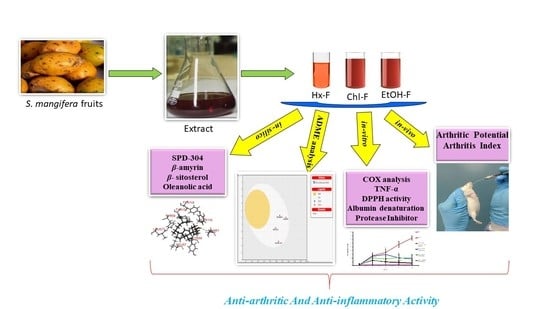
Anti-Arthritic and Anti-Inflammatory Potential of Spondias mangifera Extract Fractions: An In Silico, In Vitro and In Vivo Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Authentication
2.2. Chemicals
2.3. Molecular Docking
2.4. Prediction of Swiss Absorption, Distribution, Metabolism, Excretion and Toxicity Properties (ADME/Tox) of the phytoconstituents from Spondias mangifera
2.5. Extract and Fraction Preparation
2.6. Animals
2.7. COX Inhibition Assay
2.8. TNF-α and IL-6 Inhibition Assay
2.9. 1,1-diphenyl-2-Picrylhydrazyl Scavenging Assay
2.10. Reducing Potential Scavenging Activity
2.11. Inhibition of Albumin Denaturation
2.12. Protease Inhibition Assay
2.13. Complete Freund’s Adjuvant (CFA)-Induced Arthritis in Rats
2.14. Arthritic Score
- Normal paw = 0,
- Mild swelling and erythema of digits = 1,
- Swelling and erythema of the digits = 2,
- Severe swelling and erythema = 3,
- Gross deformity and inability to use the limb = 4.
2.15. Statistical Analysis
3. Results
3.1. Molecular Docking
3.2. Server-Based ADME Analysis
3.3. Cyclooxygenase Assay
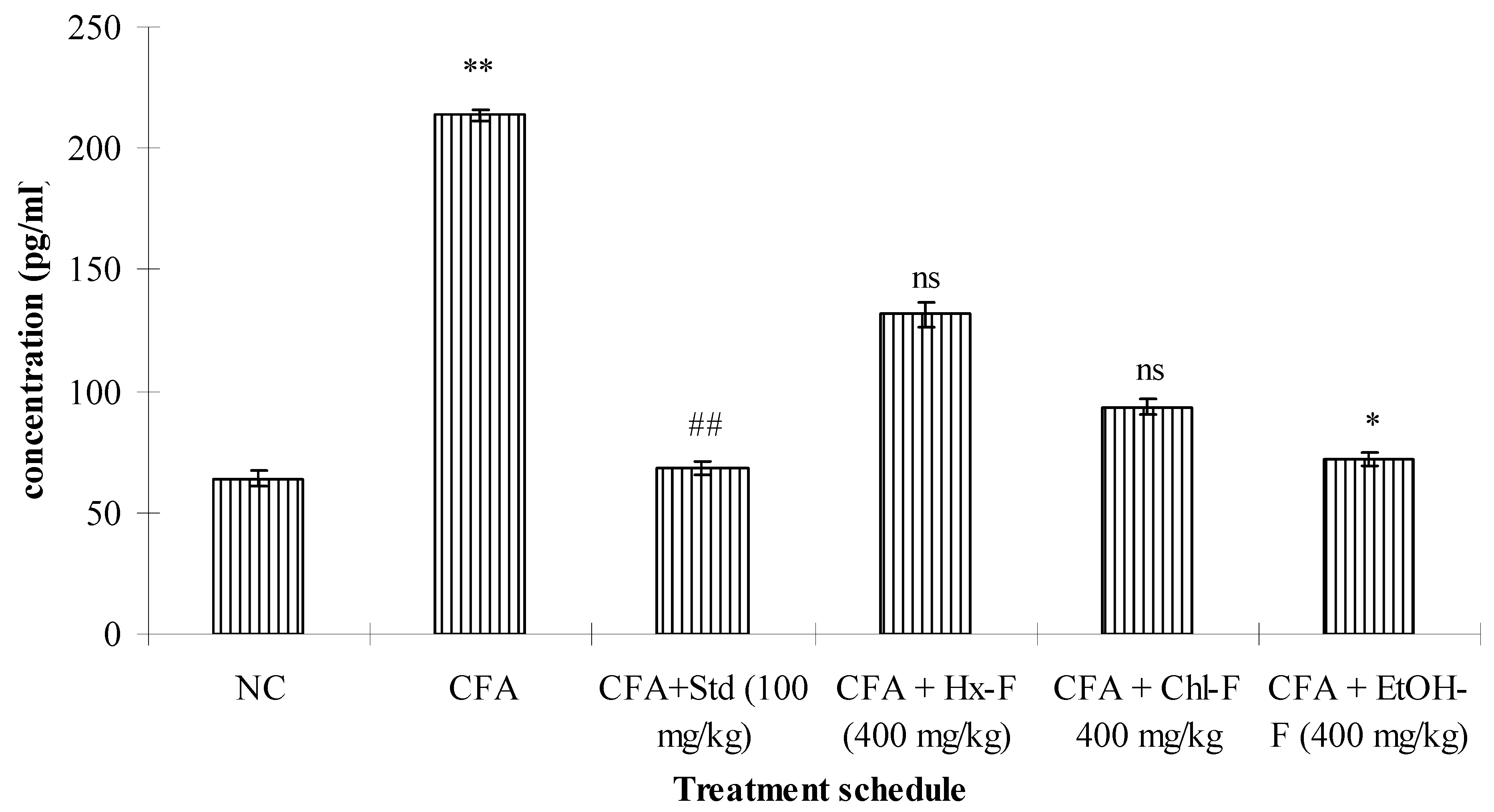
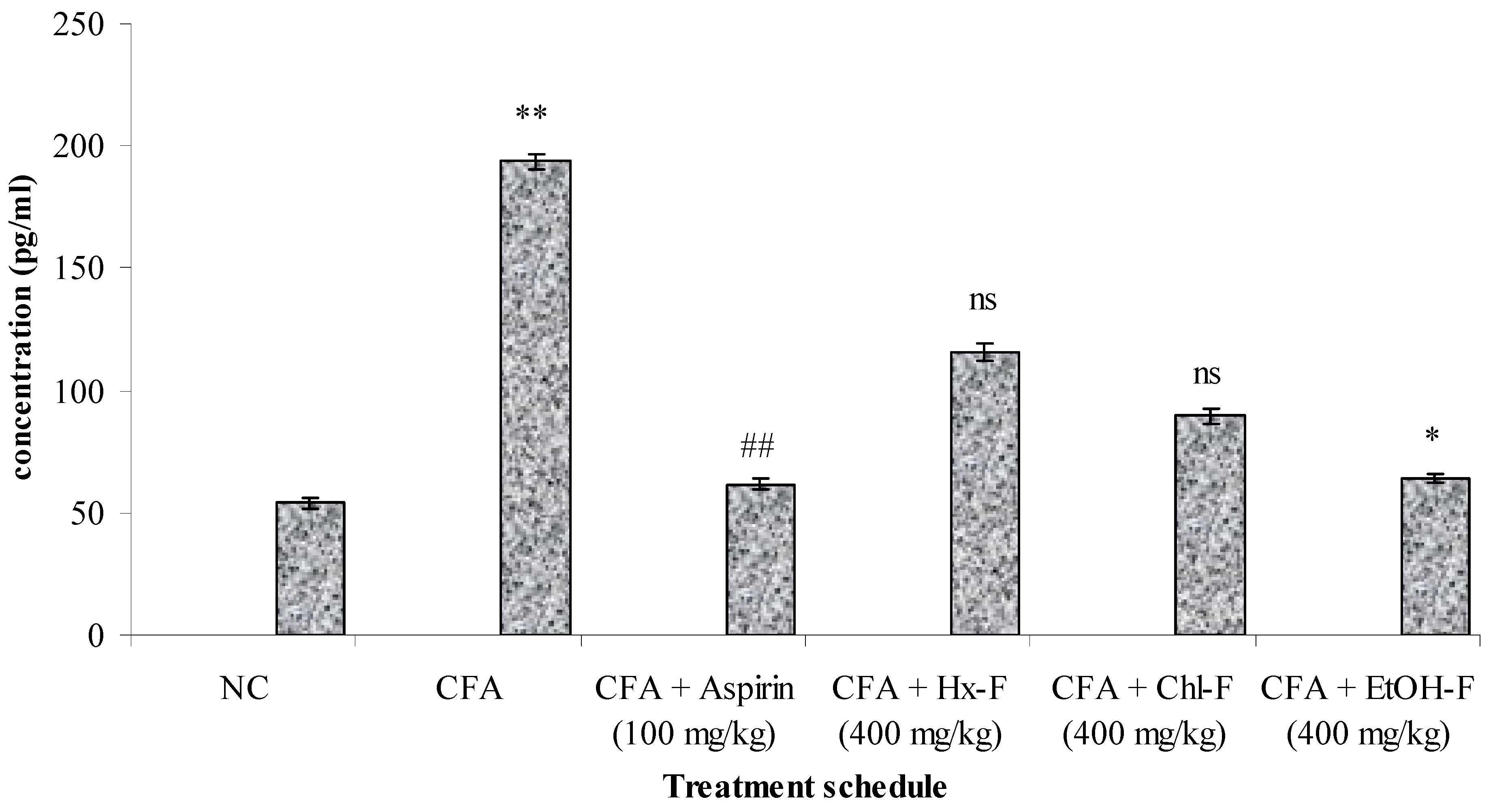
3.4. TNF-Alpha
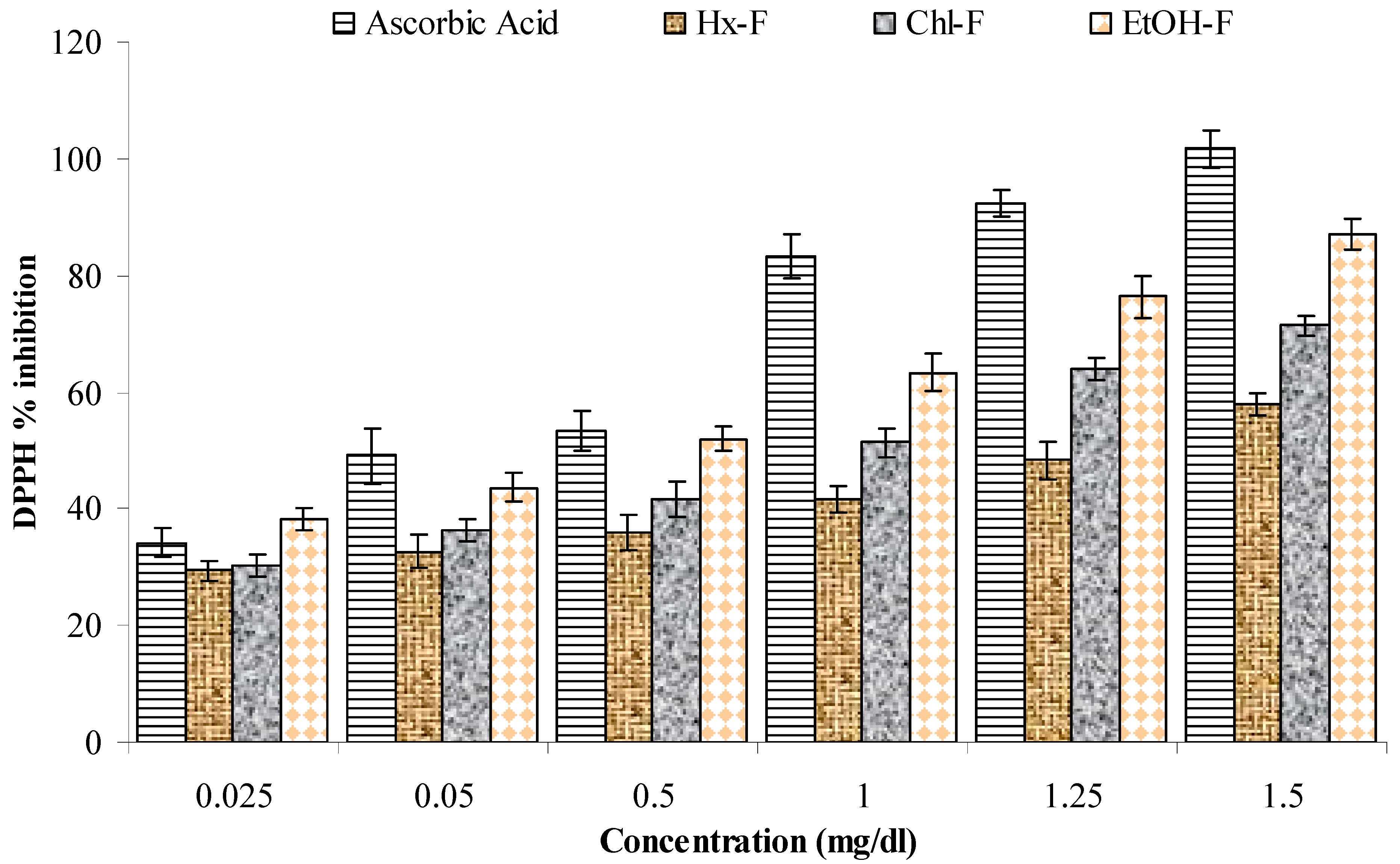
3.5. DPPH Scavenging Activity
3.6. Reducing Potential
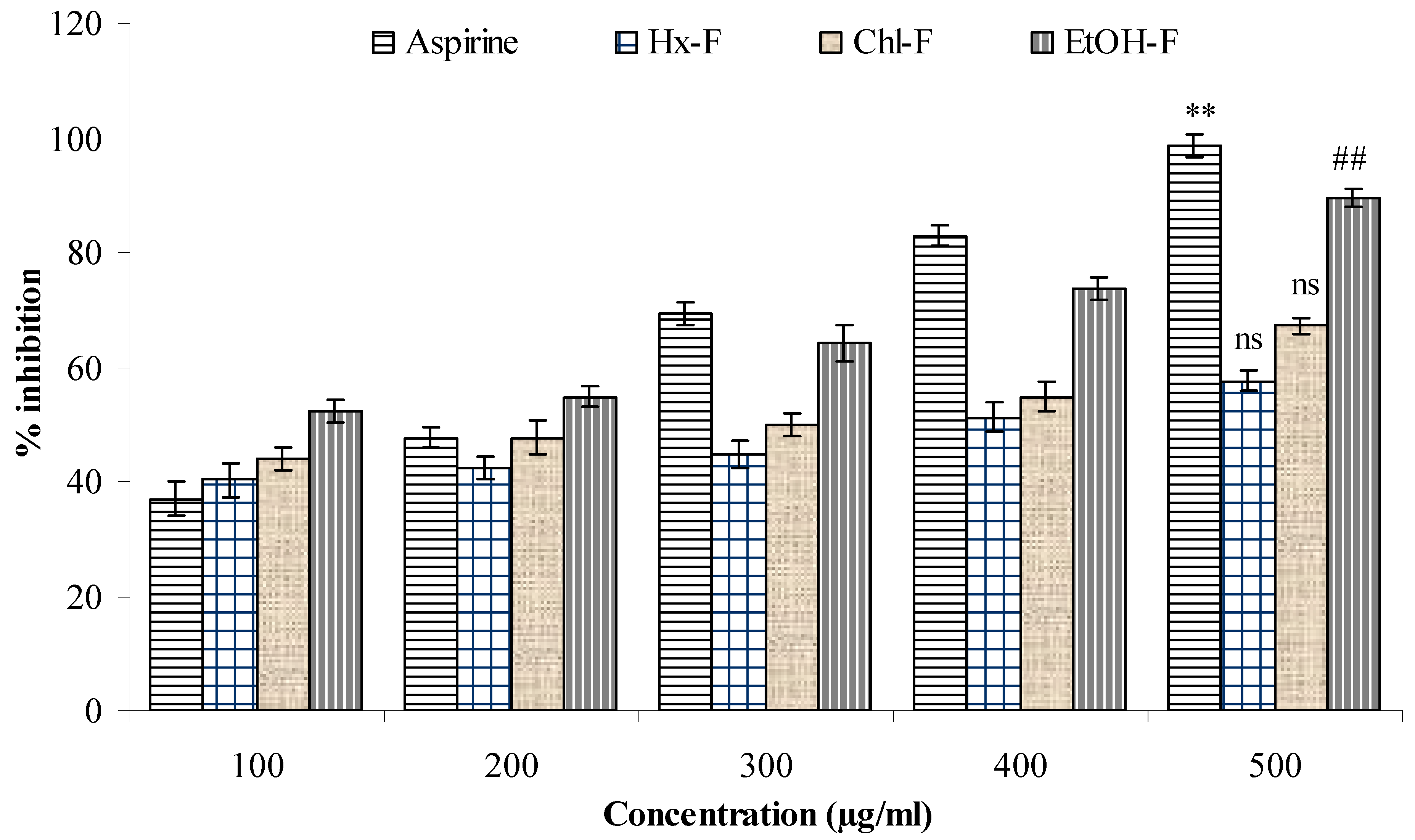
3.7. Albumin Denaturation
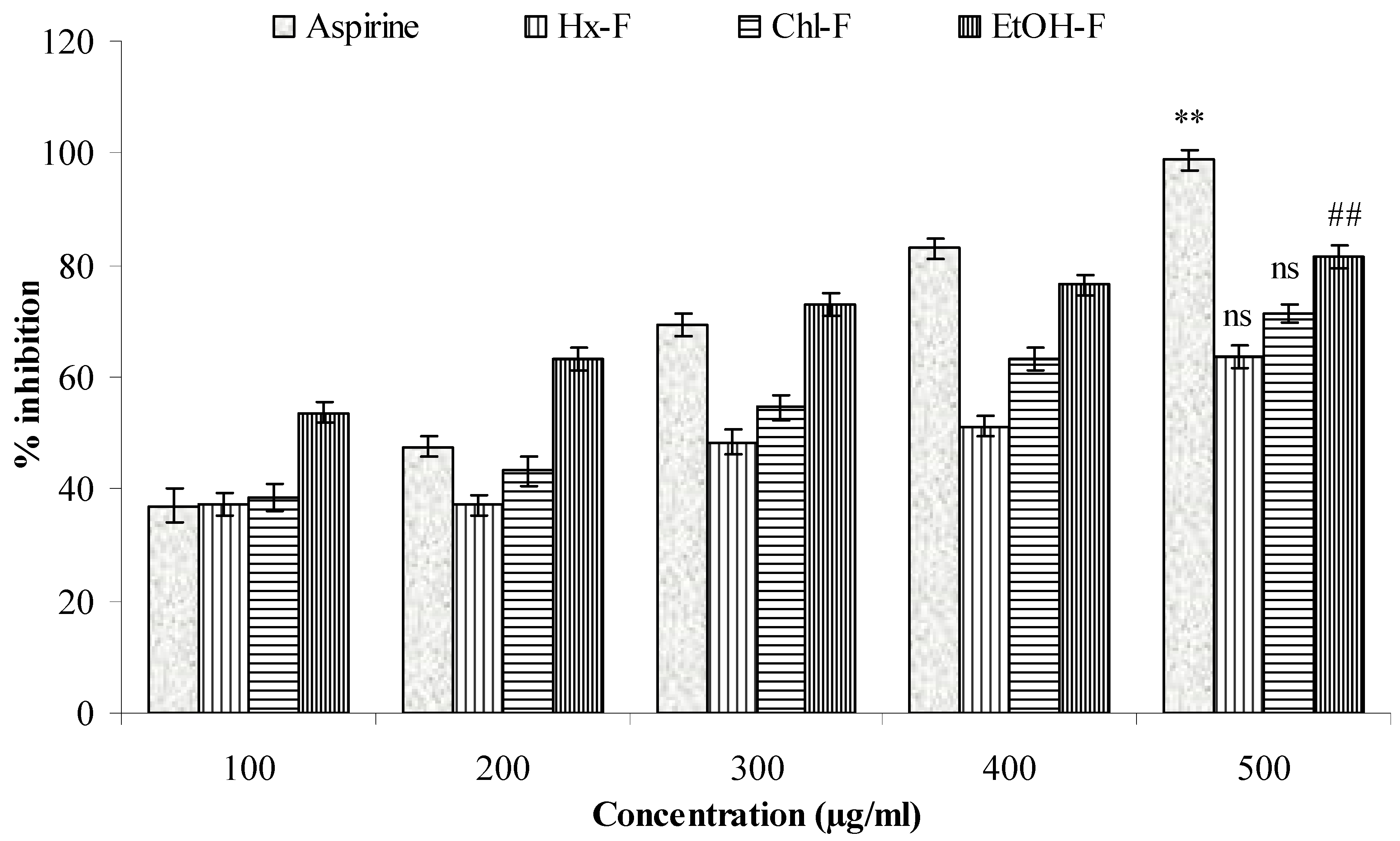
3.8. Protease Inhibitor
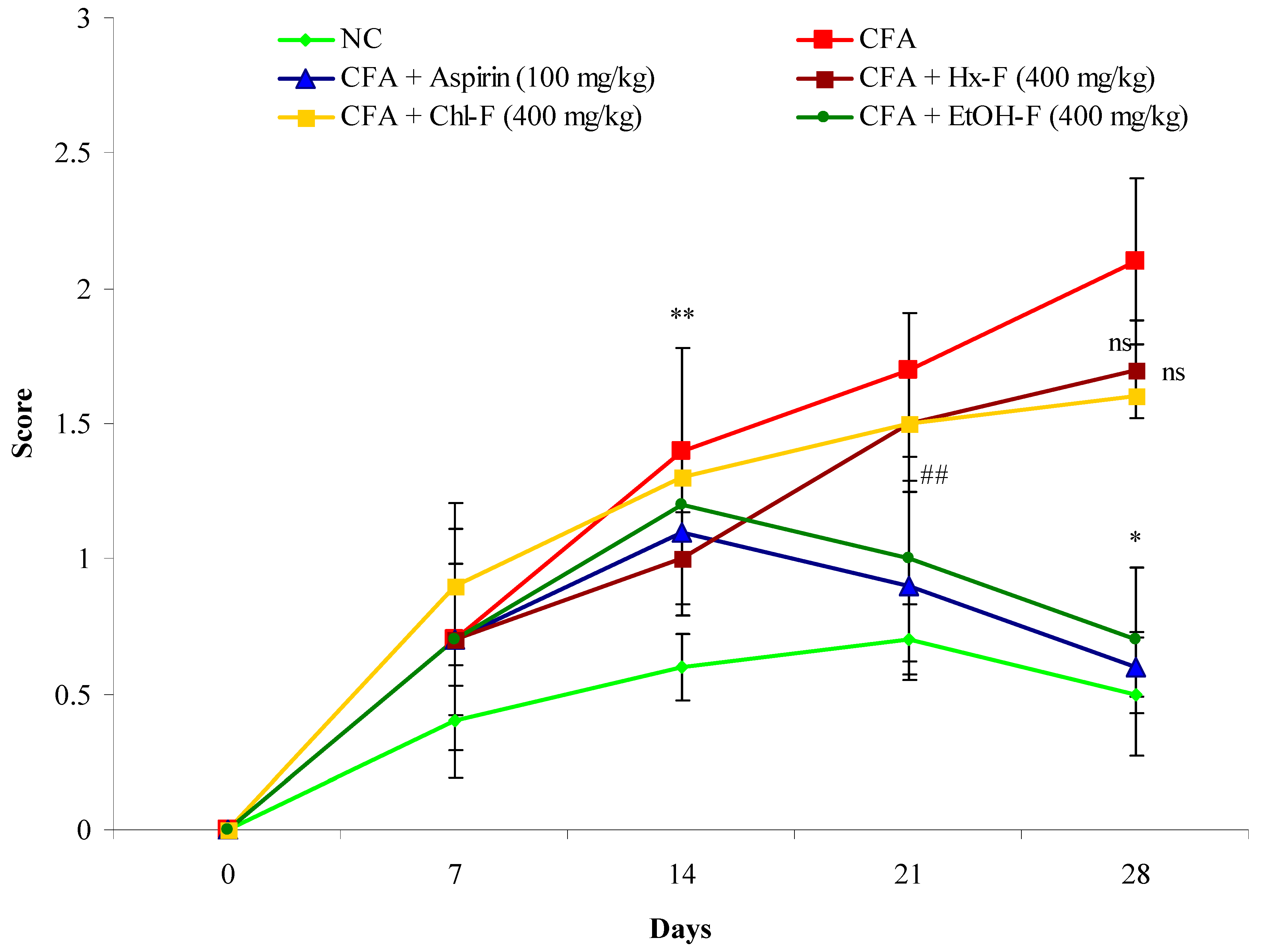
3.9. Anti-Arthritic Potential of S. mangifera against CFA-Induced Arthritis
3.10. Arthritis Index
4. Discussion
5. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sowjanya, R.; Shankar, M.; Sireesha, B.; Naik, E.A.; Yudharaj, P.; Priyadarshini, R.J. An overview on inflammation and plant having anti-inflammatory activity. Int. J. Phytopharm. Res. 2017, 7, 25–32. [Google Scholar]
- James, P.B.; Wardle, J.; Steel, A.; Adams, J. Traditional complementary and alternative medicine use in Sub-Saharan Africa: A systematic review. BMJ Glob. Health 2018, 3, e000895. [Google Scholar] [CrossRef]
