Exogenous Application of Zinc to Mitigate the Salt Stress in Vigna radiata (L.) Wilczek—Evaluation of Physiological and Biochemical Processes
Abstract
1. Introduction
2. Results
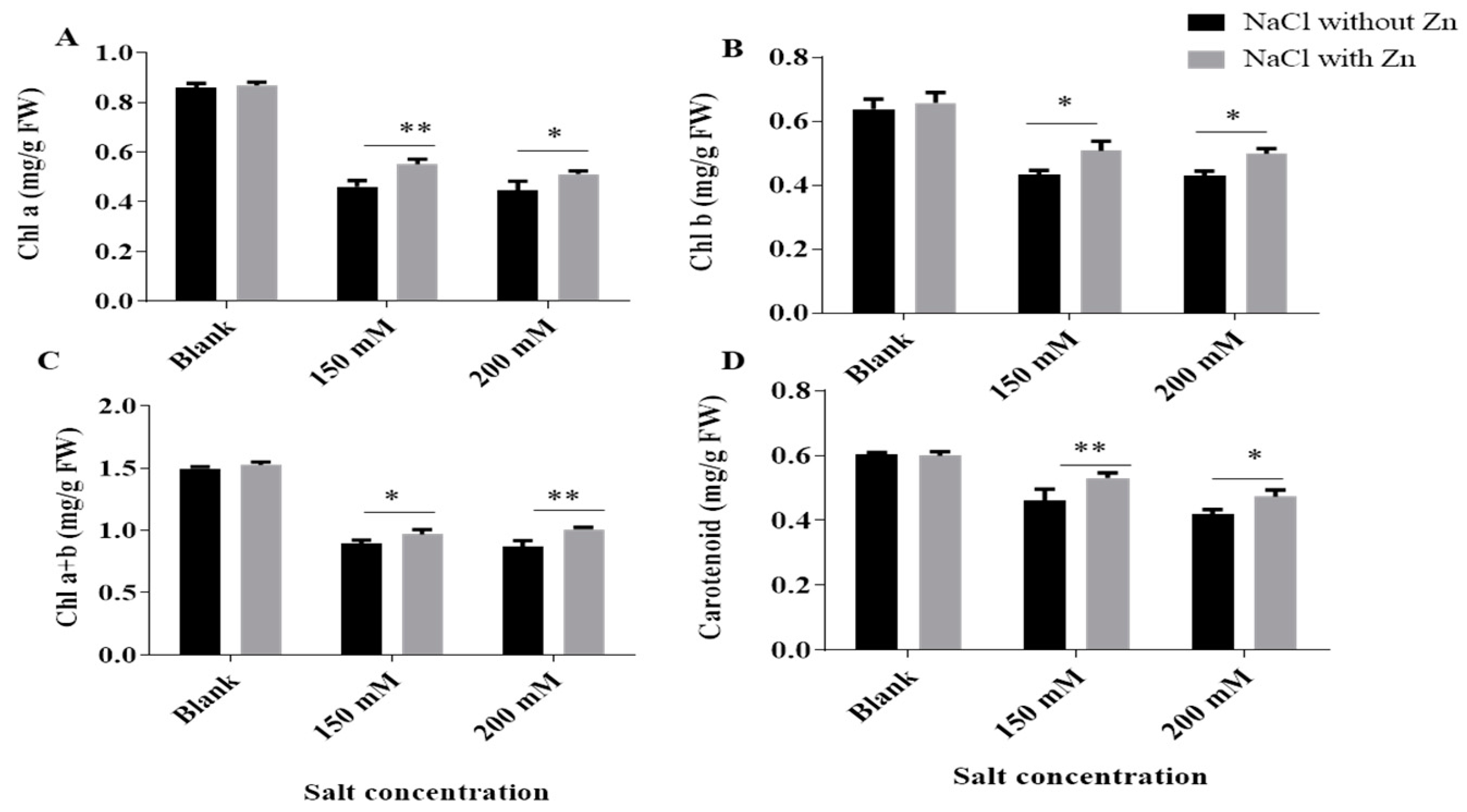
2.1. Effect of Salinity on Growth, Photosynthetic Pigments and Their Recovery by Exogenous Application of Zn
2.2. Effect of Salt Stress on Membrane Stability Index (MSI), Thiobarbituric Acid Reactive Species (TBARS) Content and Hydrogen Peroxide (H2O2) Content and Its Mitigation by Zn
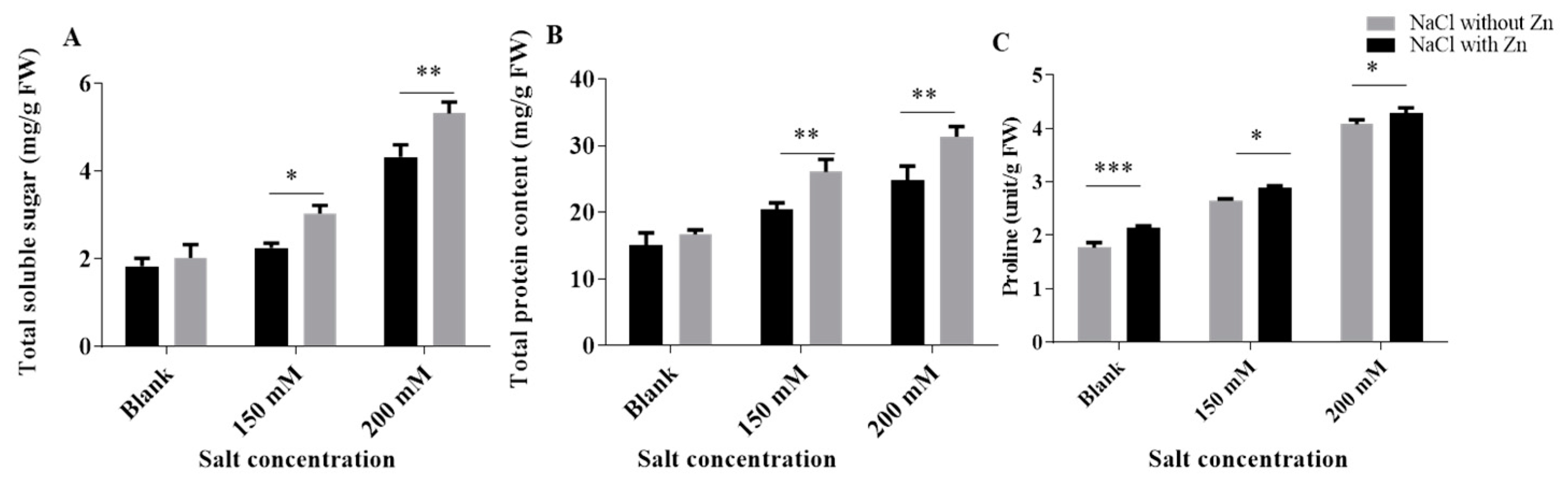
2.3. Effect of Salinity and Exogenously Applied Zn on Osmolytes (Proline, TSS and TSP)
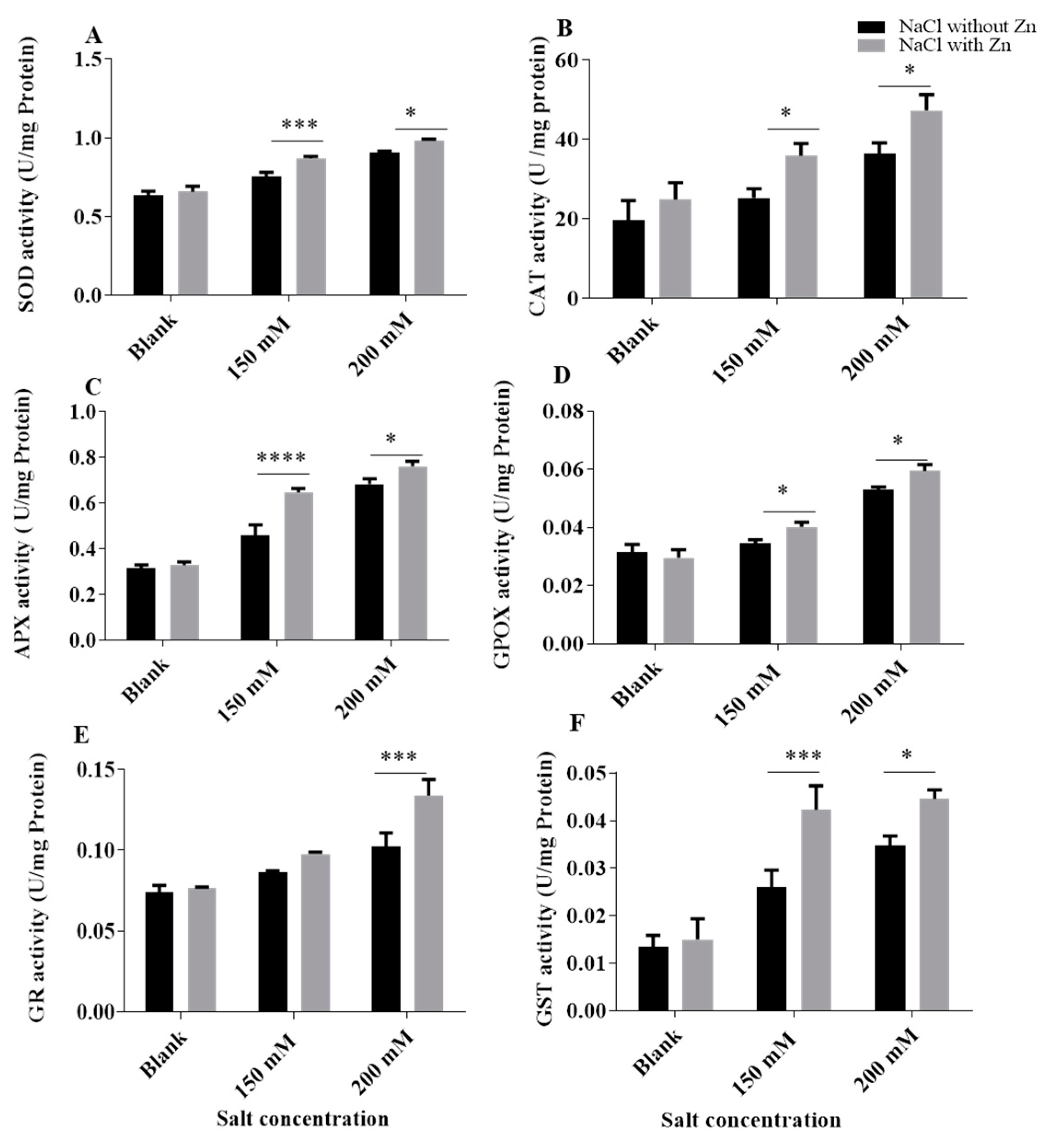
2.4. Effect of Salinity Stress and Zn on the Activity of Antioxidant Enzymes
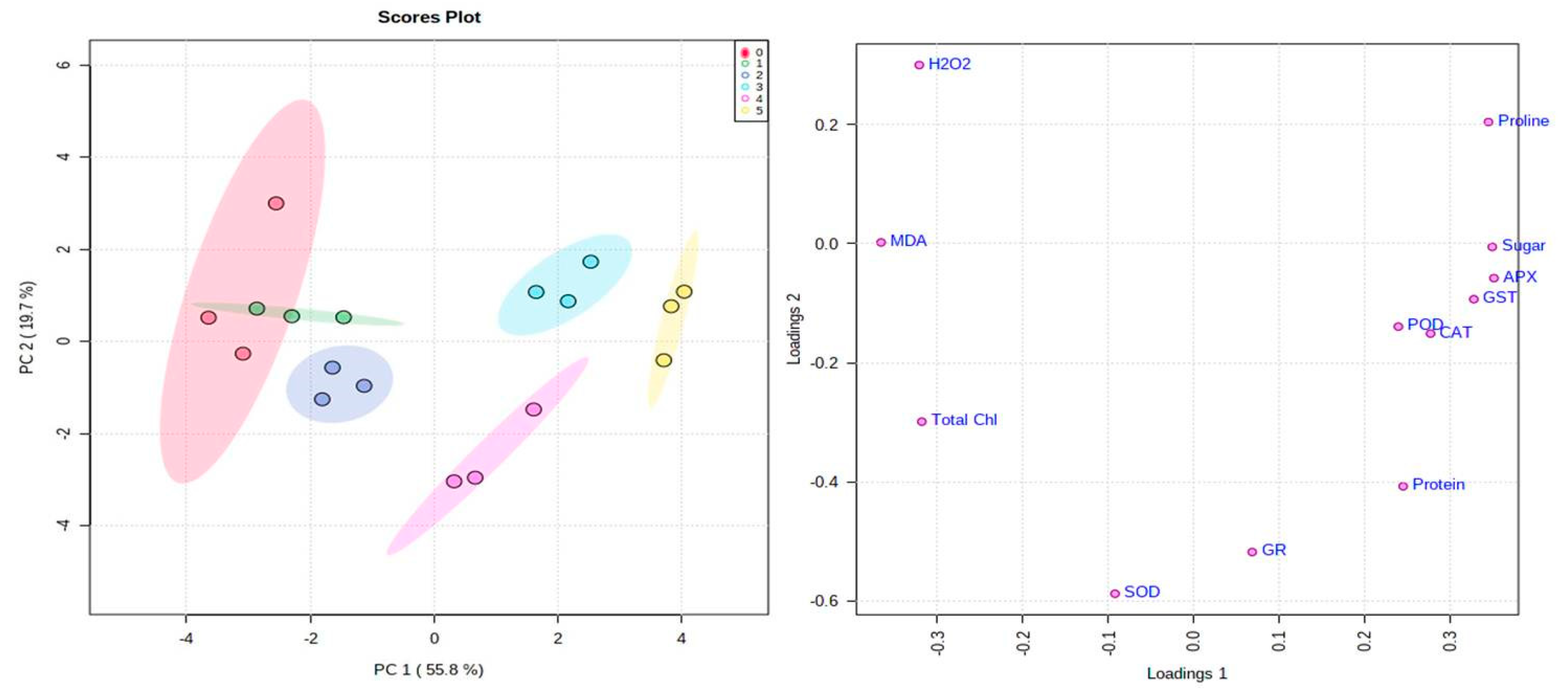
Multivariate Data Analysis
2.5. Effect of Salt and Salt with Zn on PAL (Phenylalanine Ammonia-Lyase) and TAL (Tyrosine Ammonia-Lyase)
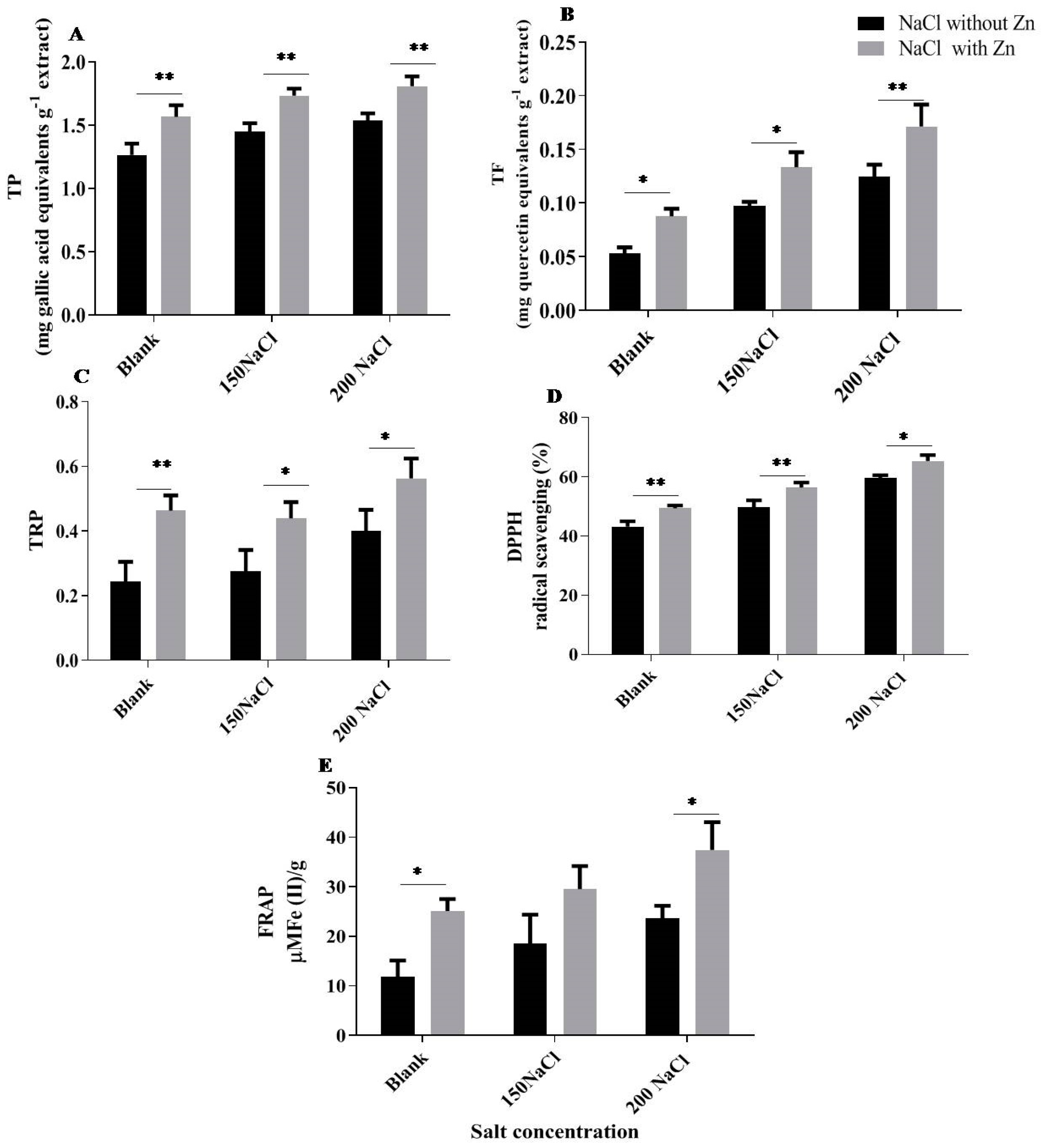
2.6. Effect of Salt and Salt with Zn on TP (Total Phenol), TF (Total Flavonoid), TRP (Total Reducing Power), DPPH (2,2-Diphenyl-1-Picrylhydrazyl) and FRAP (Ferric Reducing Antioxidant Power Assay)
3. Discussion
4. Materials and Methods
4.1. Growth and Stress Conditions
4.2. Estimation of Photosynthetic Pigments
4.3. Estimation of Membrane Stability Index (MSI)
4.4. Determination of Lipid Peroxidation (LPO) Rate
4.5. Determination of Hydrogen Peroxide (H2O2) Content
4.6. Estimation of Osmolytes
4.7. Enzymatic Assays
4.7.1. Determination of Superoxide Dismutase (SOD) Activity
4.7.2. Determination of Catalase (CAT) Activity
4.7.3. Determination of Ascorbate Peroxidase (APX) Activity
4.7.4. Determination of Guaiacol Peroxidase (POD/GPOX) Activity
4.7.5. Determination of Glutathione-s-Transferase (GST) Activity
4.7.6. Determination of Glutathione-Reductase (GR) Activity
4.8. Determination of Antioxidant Activity
4.8.1. Determination of PAL and TAL Activity
4.8.2. Total Phenol and Total Flavonoid Content
4.8.3. Determination of Total Reducing Power, DPPH and FRAP
4.8.4. Determination of Hydrogen Peroxide (H2O2) and Superoxide Radical (SOD) Scavenging Activity
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M.J. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- FAO. FAO Cereal Supply and Demand Brief; FAO: Rome, Italy, 2015. [Google Scholar]
- Hachicha, M. Salty soils and their development in Tunisia. Sci. Glob. Chang. Drought 2007, 18, 45–50. [Google Scholar]
