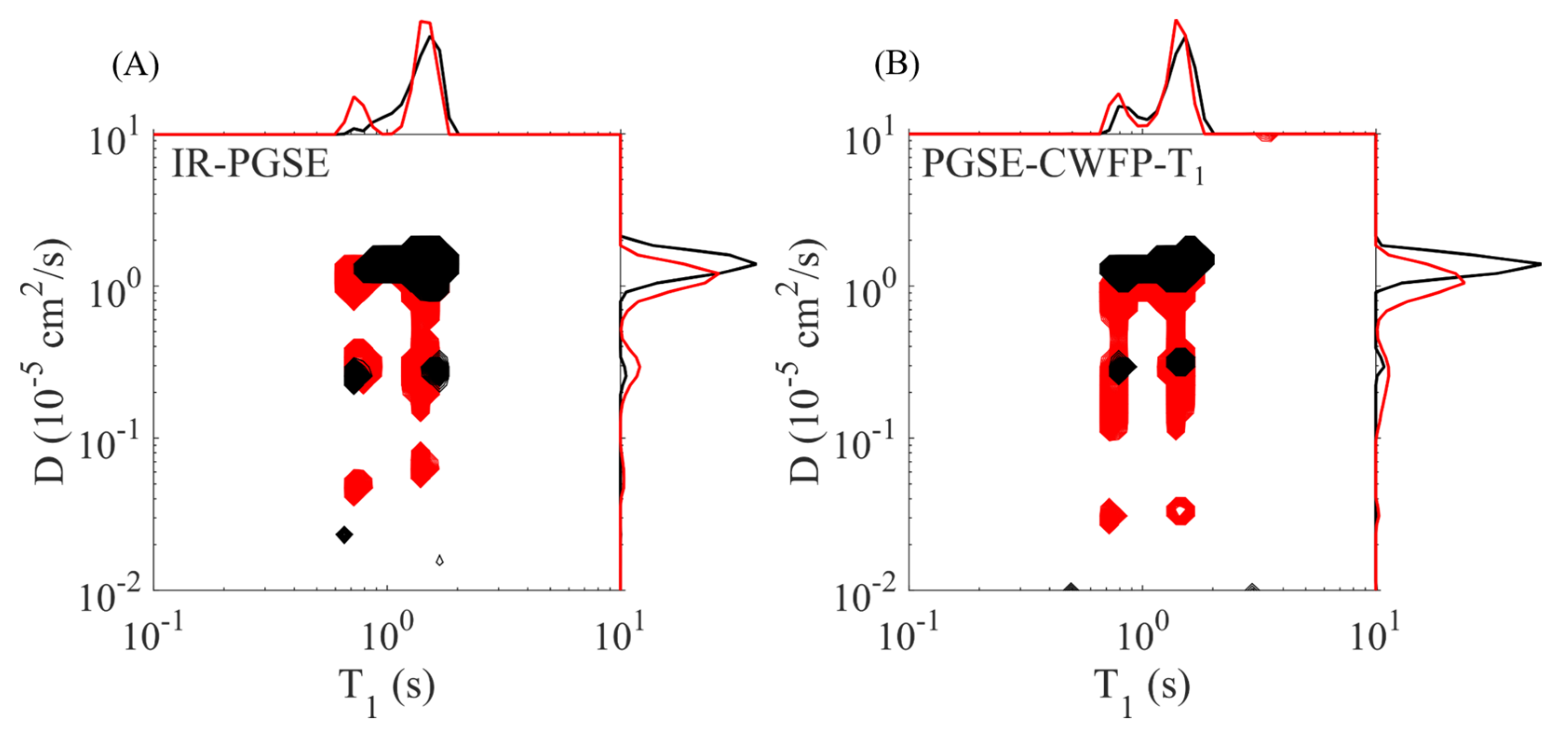
3. Two-Dimensional Methods Using the CWFP–T1 Sequence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Todt, H.; Burk, W.; Guthausen, G.; Guthausen, A.; Kamlowski, A.; Schmalbein, D. Quality control with time-domain NMR. Eur. J. Lipid Sci. Technol. 2001, 103, 835–840. [Google Scholar] [CrossRef]
- Cobo, M.F.; Deublein, E.J.; Haber, A.; Kwamen, R.; Nimbalkar, M.; Decker, F. TD–NMR in Quality Control: Standard Applications. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 1–18. [Google Scholar]
- Colnago, L.A.; Wiesman, Z.; Pages, G.; Musse, M.; Monaretto, T.; Windt, C.W.; Rondeau-Mouro, C. Low field, time domain NMR in the agriculture and agrifood sectors: An overview of applications in plants, foods and biofuels. J. Magn. Reson. 2021, 323, 106899. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, P.B. Methods of measuring spin-lattice (T1) relaxation times: An annotated bibliography. Concepts Magn. Reson. 1999, 11, 243–276. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Hürlimann, M.D.; Venkataramanan, L. Quantitative Measurement of Two-Dimensional Distribution Functions of Diffusion and Relaxation in Grossly Inhomogeneous Fields. J. Magn. Reson. 2002, 157, 31–42. [Google Scholar] [CrossRef]
- Voda, M.A.; van Duynhoven, J. Characterization of food emulsions by PFG NMR. Trends Food Sci. Technol. 2009, 20, 533–543. [Google Scholar] [CrossRef]
- Nestle, N.; Qadan, A.; Galvosas, P.; Süss, W.; Kärger, J. PFG NMR and internal magnetic field gradients in plant-based materials. Magn. Reson. Imaging 2002, 20, 567–573. [Google Scholar] [CrossRef]
- Newling, B.; Batchelor, S.N. Pulsed Field Gradient NMR Study of the Diffusion of H2O and Polyethylene Glycol Polymers in the Supramolecular Structure of Wet Cotton. J. Phys. Chem. B 2003, 107, 12391–12397. [Google Scholar] [CrossRef]
- Van Duynhoven, J.; Voda, A.; Witek, M.; Van As, H. Time-Domain NMR Applied to Food Products. In Annual Reports on NMR Spectroscopy; Academic Press: Cambridge, MA, USA, 2010; Volume 69, pp. 145–197. [Google Scholar]
- Marigheto, N.; Venturi, L.; Hibberd, D.; Wright, K.M.; Ferrante, G.; Hills, B.P. Methods for peak assignment in low-resolution multidimensional NMR cross-correlation relaxometry. J. Magn. Reson. 2007, 187, 327–342. [Google Scholar] [CrossRef]
- Montrazi, E.T.; Monaretto, T.; Bonagamba, T.J.; Colnago, L.A. New and rapid pulse sequences for two-dimensional D-T1 correlation measurements. J. Magn. Reson. 2020, 315, 106749. [Google Scholar] [CrossRef] [PubMed]