- Marquardt, P.; Seide, R.; Vissiennon, C.; Schubert, A.; Birkemeyer, C.; Ahyi, V.; Fester, K. Phytochemical characterization and in-vitro anti-inflammatory, antioxidant and antimicrobial activity of Combretum collinum Fresen leaves extracts from Benin. Molecules 2020, 25, 288. [Google Scholar] [CrossRef]
- Castro-Santos, P.; Diaz-Pena, R. Genetics of rheumatoid arthritis: A new boost is needed in Latin American populations. Rev. Bras. Reum. 2016, 56, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Y.; Li, J.; Guan, Y.; Huang, J.; Wang, Z.; Zhang, Z.; Zhang, W.; Guo, J.; Li, J.; et al. Anti-arthritic activities of ethanol extracts of Circaea mollis Sieb. & Zucc. (whole plant) in rodents. J. Ethnopharmacol. 2018, 225, 359–366. [Google Scholar] [PubMed]
- Aloke, C.; Ibiam, U.A.; Orji, O.U.; Ugwuja, E.I.; Ezeani, N.N.; Aja, P.M.; Obasi, N.A. Anti arthritic potentials of ethanol and aqueous extracts of stem bark of Cleistopholis patens on complete Freund’s adjuvant-induced rheumatoid arthritis in rats. J. Ayurveda Integr. Med. 2021, 12, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ofman, J.J.; Badamgarav, E.; Henning, J.M. Utilization of nonsteroidal anti-inflammatory drugs and antisecretory agents: A managed care claims analysis. Am. J. Med. 2004, 116, 835–842. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, bioavailability and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Brothersen, C.; McMahon, D.J.; Legako, J.; Martini, S. Comparison of milk oxidation by exposure to LED and fluorescent light. J. Dairy Sci. 2016, 99, 2537–2544. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Khalid, M.; Alqarni, M.H.; Foudah, A.I.; Akhtar, J.; Shoaib, A.; Alam, P. Evaluation of free radical scavenging potential of different bioactive fractions present in Boerhavia diffusa Linn. root extract: An in-vitro approach. J. Pharm. Res. Int. 2020, 32, 99–107. [Google Scholar] [CrossRef]
- Anonymous. The Wealth of India, A Dictionary of Indian Raw Materials Publication and Information Directorate; CSIR: New Delhi, India, 1992; Volume 10, pp. 19–21. [Google Scholar]
- Kritikar, K.R.; Basu, B.D. Indian Medicinal Plants; International book distributors: Dehradune, India, 1975; Volume III, pp. 672–675. [Google Scholar]