- Kalaji, H.; Nalborczyk, E. Gas exchange of barley seedlings growing under salinity stress. Photosynthetica 1991, 25, 197–202. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Bascola, P.R.; Menossi, M.; Jorge, R.A. Aluminum-induced oxidative stress in plants. Photochemistry 2003, 62, 181–189. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Passardi, F.; Theiler, G.; Zamocky, M.; Cosio, C.; Rouhier, N.; Teixera, F.; Margis-Pinheiro, M.; Ioannidis, V.; Penel, C.; Falquet, L.; et al. PeroxiBase: The peroxidase database. Phytochemistry 2007, 68, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Murmu, K.M.S.; Bera, C.K.K.P.S. Exogenous Proline and Glycine Betaine in Plants under Stress Tolerance. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 901–913. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F.-J. Function of Nutrients: Micronutrients, in Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- Zafar, S.; Ashraf, M.Y.; Saleem, M. Shift in Physiological and Biochemical Processes in Wheat Supplied with Zinc and Potassium under Saline Condition. J. Plant Nutr. 2017, 41, 19–28. [Google Scholar] [CrossRef]
- Parker, D.R.; Aguilera, J.J.; Thomason, D.N. Zinc-phosphorus interactions in two cultivars of tomato (Lycopersicon esculentum L.) grown in chelator-buffered nutrient solutions. Plant Soil 1992, 143, 163–177. [Google Scholar] [CrossRef]
- Sharma, P.N.; Bisht, S.S.; Kumar, N. Effect of zinc deficiency on chlorophyll content, photosynthesis and water relations of cauliflower plants. Photosynthetica 1994, 30, 353–359. [Google Scholar]
- Bettger, W.J.; O’Dell, B.L. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981, 28, 1425–1438. [Google Scholar] [CrossRef]
- Vallee, B.L.; Auld, D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M. Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Sci. 2004, 166, 1321–1327. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Zinc-dependent changes in ESR signals, NADPH oxidase and plasma membrane permeability in cotton roots. Physiol. Plant. 1988, 73, 182–186. [Google Scholar] [CrossRef]
- Marschner, H.; Cakmak, I. High Light Intensity Enhances Chlorosis and Necrosis in Leaves of Zinc, Potassium, and Magnesium Deficient Bean (Phaseolus vulgaris) Plants. J. Plant Physiol. 1989, 134, 308–315. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Graham, R.D.; Welch, R.M. Breeding for Staple Food Crops with High Micronutrient Density; International Food Policy Research Institute: Washington, DC, USA, 1996; Volume 3. [Google Scholar]