- Monaretto, T.; Montrazi, E.T.; Moraes, T.B.; Souza, A.A.; Rondeau-Mouro, C.; Colnago, L.A. Using T1 as a direct detection dimension in two-dimensional time-domain NMR experiments using CWFP regime. J. Magn. Reson. 2020, 311, 106666. [Google Scholar] [CrossRef]
- Moraes, T.B.; Monaretto, T.; Colnago, L.A. Rapid and simple determination of T1 relaxation times in time-domain NMR by Continuous Wave Free Precession sequence. J. Magn. Reson. 2016, 270, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Monaretto, T.; Andrade, F.D.; Moraes, T.B.; Souza, A.A.; de Azevedo, E.R.; Colnago, L.A. On resonance phase alternated CWFP sequences for rapid and simultaneous measurement of relaxation times. J. Magn. Reson. 2015, 259, 174–178. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, F.D.; Netto, A.M.; Colnago, L.A. Qualitative analysis by online nuclear magnetic resonance using Carr–Purcell–Meiboom–Gill sequence with low refocusing flip angles. Talanta 2011, 84, 84–88. [Google Scholar] [CrossRef]
- Van Der Weerd, L.; Claessens, M.M.A.E.; Efdé, C.; Van As, H. Nuclear magnetic resonanceimaging of membrane permeability changes in plants during osmoticstress. Plant Cell Environ. 2002, 25, 1539–1549. [Google Scholar] [CrossRef]
- Musse, M.; De Franceschi, L.; Cambert, M.; Sorin, C.; Le Caherec, F.; Burel, A.; Bouchereau, A.; Mariette, F.; Leport, L. Structural changes in senescing oilseed rape leaves at tissue and subcellular levels monitored by nuclear magnetic resonance relaxometry through water status. Plant Physiol. 2013, 163, 392–406. [Google Scholar] [CrossRef]
- Carosio, M.G.A.; Bernardes, D.F.; Andrade, F.D.; Moraes, T.B.; Tosin, G.; Colnago, L.A. Measuring thermal properties of oilseeds using time domain nuclear magnetic resonance spectroscopy. J. Food Eng. 2016, 173, 143–149. [Google Scholar] [CrossRef]
- Musse, M.; Cambert, M.; Mariette, F. NMR Study of Water Distribution inside Tomato Cells: Effects of Water Stress. Appl. Magn. Reson. 2010, 38, 455–469. [Google Scholar] [CrossRef]
- Xu, F.; Jin, X.; Zhang, L.; Chen, X.D. Investigation on water status and distribution in broccoli and the effects of drying on water status using NMR and MRI methods. Food Res. Int. 2017, 96, 191–197. [Google Scholar] [CrossRef]
- Pereira, F.M.V.; Carvalho, A.d.S.; Cabeça, L.F.; Colnago, L.A. Classification of intact fresh plums according to sweetness using time-domain nuclear magnetic resonance and chemometrics. Microchem. J. 2013, 108, 14–17. [Google Scholar] [CrossRef]
- Carosio, M.G.A.; Bernardes, D.F.; Carvalho, A.d.S.; Colnago, L.A. Non-invasive Measurements of Oilseed Temperature in Soil and Soil Thermal Diffusivity Using Time-Domain NMR Relaxometry. Appl. Magn. Reson. 2018, 49, 1119–1127. [Google Scholar] [CrossRef]