- Tandon, S.; Rastogi, R.P. Studies on the chemical constituents of Spondias pinnata. Planta Med. 1976, 29, 190. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Mehrotra, B.N. Compendium of Indian Medicinal Plant; CDRI Lucknow & National Institute of Science communication: New Delhi, India, 1979; Volume I & II, pp. 379 & 643. [Google Scholar]
- Jain, S.K.; Hajra, P.K.; Shampru, R. A survey of edible plants in bazzars of Meghalaya. Bull. Bot Surv. 1977, 2, 29–34. [Google Scholar]
- Valsaraj, R.; Pushpangadan, P.; Smitt, U.W.; Adsersel, A.; Nyman, U. Antimicrobial screening of selected medicinal plants from India. J. Ethnopharmacol. 1997, 58, 75–83. [Google Scholar] [CrossRef]
- Itokawa, H.; Hirayama, F.; Tsuruoka, S.; Mizuno, K.; Nitta, A. Studies on antitumor activity of Indonesian medicinal plants. Shoyakugaku Zasshi 1990, 44, 58–62. [Google Scholar]
- Rao, G.B.; Raju, N.J. Investigation of hepatoprotective activity of Spondias pinnata. Int. J. Pharm. Sci. Res. 2010, 1, 193–198. [Google Scholar]
- Kandali, R.; Kumar, B.K. Evaluation of nutraceutical potentiality of a minor fruit of Assam-Spondias pinnata Kurz. In The Souvenir cum Abstract of “Vale Addition of Bio-Resources of NE India, Post-Harvest Technology and Cold Chain”; Department of Botany, Guwahati University: Guwahati, India, 2006; p. 99. [Google Scholar]
- Barua, U.; Hore, D.K.; Sarma, R. Wild edible plants of Majuli and Darrang districts of Assam. Indian J. Tradit. Knowl. 2007, 6, 191–194. [Google Scholar]
- Sidhu, R.S.; Lee, J.Y.; Yuan, C.; Smith, W.L. Comparison of cyclooxygenase-1 crystal structures: Cross-talk between monomers comprising cyclooxygenase-1 homodimers. Biochemistry 2010, 49, 7069–7079. [Google Scholar] [CrossRef]
- Orlando, B.J.; Lucido, M.J.; Malkowski, M.G. The structure of ibuprofen bound to cyclooxygenase-2. J. Struct. Biol. 2015, 189, 62–66. [Google Scholar] [CrossRef]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C.; et al. Small-molecule inhibition of TNF-alpha. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Pridiction of drug absorption using multivariate statistic. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Viji, V.; Helen, A. Inhibition of lipoxygenases and cyclooxygenase-2 enzymes by extracts isolated from Bacopa monniera (L.) Wettst. J. Ethnopharmacol. 2008, 118, 305–311. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 2005, 53, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Siddiqui, H.H.; Fareed, S. Free radical scavenging and total phenolic content of Saccharum spontaneum L. root extracts. Int. J. Res. Pharm. Chem. 2011, 1, 1160–1166. [Google Scholar]
- Hina, I.; Rose, C. In-vitro anti-inflammatory and antiarthritic activity of Pergularia daemia Leaves and Roots. Int. J. Drug Dev. Res. 2018, 10, 10–13. [Google Scholar]
- Dharmalingam, S.R.; Chidambaram, K.; Ramamurthy, S.; Nadaraju, S. Effects of nanosuspension and inclusion complex techniques on the in-vitro protease inhibitory activity of naproxen. Braz. J. Pharm. Sci. 2014, 50, 165–171. [Google Scholar] [CrossRef][Green Version]
- Jubie, S.; Jawahar, N.; Koshy, R.; Gowramma, B.; Murugan, V.; Suresh, B. Anti–arthritic activity of bark extracts of Alangium salviifolium Wang. Rasayan J. Chem. 2008, 1, 433–442. [Google Scholar]
- Patil, M.V.K.; Kandhare, A.D.; Bhise, S.D. Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L. ethanolic extract in Freund’s complete adjuvant induced arthritis. Biomed. Aging Pathol. 2012, 2, 6–15. [Google Scholar] [CrossRef]
- Arif, M.; Rahman, M.A.; Imran, M.; Khalid, M.; Khushtar, M. An insight of Spondias mangifera Willd: An underutilized medicinal plant with immense nutraceutical and therapeutic potentials. Int. J. Res. Pharm. Sci. 2015, 6, 17–26. [Google Scholar]
- Chen, S.; Feng, Z.; Wang, Y.; Ma, S.; Hu, Z.; Yang, P.; Chai, Y.; Xie, X. Discovery of novel ligands for TNF-α and TNF Receptor-1 through structure-based virtual screening and biological assay. J. Chem. Inf. Model. 2017, 57, 1101–1111. [Google Scholar] [CrossRef]
- Mahanthesh, M.T.; Ranjith, D.; Raghavendra, Y.; Jyothi, R.; Narappa, G.; Ravi, M.V. Swiss ADME prediction of phytochemicals present in Butea monosperma (Lam) Taub. J. Pharmacol. Phytochem. 2020, 9, 1799–1809. [Google Scholar]
