Green Light Improves Photosystem Stoichiometry in Cucumber Seedlings (Cucumis sativus) Compared to Monochromatic Red Light
Abstract
1. Introduction
2. Results
2.1. Photosynthesis
2.2. Chlorophyll Fluorescence
2.3. Shoot Characteristics
2.4. Stomatal Characteristics
3. Discussion
3.1. Chlorophyll Fluorescence
3.2. Stomatal Conductance
3.3. Morphology
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Sellaro, R.; Crepy, M.; Trupkin, S.A.; Karayekov, E.; Buckovsky, A.S.; Rossi, C.; Casal, J.J. Cryptochrome as a Sensor of the Blue/Green Ratio of Natural Radiation in Arabidopsis. Plant Physiol. 2010, 154, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kasperbauer, M.J. Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol. 1987, 85, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Massa, G.; Graham, T.; Haire, T.; Flemming II, C.; Gerard, N.; Wheeler, R. Light-emitting diode light transmission through leaf tissue of seven different crops. HortScience 2015, 50, 501–506. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar]
- Evans, J.R. The dependence of quantum yield on wavelength and growth irradiance. Aust. J. Plant Physiol. 1987, 14, 69–79. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. ASABE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-response of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, X.; Gao, L.; Chen, Q.; Mei, Q. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Trouwborst, G.; Oosterkamp, J.; Hogewoning, S.W.; Harbinson, J.; van Ieperen, W. The responses of light interception, photosynthesis and fruit yield of cucumber to LED-lighting within the canopy. Physiol. Plant. 2010, 138, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Growth and morphological response of cucumber seedlings to supplemental red and blue photon flux ratios under varied solar daily light integrals. Sci. Hortic. 2014, 173, 92–99. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. 1995, 120, 808–813. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthetic characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Folta, K.M. Green light augments far-red-light-induced shade response. Plant Growth Regul. 2013, 77, 145–155. [Google Scholar] [CrossRef]