- Borgia, G.C.; Brown, R.J.S.; Fantazzini, P. Uniform-Penalty Inversion of Multiexponential Decay Data: II. Data Spacing, T2 Data, Systematic Data Errors, and Diagnostics. J. Magn. Reson. 2000, 147, 273–285. [Google Scholar] [CrossRef]
- Schwenk, A. NMR pulse technique with high sensitivity for slowly relaxing systems. J. Magn. Reson. 1971, 5, 376–389. [Google Scholar] [CrossRef]
- Azeredo, R.B.V.; Colnago, L.A.; Souza, A.A.; Engelsberg, M. Continuous wave free precession: Practical analytical tool for low-resolution nuclear magnetic resonance measurements. Anal. Chim. Acta 2003, 478, 313–320. [Google Scholar] [CrossRef]
- Azeredo, R.B.V.; Colnago, L.A.; Engelsberg, M. Quantitative analysis using steady-state free precession nuclear magnetic resonance. Anal. Chem. 2000, 72, 2401–2405. [Google Scholar]
- Moraes, T.B.; Monaretto, T.; Colnago, L.A. Applications of Continuous Wave Free Precession Sequences in Low-Field, Time-Domain NMR. Appl. Sci. 2019, 9, 1312. [Google Scholar] [CrossRef]
- Colnago, L.A.; Azeredo, R.B.V.; Marchi Netto, A.; Andrade, F.D.; Venâncio, T. Rapid analyses of oil and fat content in agri-food products using continuous wave free precession time domain NMR. Magn. Reson. Chem. 2011, 49, S113–S120. [Google Scholar] [CrossRef]
- Ernst, R.R.; Anderson, W.A. Application of Fourier Transform Spectroscopy to Magnetic Resonance. Rev. Sci. Instrum. 1966, 37, 93–102. [Google Scholar] [CrossRef]
- Moraes, T.B.; Colnago, L.A. Simulação de sinais de RMN através das equações de Bloch. Química Nova 2014, 37, 1410–1416. [Google Scholar]
- Venâncio, T.; Engelsberg, M.; Azeredo, R.B.V.; Alem, N.E.R.; Colnago, L.A. Fast and simultaneous measurement of longitudinal and transverse NMR relaxation times in a single continuous wave free precession experiment. J. Magn. Reson. 2005, 173, 34–39. [Google Scholar] [CrossRef]
- Colnago, L.A.; Engelsberg, M.; Souza, A.A.; Barbosa, L.L. High-Throughput, Non-Destructive Determination of Oil Content in Intact Seeds by Continuous Wave-Free Precession NMR. Anal. Chem. 2007, 79, 1271–1274. [Google Scholar] [CrossRef]
- Ribeiro, F.Z.; Marconcini, L.V.; de Toledo, I.B.; de Vasconcellos Azeredo, R.B.; Barbosa, L.L.; Colnago, L.A. Nuclear magnetic resonance water relaxation time changes in bananas during ripening: A new mechanism. J. Sci. Food Agric. 2010, 90, 2052–2057. [Google Scholar] [CrossRef]
- de Andrade, F.D.; Marchi Netto, A.; Colnago, L.A. Use of Carr–Purcell pulse sequence with low refocusing flip angle to measure T1 and T2 in a single experiment. J. Magn. Reson. 2012, 214, 184–188. [Google Scholar] [CrossRef]