- Alharby, H.F.; Al-Zahrani, H.S.; Hakeem, K.R.; Iqbal, M. Identification of physiological and biochemical markers for salt (NaCl) stress in the seedlings of mungbean [Vigna radiata (L.) Wilczek] genotypes. Saudi J. Biol. Sci. 2019, 26, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Shah, W.H.; Rasool, A.; Saleem, S.; Mushtaq, N.U.; Tahir, I.; Hakeem, K.R.; Rehman, R.U. Understanding the Integrated Pathways and Mechanisms of Transporters, Protein Kinases, and Transcription Factors in Plants under Salt Stress. Int. J. Genom. 2021, 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Račková, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics 2012, 5, 60. [Google Scholar]
- Hussein, M.M.; Abou-Baker, N.H. Growth and mineral status of moringa plants as affected by silicate and salicylic acid under salt stress. Int. J. Plant Soil Sci. 2014, 3, 163–177. [Google Scholar] [CrossRef]
- Amira, S.S.; Souad, A.E.F.; Essam, D.; Soliman, A.S.; El-Feky, S.A.; Darwish, E. Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J. Hortic. For. 2015, 7, 36–47. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abou-Baker, N.H. The contribution of nano-zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef]
- Hafeez, B.; Khanif, Y.M.; Saleem, M. Role of Zinc in Plant Nutrition- A Review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Galal, A. Physico-chemical changes in karkade (Hibiscus sabdariffa L.) seedlings responding to salt stress. Acta Biol. Hung. 2017, 68, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A. Physiological responses of soybean (Glycine max L.) to zinc application under salinity stress. Aust. J. Crop Sci. 2011, 5, 1441–1447. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Egamberdieva, D.; Bhardwaj, R.; Ashraf, M. Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J. Plant Interactions 2017, 12, 429–437. [Google Scholar] [CrossRef]
- El-Tayeb, M.A. Response of barley grains to the interactive e.ect of salinity and salicylic acid. Plant Growth Regul. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- Fang, Z.; Solomos, T.; Bouwkamp, J.C. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Bot. 1998, 49, 503–510. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Badakhshan, H. Effects of Zinc Application on Growth, Absorption and Distribution of Mineral Nutrients Under Salinity Stress in Soybean (Glycine Max L.). J. Plant Nutr. 2014, 37, 2255–2269. [Google Scholar] [CrossRef]
- Samreen, T.; Humaira; Shah, H.U.; Ullah, S.; Javid, M. Zinc effect on growth rate, chlorophyll, protein and mineral contents of hydroponically grown mungbeans plant (Vigna radiata). Arab. J. Chem. 2017, 10, S1802–S1807. [Google Scholar] [CrossRef]