- Jung, C.; Jung, H.; Shin, Y.; Park, J.; Jun, C. Eleutherococcus senticosus extract attenuates LPS-induced iNOS expression through the inhibition of Akt and JNK pathways in murine macropage. J. Etnopharmacol. 2007, 113, 183–187. [Google Scholar] [CrossRef]
- Barbara, O.; Correa, H.O.; Azevedo, E.P.C.; Padua, R.M.; Oliveira, V.L.S.; Oliveira, T.H.C.; Daiane-Boff Dias, A.C.F.; De Souza, D.G.; Amaral, F.A.; Teixeira, M.M.; et al. In-vitro TNF-α Inhibitory Activity of Brazilian Plants and Anti-Inflammatory Effect of Stryphnodendron adstringens in an Acute Arthritis Model. Evid. Based Complementary Altern. Med. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Naik, S.; Sheth, U. Inflammatory process and screening methods for anti-inflammatory agents—A review. J. Postgrad. Med. 1976, 22, 5–21. [Google Scholar]
- Onoja, S.O.; Omeh, Y.N.; Ezeja, M.I.; Chukwu, M.N. Evaluation of the in-vitro and in-vivo Antioxidant Potentials of Aframomum melegueta methanolic seed extract. J. Trop. Med. 2014, 2014, 159343. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Dash, G.K.; Mondal, S.; Dash, S.K. Antioxidative and antimicrobial study of Spondias mangifera Willd root. Int. J. Pharm. Pharm. Sci. 2010, 2, 68–71. [Google Scholar]
- Atere, T.G.; Akinloye, O.A.; Ugbaja, R.N.; Ojo, D.A.; Dealtry, G. In-vitro antioxidant capacity and free radical scavenging evaluation of standardized extract of Costus afer leaf. Food Sci. Hum. Wellness 2018, 7, 266–272. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gangwar, M.; Chaturvedi, A.P.; Sinha, A.S.; Tripathi, Y.B. Comparative analysis of phenolic and flavonoid content of Jatropha curcas Linn. Plant. Arch. 2012, 12, 823–826. [Google Scholar]
- Shakeel, S.; Khan, U.; Gul, S.; Sheikh, Z.A.; Zaidi, S.F.; Lateef, M. Evaluation of in vitro antioxidant capacity and reducing potential of polyherbal drug. Entoban. Afr. J. Pharm. Pharmacol. 2015, 9, 982–987. [Google Scholar]
- Modi, C.M.; Bhatt, P.R.; Pandya, K.B.; Patel, H.B.; Patel, U.D. Comparative evaluation of in vitro anti-inflammatory activity of different extracts of selected medicinal plants from Saurashtra region, Gujarat, India. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1686–1698. [Google Scholar] [CrossRef]