- Golovatskaya, I.F.; Karnachuk, R.A. Role of green light in physiological activity of plants. Russ. J. Plant Physiol. 2014, 62, 727–740. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- McCoshum, S.; Kiss, J.Z. Green light affects blue-light-based phototropism in hypocotyls of Arabidopsis thaliana. J. Torrey Bot. Soc. 2011, 138, 409–417. [Google Scholar] [CrossRef]
- Frechilla, S.; Talbott, L.D.; Bogomolni, R.A.; Zeiger, E. Reversal of blue light-stimulated stomatal opening by green light. Plant Cell Physiol. 2000, 41, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Talbott, L.D.; Nikolova, G.; Ortix, A.; Shmayevich, I.; Zeiger, E. Green light reversal of blue-light-stimulated stomatal opening is found in a diversity of plant species. Am. J. Bot. 2002, 89, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Hemming, S.; Mohammadkhani, V.; Dueck, T. Diffuse greenhouse covering materials—Material technology, measurements and evaluation of optical properties. Acta Hortic. 2008, 797, 469–476. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Tomato seedling growth and morphological responses to supplemental LED lighting red:Blue ratios under varied daily solar light integrals. Acta Hortic. 2012, 956, 187–194. [Google Scholar] [CrossRef]
- Sabir, N.; Singh, B. Protected cultivation of vegetables in global arena: A review. Indian J. Agric. Sci. 2013, 83, 123–135. [Google Scholar]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef]
- Jinxiu, S.; Qingwu, M.; Weifan, D.; Dongxian, H. Effects of light quality on growth and development of cucumber seedlings in controlled environment. Int. J. Agric. Biol. 2017, 10, 312–318. [Google Scholar]
- Shibuya, T.; Endo, R.; Yuba, T.; Kitaya, Y. The photosynthetic parameters of cucumber as affected by irradiances with different red:far-red ratios. Biol. Plant. 2015, 59, 198–200. [Google Scholar] [CrossRef]