- Logani, M.K.; Davies, R.E. Lipid oxidation: Biologic effects and antioxidants—A review. Lipids 1980, 15, 485–495. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahemi, M.; Eshghi, S.; Kholdebarin, B. Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L.‘Badami’) seedlings. Turk. J. Agric. For. 2010, 34, 349–359. [Google Scholar]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2012, 35, 1039–1050. [Google Scholar] [CrossRef]
- Hussein, M.; Embiale, A.; Husen, A.; Arem, I.M.; Iqbal, M. Salinity-induced modulation of plant growth and photosynthetic parameters in faba bean (Vicia faba) cultivars. Pak. J. Bot. 2017, 49, 867–877. [Google Scholar]
- Shahriaripour, R.; Pour, A.T.; Mozaffari, V.; Dashti, H.; Adhami, E. Effects of salinity and soil zinc application on growth and chemical composition of pistachio seedlings. J. Plant Nutr. 2010, 33, 1166–1179. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahemi, M.; Maftoun, M.; Panahi, B.; Karimi, S.; Ramezanian, A.; Vaezpour, M. Zinc influence and salt stress on photosynthesis, water relations, and carbonic anhydrase activity in pistachio. Sci. Hortic. 2009, 123, 272–279. [Google Scholar] [CrossRef]
- Galal, A. Exogenous application of zinc mitigates the deleterious effects in eggplant grown under salinity stress. J. Plant Nutr. 2019, 42, 915–927. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Abebe, T.; Guenzi, A.C.; Martin, B.; Cushman, J.C. Tolerance of Mannitol-Accumulating Transgenic Wheat to Water Stress and Salinity. Plant Physiol. 2003, 131, 1748–1755. [Google Scholar] [CrossRef]
- Shah, W.H.; Rasool, A.; Tahir, I.; Rehman, R.U. Exogenously applied selenium (Se) mitigates the impact of salt stress in Setaria italica L. and Panicum miliaceum L. Nucleus 2020, 63, 327–339. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Yuan, Y.-Z.; Ou, J.-Q.; Lin, Q.-H.; Zhang, C.-F. Glutamine synthetase and glutamate dehydrogenase contribute differentially to proline accumulation in leaves of wheat (Triticum aestivum) seedlings exposed to different salinity. J. Plant Physiol. 2007, 164, 695–701. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: Berlin, Germany, 2014; pp. 25–55. [Google Scholar]
- Ahanger, M.A.; Agarwal, R.M.; Tomar, N.S.; Shrivastava, M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent). J. Plant Interact. 2015, 10, 211–223. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.-S.P. Alleviation of Cadmium Toxicity in Brassica juncea L. (Czern. & Coss.) by Calcium Application Involves Various Physiological and Biochemical Strategies. PLoS ONE 2015, 10, e0114571. [Google Scholar] [CrossRef]