- Mann, G. Chemistry of the Proteins; Macmillan and Co. Limited: London, UK; New York, NY, USA, 1906; pp. 336–344. [Google Scholar]
- Mizushima, Y.; Kobayashi, M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 1968, 20, 169–173. [Google Scholar] [CrossRef]
- Chou, C.T. The antiinflammatory effect of an extract of Tripterygium wilfordii Hook F on adjuvant-induced paw oedema in rats and inflammatory mediators’ release. Phytother. Res. 1997, 11, 152–154. [Google Scholar] [CrossRef]
- Phusricom, S.; Chatuphonprasert, W.; Monthakantirat, O.; Pearaksa, P.; Jarukamjorn, K. Alternanthera sessilis and Alternanthera bettzickiana improved superoxide dismutase and catalase activities in the livers of ovariectomized mice. J. Appl. Biopharm. Pharm. 2013, 1, 64–71. [Google Scholar]
- Sunmathi, D.; Sivakumar, R.; Ravikumar, K. In-vitro antiinflammatory and antiarthritic activity of ethanolic leaf extract of Alternanthera sessilis (L.) R. BR. ex DC and Alternanthera philoxeroides (Mart.) Griseb. Int. J. Adv. Pharm. Biol. Chem. 2016, 5, 109–115. [Google Scholar]
- Rastogim, S.; Iqbal, M.S.; Ohri, D. In-vitro study of anti-inflammatory and antioxidant activity of some medicinal plants and their interrelationship. Asian J. Pharm. Clin. Res. 2018, 11, 195–202. [Google Scholar] [CrossRef][Green Version]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in-vitro anti-inflammatory activity of coffee against the denaturation of the protein. Asian Pac. J. Trop. Biomed. 2012, 2, 178–180. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Analysis of rutin, carotene, and lutein content and evaluation of antioxidant activities of six edible leaves on free radicals and reactive oxygenspecies. J. Food Biochem. 2018, 42, e12579. [Google Scholar] [CrossRef]
- Singh, S.; Nair, V.; Gupta, Y. Antiarthritic activity of majoon suranjan (a polyherbal Unani formulation) in rat. Indian J. Med. Res. 2011, 134, 384. [Google Scholar] [PubMed]