- Bergstrand, K.J.; Suthaparan, A.; Mortensen, L.M.; Gislerød, H.G. Photosynthesis in horticultural plants in relation to light quality and CO2 concentration. Eur. J. Hortic. Sci. 2016, 81, 237–242. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Emerson, R.; Chalmers, R.; Cederstrand, C. Some factors influencing the long-wave limit of photosynthesis. Proc. Natl. Acad. Sci. USA 1957, 43, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Croce, R. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef]
- Murakami, K.; Matsuda, R.; Fujiwara, K. A mathematical model of photosynthetic electron transport in response to the light spectrum based on excitation energy distributed to photosystems. Plant Cell Physiol. 2018, 59, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, K.J.; Mortensen, L.M.; Suthaparan, A.; Gislerød, H.G. Acclimatisation of greenhouse crops to differing light quality. Sci. Hortic. 2016, 204, 1–7. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and nonphotochemical components—Calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Galka, P.; Santabarbara, S.; Khuong, T.T.H.; Degand, H.; Morsomme, P.; Jennings, R.C.; Boekema, E.J.; Caffarri, S. Functional analyses of the plant photosystem I-light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. Plant Cell 2012, 24, 2963–2978. [Google Scholar] [CrossRef]
- Fujita, Y. A study on the dynamic features of photosystem stoichiometry: Accomplishments and problems for future studies. Photosynth. Res. 1997, 53, 83–93. [Google Scholar] [CrossRef]
- Yamazaki, J.; Suzuki, T.; Maruta, E.; Kamimura, Y. The stoichiometry and antenna size of the two photosystems in marine green algae, Bryopsis maxima and Ulva pertusa, in relation to the light environment of their natural habitat. J. Exp. Bot. 2005, 56, 1517–1523. [Google Scholar] [CrossRef]