- Ahmad, P.; Latef, A.A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.-S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, C.S.; Suryanarayana, T.; Reddy, A.R. DNA helix destabilization by proline and betaine: Possible role in the salinity tolerance process. FEBS Lett. 1997, 410, 201–205. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Aziz, A.; Akram, N.A.; Ashraf, M. Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary me-tabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem. 2018, 123, 192–203. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Brini, F. Comparison of an antioxidant system in tolerant and susceptible wheat seedlings in response to salt stress. Span. J. Agric. Res. 2018, 15, e0805. [Google Scholar] [CrossRef]
- Iqbal, M.N.; Rasheed, R.; Ashraf, M.A.; Hussain, I. Exogenously applied zinc and copper mitigate salinity effect in maize (Zea mays L.) by improving key physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2018, 25, 23883–23896. [Google Scholar] [CrossRef]
- Amiri, A.; Baninasab, B.; Ghobadi, C.; Khoshgoftarmanesh, A.H. Zinc soil application enhances photosynthetic capacity and antioxidant enzyme activities in almond seedlings affected by salinity stress. Photosynthetica 2016, 54, 267–274. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of nano-particles of FeSO4 on the growth and ion content of sunflower under saline condition. J. Plant Nutr. 2017, 40, 615–623. [Google Scholar] [CrossRef]
- Mallik, S.; Nayak, M.; Sahu, B.B.; Panigrahi, A.K.; Shaw, B.P. Response of antioxidant enzymes to high NaCl concentration in different salt-tolerant plants. Biol. Plant. 2011, 55, 191–195. [Google Scholar] [CrossRef]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot. 2004, 55, 1105–1113. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Fariduddin, Q. Salt-induced modulation in growth, photosynthesis and antioxidant system in two varieties of Brassica juncea. Saudi J. Biol. Sci. 2013, 20, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Najami, N.; Janda, T.; Barriah, W.; Kayam, G.; Tal, M.; Guy, M.; Volokita, M. Ascorbate peroxidase gene family in tomato: Its identification and characterization. Mol. Genet. Genom. 2007, 279, 171–182. [Google Scholar] [CrossRef]
- Abbaspour, H. Effect of salt stress on lipid peroxidation, antioxidative enzymes, and proline accumulation in pistachio plants. J. Med. Plants Res. 2012, 6, 526–529. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No. 111: Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Dixon, D.P.; Skipsey, M.; Edwards, R. Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 2010, 71, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Gapińska, M.; Skłodowska, M.; Gabara, B. Effect of short- and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol. Plant. 2007, 30, 11–18. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family–an evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.-Z.; Zhao, L.; Yin, L.J.; Chen, M.; Wang, Q.Y.; Li, L.C.; Xu, Z.S.; Ma, Y.Z. Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis. PLoS ONE 2013, 8, e73989. [Google Scholar] [CrossRef]
- Passaia, G.; Fonini, L.S.; Caverzan, A.; Jardim-Messeder, D.; Christoff, A.P.; Gaeta, M.L.; Mariath, J.E.D.A.; Margis, R.; Margis-Pinheiro, M. The mitochondrial glutathione peroxidase GPX3 is essential for H2O2 homeostasis and root and shoot development in rice. Plant Sci. 2013, 208, 93–101. [Google Scholar] [CrossRef]
- Passaia, G.; Caverzan, A.; Fonini, L.S.; Carvalho, F.E.L.; Silveira, J.A.G.; Margis-Pinheiro, M. Chloroplastic and mitochondrial GPX genes play a critical role in rice development. Biol. Plant. 2014, 58, 375–378. [Google Scholar] [CrossRef]
- Yamane, K.; Mitsuya, S.; Kawasaki, M.; Taniguchi, M.; Miyake, H. Antioxidant Capacity and Damages Caused by Salinity Stress in Apical and Basal Regions of Rice Leaf. Plant Prod. Sci. 2009, 12, 319–326. [Google Scholar] [CrossRef]
- Rao, A.S.V.C.; Reddy, A.R. Glutathione Reductase: A Putative Redox Regulatory System in Plant Cells. In Sulfur Assimilation and Abiotic Stress in Plants; Springer: Berlin, Germany, 2008; pp. 111–147. [Google Scholar]
- Rao, K.; Raghavendra, A.; Reddy, K. Physiology and Molecular Biology of Stress Tolerance; Springer: Berlin, Germany, 2006. [Google Scholar]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Saeidnejad, A.H.; Kafi, M.; Pessarakli, M. Interactive Effects of Salinity Stress and Zn Availability on Physiological Properties, Antioxidants Activity and Micronutrients’ Content of Wheat (Triticum aestivum) plants. Commun. Soil Sci. Plant Anal. 2016, 47, 1048–1057. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Neto, A.D.D.A.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crop. Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.J.; D’Cunha, G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Bačkor, M. Phenylalanine Ammonia-Lyase and Phenolic Compounds in Chamomile Tolerance to Cadmium and Copper Excess. Water Air Soil Pollut. 2007, 185, 185–193. [Google Scholar] [CrossRef]
- Luo, Z.-B.; He, X.-J.; Chen, L.; Tang, L. Effects of zinc on growth and antioxidant responses in Jatropha curcas seedlings. Int. J. Agric. Biol. 2010, 12, 119–124. [Google Scholar]