- Balasubramaniam, L.; Manivannan, R.; Paramaguru, R.; Mazumder, P.M.; Vijayakumar, M. Evaluation of anti-inflammatory and antioxidant activities of stem bark of Todalia asiatica (L) lam. using different experimental models. Pharmacologia 2012, 3, 144–149. [Google Scholar]
- Ghosh, S.; Derle, A.; Ahire, M.; More, P.; Jagtap, S.; Phadatare, S.D.; Patil, A.B.; Jabgunde, A.M.; Sharma, G.K.; Shinde, V.S.; et al. Phytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbifera. PLoS ONE 2013, 8, e82529. [Google Scholar] [CrossRef]
- Karagoz, A.; Artun, F.T.; Ozcan, G.; Melikoglu, G.; Anil, S.; Kultur, S.; Sutlupınar, N. In-vitro evaluation of antioxidant activity of some plant methanol extracts. Biotechnol. Biotechnol. Equip. 2015, 29, 1184–1189. [Google Scholar] [CrossRef]
- Zheng, C.J.; Zhao, X.X.; Ai, H.W.; Lin, B.; Han, T.; Jiang, Y.P.; Xing, X.; Qin, L.P. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund’s adjuvant induced arthritis in rats. Phytomedicine 2014, 21, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Sharma, S.; Sharma, K.; Gupta, G. Evaluation of Antiarthritic Activity of Butanol Fraction of Punica granatum Linn. Rind Extract Against Freund’s Complete Adjuvant-Induced Arthritis in Rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 53. [Google Scholar] [CrossRef]
- Rajendran, R.; Kumar, K.E. Anti-arthritic activity of Premna serratifolia Linn., wood against adjuvant induced arthritis. Avicenna J. Med. Biotechnol. 2010, 2, 101. [Google Scholar] [PubMed]
- Uttra, A.M.; Shahzad, M.; Shabbir, A.; Jahan, S.; Bukhari, I.A.; Assiri, A.M. Ribes orientale: A novel therapeutic approach targeting rheumatoid arthritis with reference to proinflammatory cytokines, inflammatory enzymes and anti-inflammatory cytokines. J. Ethnopharmacol. 2019, 237, 92–107. [Google Scholar] [CrossRef]
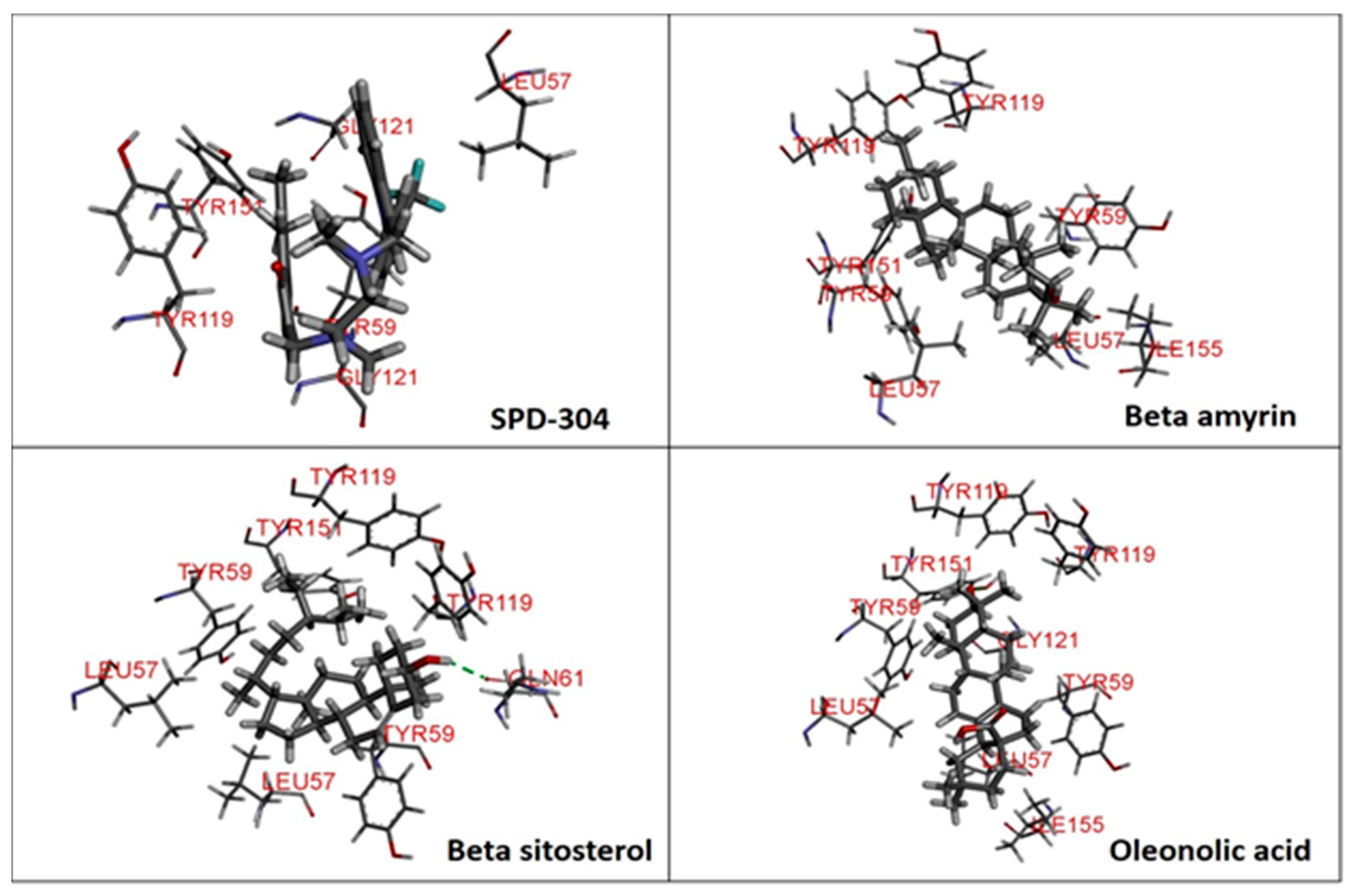




| Ligands | Chemical Structure | Binding Energy Score (Kcal/Mol) | Interaction (H-Bond) | Interaction (Hydrophobic) |
|---|---|---|---|---|
| SPD-304 | 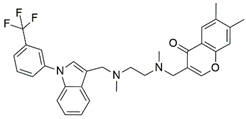 | −8.137 | Gly A: 121 | Leu A: 57, Tyr B: 59, Tyr B: 119, Gly B: 121, Tyr B: 151 |
| β-amyrin | 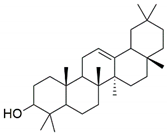 | −7.517 | - | Leu A: 57, Tyr A: 59, Tyr A: 119, Ile A: 155, Leu B: 57, Tyr B: 59, Tyr B: 119, Tyr B: 151 |