- Monaretto, T.; Souza, A.; Moraes, T.B.; Bertucci-Neto, V.; Rondeau-Mouro, C.; Colnago, L.A. Enhancing signal-to-noise ratio and resolution in low-field NMR relaxation measurements using post-acquisition digital filters. Magn. Reson. Chem. 2019, 57, 616–625. [Google Scholar] [CrossRef]
- Venkataramanan, L.; Yi-Qiao, S.; Hurlimann, M.D. Solving Fredholm integrals of the first kind with tensor product structure in 2 and 2.5 dimensions. IEEE Trans. Signal Process. 2002, 50, 1017–1026. [Google Scholar] [CrossRef]
- Song, Y.Q.; Venkataramanan, L.; Hürlimann, M.D.; Flaum, M.; Frulla, P.; Straley, C. T1–T2 Correlation Spectra Obtained Using a Fast Two-Dimensional Laplace Inversion. J. Magn. Reson. 2002, 154, 261–268. [Google Scholar] [CrossRef]
- Song, Y.-Q. A 2D NMR method to characterize granular structure of dairy products. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 55, 324–334. [Google Scholar] [CrossRef]
- Hürlimann, M.D.; Burcaw, L.; Song, Y.-Q. Quantitative characterization of food products by two-dimensional D–T2 and T1–T2 distribution functions in a static gradient. J. Colloid Interface Sci. 2006, 297, 303–311. [Google Scholar] [CrossRef]
- Rondeau Mouro, C. 2D TD–NMR Analysis of Complex Food Products. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer: London, UK, 2018; pp. 1483–1502. [Google Scholar]
- Meiri, N.; Berman, P.; Colnago, L.A.; Moraes, T.B.; Linder, C.; Wiesman, Z. Liquid-phase characterization of molecular interactions in polyunsaturated and n-fatty acid methyl esters by (1)H low-field nuclear magnetic resonance. Biotechnol. Biofuels 2015, 8, 96. [Google Scholar] [CrossRef]
- Berman, P.; Meiri, N.; Colnago, L.A.; Moraes, T.B.; Linder, C.; Levi, O.; Parmet, Y.; Saunders, M.; Wiesman, Z. Study of liquid-phase molecular packing interactions and morphology of fatty acid methyl esters (biodiesel). Biotechnol. Biofuels 2015, 8, 12. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaretto, T.; Moraes, T.B.; Colnago, L.A. Recent 1D and 2D TD–NMR Pulse Sequences for Plant Science. Plants 2021, 10, 833. https://doi.org/10.3390/plants10050833
Monaretto T, Moraes TB, Colnago LA. Recent 1D and 2D TD–NMR Pulse Sequences for Plant Science. Plants. 2021; 10(5):833. https://doi.org/10.3390/plants10050833
Chicago/Turabian StyleMonaretto, Tatiana, Tiago Bueno Moraes, and Luiz Alberto Colnago. 2021. "Recent 1D and 2D TD–NMR Pulse Sequences for Plant Science" Plants 10, no. 5: 833. https://doi.org/10.3390/plants10050833
APA StyleMonaretto, T., Moraes, T. B., & Colnago, L. A. (2021). Recent 1D and 2D TD–NMR Pulse Sequences for Plant Science. Plants, 10(5), 833. https://doi.org/10.3390/plants10050833