- Dietzel, L.; Bräutigam, K.; Pfannschmidt, T. Photosynthetic acclimation: State transitions and adjustment of photosystem stoichiometry—Functional relationships between short-term and long-term light quality acclimation in plants. FEBS J. 2008, 275, 1080–1088. [Google Scholar] [CrossRef]
- Schöttler, M.A.; Tóth, S.Z. Photosynthetic complex stoichiometry dynamics in higher plants: Environmental acclimation and photosynthetic flux control. Front. Plant Sci. 2014, 5, 188. [Google Scholar] [PubMed]
- Croce, R.; van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef]
- Schöttler, M.A.; Tóth, S.Z.; Boulouis, A.; Kahlau, S. Photosynthetic complex stoichiometry dynamics in higher plants: Biogenesis, function, and turnover of ATP synthase and the cytochrome B6f complex. J. Exp. Bot. 2015, 66, 2373–2400. [Google Scholar] [CrossRef] [PubMed]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J.; Aphalo, P.J.; Sanchez, R.A. Phytochrome effects on leaf growth and chlorophyll content in Petunia axilaris. Plant Cell Environ. 1987, 10, 509–514. [Google Scholar] [CrossRef]
- Jeong, H.W.; Lee, H.R.; Kim, H.M.; Hwang, H.S.; Hwand, S.J. Using light quality for growth control of cucumber seedlings in closed-type plant production system. Plants 2020, 9, 639. [Google Scholar] [CrossRef]
- Paul, L.J.; Kurana, J.P. Phytochrome-mediated light signaling in plants: Emerging trends. Physiol. Mol. Biol. Plants 2008, 14, 9–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kahlen, K.; Stützel, H. Simplification of a light-based model for estimating final internode length in greenhouse cucumber canopies. Ann. Bot. 2011, 108, 1055–1063. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Arnon, D.I.; Hoagland, D.R. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940, 50, 463–485. [Google Scholar]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 2019–2218. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS Studio 3.8; SAS Institute Inc.: Cary, NC, USA, 2018. [Google Scholar]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbo, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G. Plants and Microclimate, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992; pp. 137–140. [Google Scholar]