- Maldonado, R.; Goñi, O.; Escribano, M.; Merodio, C. Regulation of phenylalanine ammonia-lyase enzyme in annona fruit: Kinetic characteristics and inhibitory effect of ammonia. J. Food Biochem. 2007, 31, 161–178. [Google Scholar] [CrossRef][Green Version]
- Gholizadeh, A. Effects of drought on the activity of phenylalanine ammonia lyase in the leaves and roots of maize inbreds. Aust. J. Basic Appl. Sci. 2011, 5, 952–956. [Google Scholar]
- Koç, E.; İşlek, C.; Büyükkartal, H.N. Comparasion of phenylalanine ammonia lyase response to lead and zinc stress in different wheat genotypes. Commun. Fac. Sci. Univ. Ank. Ser. C 2018, 27, 37–44. [Google Scholar]
- Wadhwa, N.; Joshi, U.N.; Mehta, N. Zinc Induced Enzymatic Defense Mechanisms in Rhizoctonia Root Rot Infected Clusterbean Seedlings. J. Bot. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under s alinity stress. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, J.; Yanishlieva, N.; Gordon, M.H. Antioxidants in Food: Practical Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under Moderate Salinity Increases Phenolic Content and Antioxidant Activity in Rapeseed (Brassica napus var oleifera Del.) Sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Primary, Secondary Metabolites, Photosynthetic Capacity and Antioxidant Activity of the Malaysian Herb Kacip Fatimah (Labisia Pumila Benth) Exposed to Potassium Fertilization under Greenhouse Conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A.; Wahab, P.E.M.; Halim, M.R.A. Effect of Different Light Intensities on Total Phenolics and Flavonoids Synthesis and Anti-oxidant Activities in Young Ginger Varieties (Zingiber officinale Roscoe). Int. J. Mol. Sci. 2010, 11, 3885–3897. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M. In vitro evaluation of phenolic and osmolite compounds, ionic content, and antioxidant activity in safflower (Carthamus tinctorius L.) under salinity stress. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 357–368. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Sharma, V.; Ramawat, K.G. Salt stress enhanced antioxidant response in callus of three halophytes (Salsola baryosma, Trianthema triquetra, Zygophyllum simplex) of Thar Desert. Biologia 2014, 69, 178–185. [Google Scholar] [CrossRef]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). BioMed Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri-Tirani, M.; Dayani, S. In vitro effect of zinc oxide nanoparticles on Nicotiana tabacum callus compared to ZnO micro particles and zinc sulfate (ZnSO4). Plant Cell Tissue Organ Cult. (PCTOC) 2019, 140, 279–289. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008, 107, 1413–1420. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-culture Method for Growing Plants without Soil. Californian Agricultural Experimental Station. Circular No. 347; University of California: Berkeley, CA, USA, 1950. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Orlando, FL, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Tabot, P.; Adams, J. Early responses of Bassia diffusa (Thunb.) Kuntze to submergence for different salinity treatments. South Afr. J. Bot. 2013, 84, 19–29. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Dey, P.M. Oligosaccharides. In Methods in Plant Biochemistry; Elsevier: Amsterdam, The Netherlands, 1990; pp. 189–218. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-Transferases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1981; pp. 398–405. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Haard, N.F.; Wasserman, B. Induction of phenylalanine ammonia lyase in sweet potato (Ipomoea batatas) root by light irradiation and black rot infection. Physiol. Plant Pathol. 1976, 8, 207–213. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Moon, J.-H.; Terao, J. Antioxidant Activity of Caffeic Acid and Dihydrocaffeic Acid in Lard and Human Low-Density Lipoprotein†. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Bahramian, F.; Bekhradnia, A.R. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 2010, 23, 29–34. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahrani, H.S.; Alharby, H.F.; Hakeem, K.R.; Rehman, R.U. Exogenous Application of Zinc to Mitigate the Salt Stress in Vigna radiata (L.) Wilczek—Evaluation of Physiological and Biochemical Processes. Plants 2021, 10, 1005. https://doi.org/10.3390/plants10051005
Al-Zahrani HS, Alharby HF, Hakeem KR, Rehman RU. Exogenous Application of Zinc to Mitigate the Salt Stress in Vigna radiata (L.) Wilczek—Evaluation of Physiological and Biochemical Processes. Plants. 2021; 10(5):1005. https://doi.org/10.3390/plants10051005
Chicago/Turabian StyleAl-Zahrani, Hassan S., Hesham F. Alharby, Khalid Rehman Hakeem, and Reiaz Ul Rehman. 2021. "Exogenous Application of Zinc to Mitigate the Salt Stress in Vigna radiata (L.) Wilczek—Evaluation of Physiological and Biochemical Processes" Plants 10, no. 5: 1005. https://doi.org/10.3390/plants10051005
APA StyleAl-Zahrani, H. S., Alharby, H. F., Hakeem, K. R., & Rehman, R. U. (2021). Exogenous Application of Zinc to Mitigate the Salt Stress in Vigna radiata (L.) Wilczek—Evaluation of Physiological and Biochemical Processes. Plants, 10(5), 1005. https://doi.org/10.3390/plants10051005