| β-sitosterol | 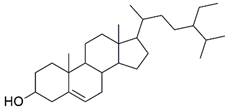 | −9.190 | Gln A: 61 | Leu A: 57, Tyr A: 59, Tyr A: 119, Leu B: 57, Tyr B: 59, Tyr B: 119, Tyr B: 151 |
| Oleonolic acid | 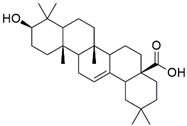 | −8.178 | Gly A: 121 Tyr B: 151 | Leu A: 57, Tyr A: 59, Tyr A: 119, Ile A: 155, Leu B: 57, Tyr B: 59, Tyr B: 119 |
| Molecule | MW | Ali Class | GI Absorption | BBB Permeant | Log Kp (cm/s) | Lipinski #Violations |
|---|---|---|---|---|---|---|
| Gallic acid | 170.12 | Soluble | High | No | −6.84 | 0 |
| Kaempferol | 286.24 | Soluble | High | No | −6.70 | 0 |
| Quercetin | 302.24 | Soluble | High | No | −7.05 | 0 |
| Ascorbic acid | 176.12 | Very soluble | High | No | −8.54 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.; Alqarni, M.H.; Shoaib, A.; Arif, M.; Foudah, A.I.; Afzal, O.; Ali, A.; Ali, A.; Alqahtani, S.S.; Altamimi, A.S.A. Anti-Arthritic and Anti-Inflammatory Potential of Spondias mangifera Extract Fractions: An In Silico, In Vitro and In Vivo Approach. Plants 2021, 10, 825. https://doi.org/10.3390/plants10050825
Khalid M, Alqarni MH, Shoaib A, Arif M, Foudah AI, Afzal O, Ali A, Ali A, Alqahtani SS, Altamimi ASA. Anti-Arthritic and Anti-Inflammatory Potential of Spondias mangifera Extract Fractions: An In Silico, In Vitro and In Vivo Approach. Plants. 2021; 10(5):825. https://doi.org/10.3390/plants10050825
Chicago/Turabian StyleKhalid, Mohammad, Mohammed H. Alqarni, Ambreen Shoaib, Muhammad Arif, Ahmed I. Foudah, Obaid Afzal, Abuzer Ali, Amena Ali, Saad S. Alqahtani, and Abdulmalik S. A. Altamimi. 2021. "Anti-Arthritic and Anti-Inflammatory Potential of Spondias mangifera Extract Fractions: An In Silico, In Vitro and In Vivo Approach" Plants 10, no. 5: 825. https://doi.org/10.3390/plants10050825
APA StyleKhalid, M., Alqarni, M. H., Shoaib, A., Arif, M., Foudah, A. I., Afzal, O., Ali, A., Ali, A., Alqahtani, S. S., & Altamimi, A. S. A. (2021). Anti-Arthritic and Anti-Inflammatory Potential of Spondias mangifera Extract Fractions: An In Silico, In Vitro and In Vivo Approach. Plants, 10(5), 825. https://doi.org/10.3390/plants10050825