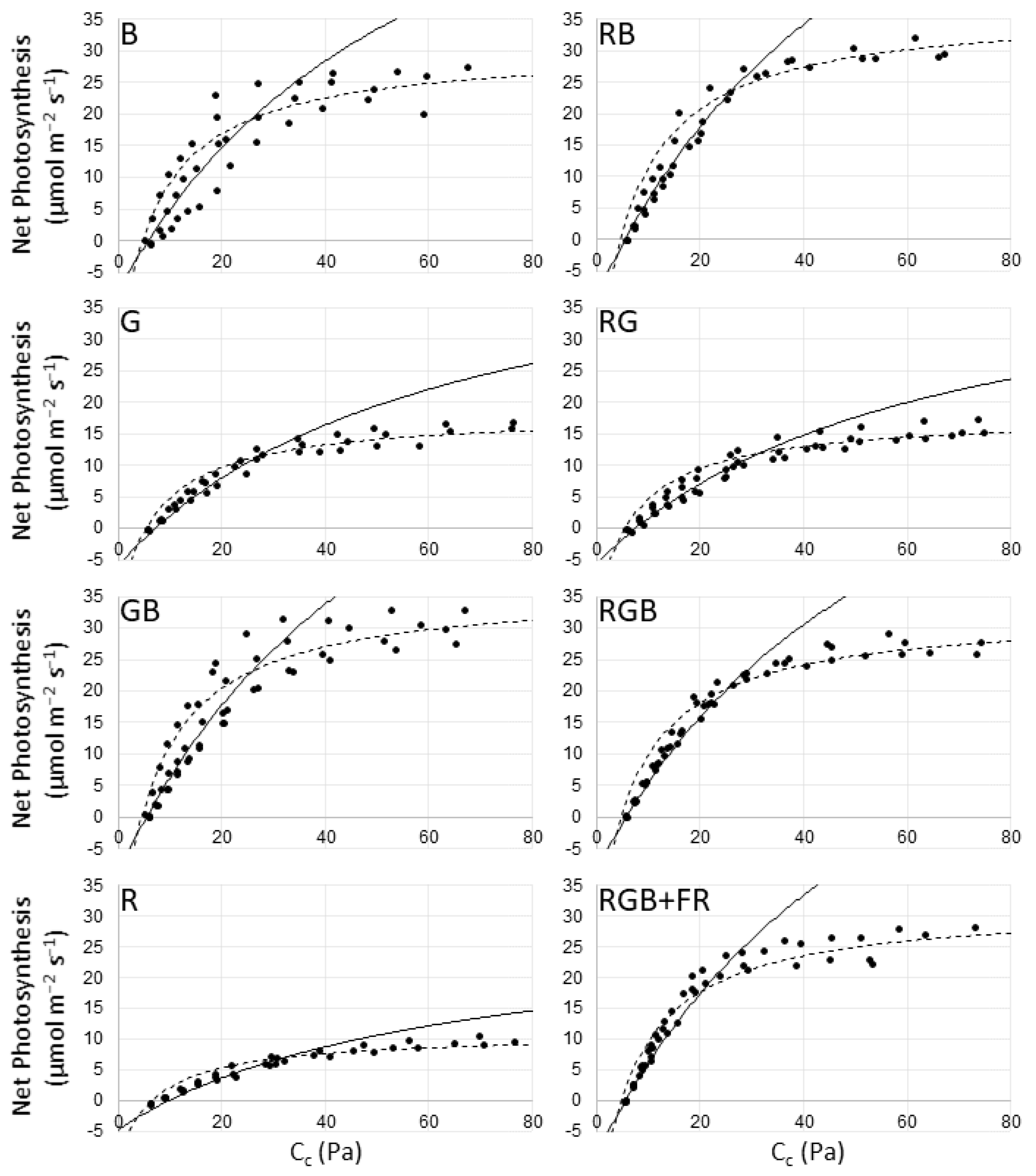
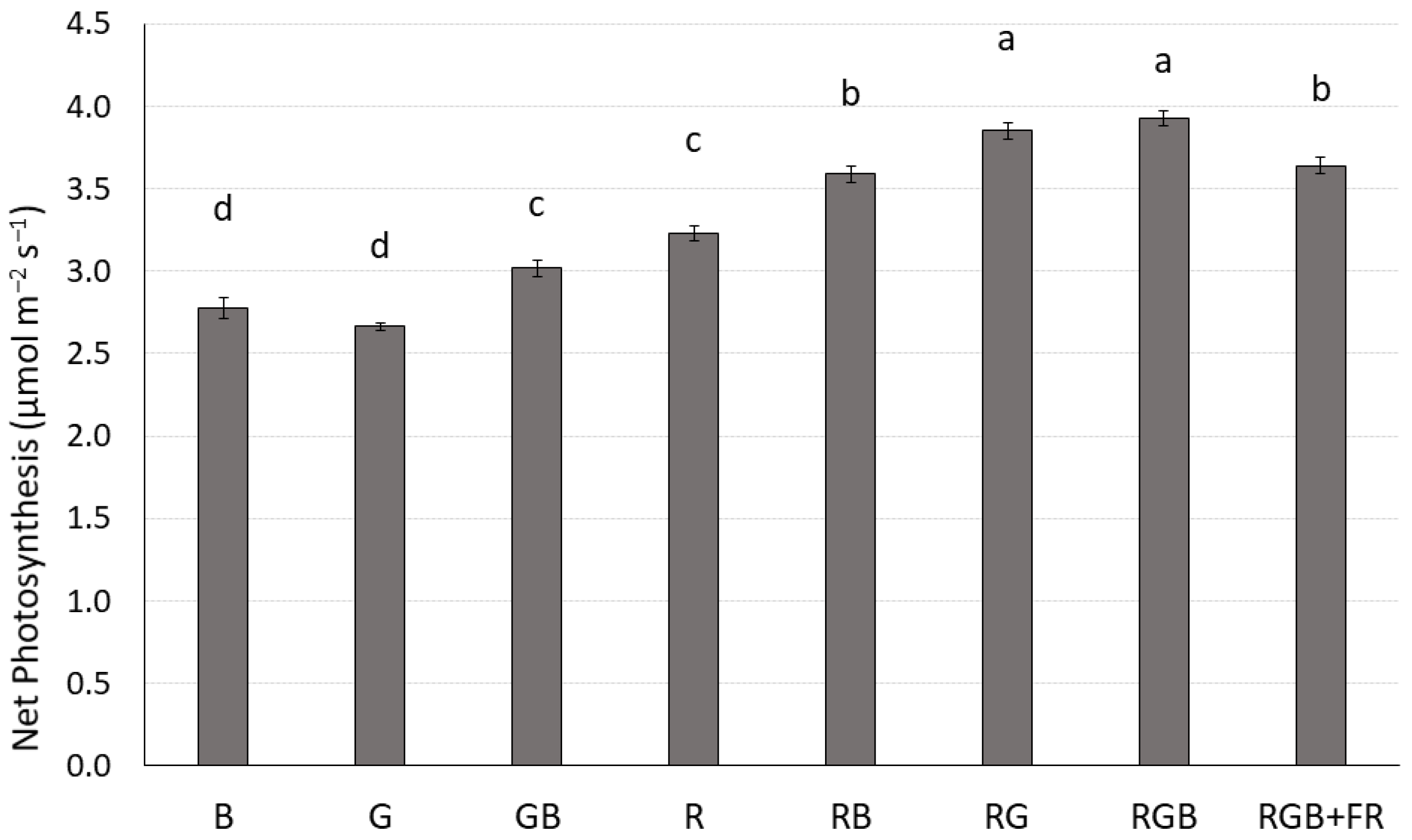

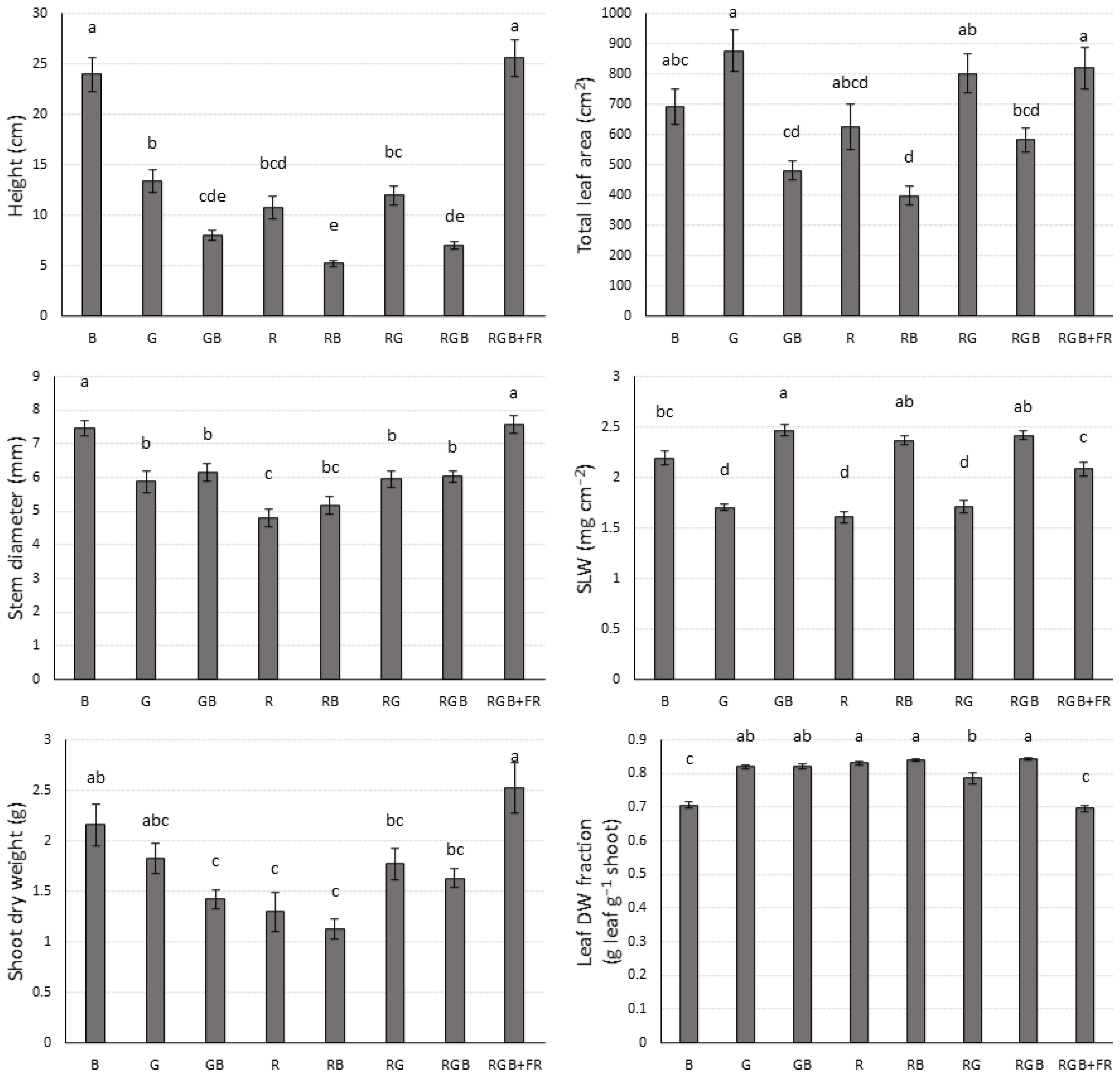


| Treatment | J | Vcmax |
|---|---|---|
| µmol m−2 s−1 | µmol m−2 s−1 | |
| B | 132.0 ± 5.3 c | 87.0 ± 4.6 b |
| G | 83.4 ± 1.2 d | 53.6 ± 1.2 c |
| GB | 155.5 ± 4.3 ab | 102.1 ± 3.6 ab |
| R | 54.3 ± 0.7 e | 32.2 ± 0.8 d |
| RB | 157.3 ± 2.8 a | 103.0 ± 2.3 a |
| RG | 82.1 ± 1.3 d | 49.0 ± 1.2 c |
| RGB | 140.5 ± 1.6 bc | 93.2 ± 1.2 b |
| RGB + FR | 137.7 ± 2.7 c | 101.9 ± 2.1 a |
| Treatment | ΦPSII | Fv/Fm | ΦNPQ | ΦNO | qP | qL |
|---|---|---|---|---|---|---|
| B | 0.26 ± 0.01 b | 0.81 ± 0.01 a | 0.48 ± 0.01 c | 0.26 ± 0.00 f | 0.43 ± 0.01 b | 0.23 ± 0.01 ab |
| G | 0.14 ± 0.00 c | 0.80 ± 0.00 a | 0.54 ± 0.00 a | 0.32 ± 0.00 c | 0.24 ± 0.01 c | 0.12 ± 0.00 d |
| GB | 0.29 ± 0.01 a | 0.82 ± 0.00 a | 0.44 ± 0.01 e | 0.26 ± 0.00 f | 0.46 ± 0.01 a | 0.24 ± 0.01 a |
| R | 0.09 ± 0.00 e | 0.79 ± 0.01 a | 0.53 ± 0.00 a | 0.38 ± 0.00 a | 0.14 ± 0.00 e | 0.06 ± 0.00 f |
| RB | 0.26 ± 0.01 b | 0.82 ± 0.01 a | 0.47 ± 0.01 cd | 0.26 ± 0.00 f | 0.42 ± 0.01 b | 0.22 ± 0.01 bc |
| RG | 0.12 ± 0.00 d | 0.80 ± 0.01 a | 0.51 ± 0.00 b | 0.37 ± 0.00 b | 0.19 ± 0.01 d | 0.08 ± 0.00 e |
| RGB | 0.27 ± 0.01 b | 0.82 ± 0.00 a | 0.43 ± 0.01 e | 0.29 ± 0.00 d | 0.42 ± 0.01 b | 0.20 ± 0.01 c |
| RGB + FR | 0.27 ± 0.01 b | 0.81 ± 0.01 a | 0.45 ± 0.01 de | 0.28 ± 0.00 e | 0.43 ± 0.01 b | 0.23 ± 0.01 b |
| Treatment | Abaxial | Adaxial | AB:AD | iWUE | Water Content |
|---|---|---|---|---|---|
| Stomata/mm2 | Stomata/mm2 | µmol CO2 mol−1 H2O | g H2O 100 g−1 FW | ||
| B | 402 ± 22 bcd | 243 ± 16 bc | 1.66 ± 0.11 b | 12.5 ± 1.4 d | 92.1 ± 0.2 a |
| G | 378 ± 26 cde | 153 ± 20 de | 2.67 ± 0.13 a | 37.1 ± 1.5 b | 92.4 ± 0.2 a |
| GB | 507 ± 25 a | 349 ± 18 a | 1.55 ± 0.12 b | 17.9 ± 1.4 cd | 91.0 ± 0.2 b |
| R | 295 ± 25 e | 116 ± 19 e | 2.76 ± 0.13 a | 37.4 ± 1.4 b | 92.7 ± 0.4 a |
| RB | 490 ± 22 ab | 254 ± 16 b | 1.99 ± 0.11 b | 22.6 ± 1.4 c | 90.6 ± 0.1 b |
| RG | 312 ± 24 de | 175 ± 18 cde | 1.82 ± 0.12 b | 47.0 ± 1.3 a | 92.0 ± 0.3 a |
| RGB | 486 ± 25 abc | 287 ± 19 ab | 1.73 ± 0.13 b | 20.9 ± 1.4 c | 90.6 ± 0.2 b |
| RGB + FR | 363 ± 21 de | 224 ± 16 bcd | 1.66 ± 0.11 b | 23.0 ± 1.4 c | 92.3 ± 0.3 a |
| Traditional method | Treatment | Red (600–700 nm) | Green (500–600 nm) | Blue (400–500 nm) |
| B | 0 | 1 | 99 | |
| G | 0 | 93 | 6 | |
| GB | 0 | 37 | 63 | |
| R | 100 | 0 | 0 | |
| RB | 47 | 1 | 52 | |
| RG | 65 | 31 | 3 | |
| RGB | 38 | 22 | 40 | |
| RGB + FR | 40 | 20 | 40 | |
| Bar method | Treatment | Red (623–684 nm) | Green (486–582 nm) | Blue (432–500 nm) |
| B | 0 | 0 | 100 | |
| G | 0 | 100 | 0 | |
| GB | 0 | 38 | 62 | |
| R | 100 | 0 | 0 | |
| RB | 47 | 0 | 53 | |
| RG | 65 | 35 | 0 | |
| RGB | 38 | 23 | 39 | |
| RGB + FR | 40 | 21 | 39 |
| Treatment | YPF | PSS |
|---|---|---|
| µmol m−2 s−1 | Pfr:Ptotal | |
| B | 88 | 0.51 |
| G | 94 | 0.83 |
| GB | 90 | 0.62 |
| R | 114 | 0.89 |
| RB | 98 | 0.86 |
| RG | 106 | 0.88 |
| RGB | 102 | 0.86 |
| RGB + FR | 104 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claypool, N.B.; Lieth, J.H. Green Light Improves Photosystem Stoichiometry in Cucumber Seedlings (Cucumis sativus) Compared to Monochromatic Red Light. Plants 2021, 10, 824. https://doi.org/10.3390/plants10050824
Claypool NB, Lieth JH. Green Light Improves Photosystem Stoichiometry in Cucumber Seedlings (Cucumis sativus) Compared to Monochromatic Red Light. Plants. 2021; 10(5):824. https://doi.org/10.3390/plants10050824
Chicago/Turabian StyleClaypool, Nicholas B., and J. Heinrich Lieth. 2021. "Green Light Improves Photosystem Stoichiometry in Cucumber Seedlings (Cucumis sativus) Compared to Monochromatic Red Light" Plants 10, no. 5: 824. https://doi.org/10.3390/plants10050824
APA StyleClaypool, N. B., & Lieth, J. H. (2021). Green Light Improves Photosystem Stoichiometry in Cucumber Seedlings (Cucumis sativus) Compared to Monochromatic Red Light. Plants, 10(5), 824. https://doi.org/10.3390/plants10050824






