Shade Avoidance and Light Foraging of a Clonal Woody Species, Pachysandra terminalis
Abstract
1. Introduction
2. Results
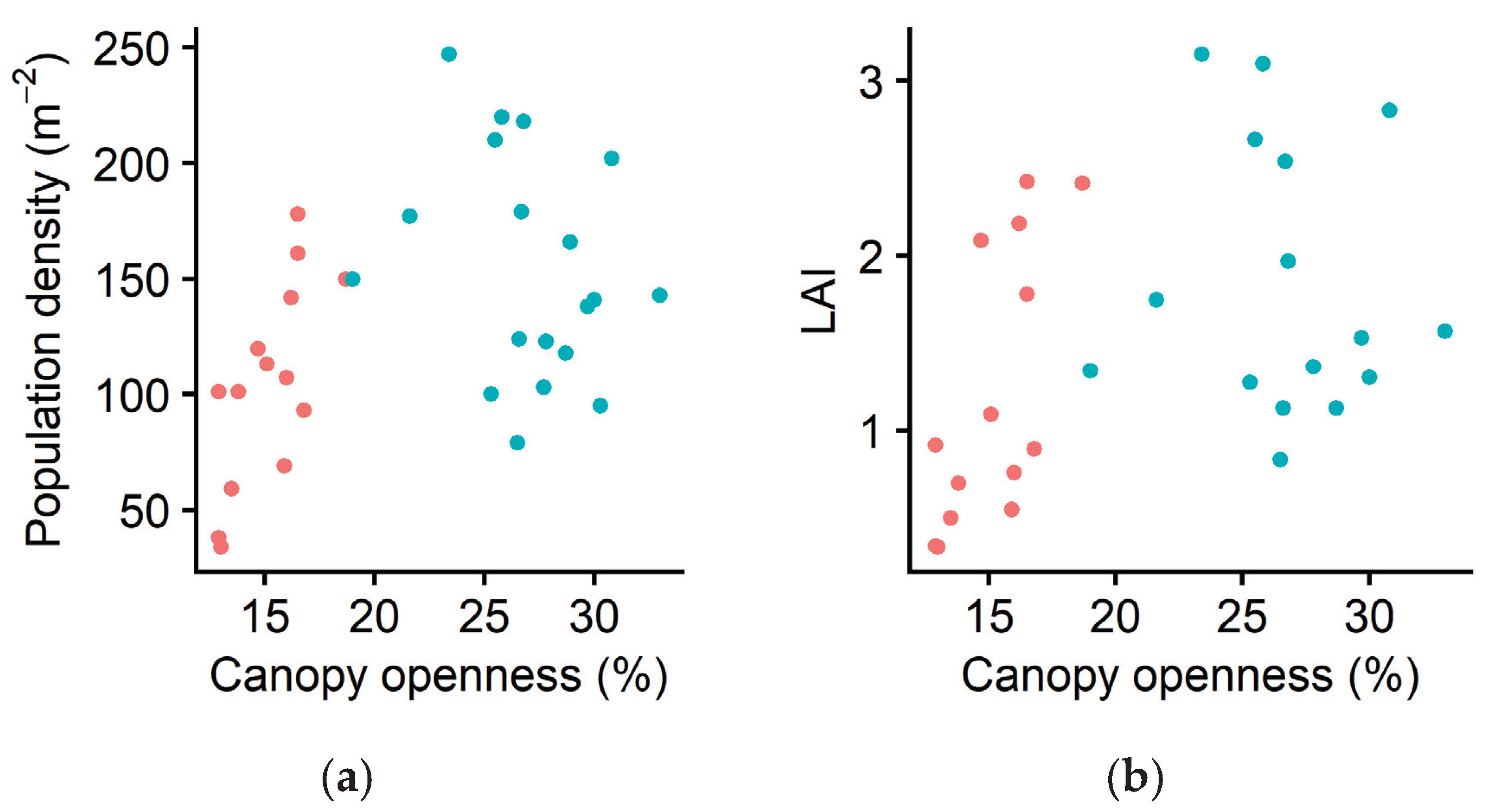
2.1. Ramet Population Density
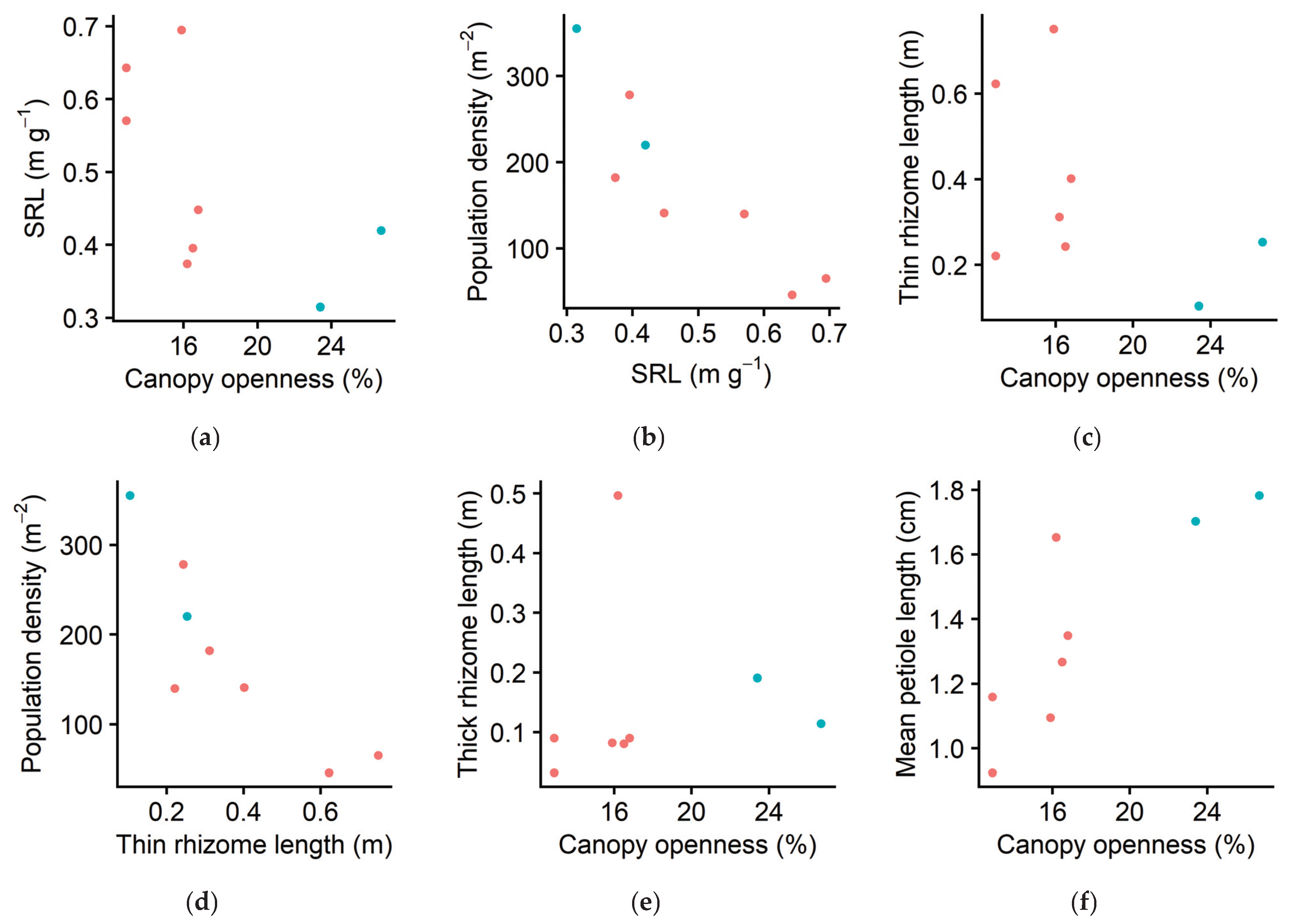
2.2. Morphological Plasticity
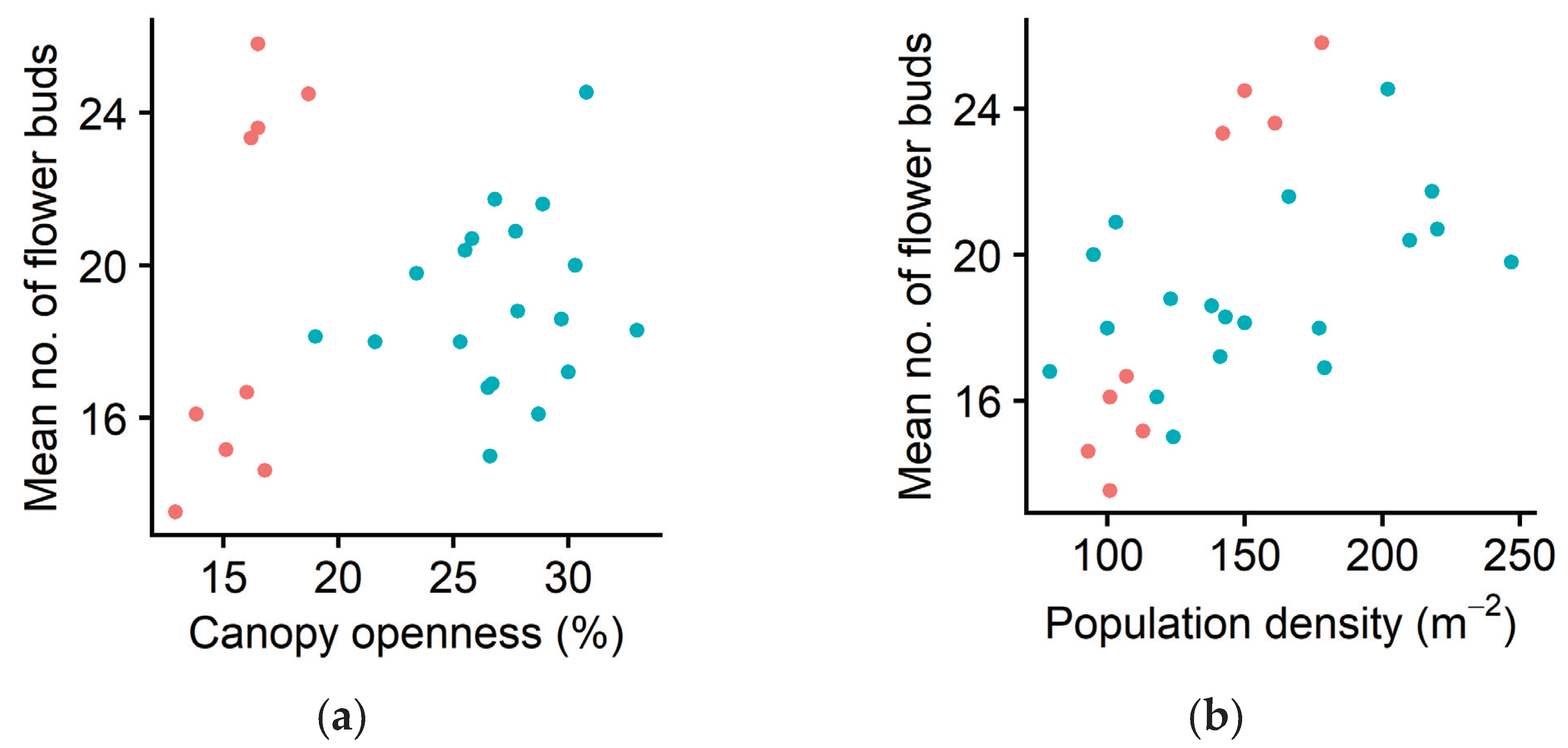
2.3. Sexual Reproduction
3. Discussion
4. Materials and Methods
4.1. Study Sites
4.2. Sampling Strategy
4.3. Measurement of Canopy Openness
4.4. Ramet Population Density in Spring and Sexual Reproduction
4.5. Measurement of LAI
4.6. Measurement of Rhizome Length and Ramet Population Density in Autumn
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrahamson, W.G. Reproductive strategies in dewberries. Ecology 1975, 56, 721–726. [Google Scholar] [CrossRef]
- Alpert, P.; Stuefer, J.F. Division of labour in clonal plants. In The Ecology and Evolution of Clonal Plants; de Kroon, H., van Groenendael, J., Eds.; Backhuys: Leiden, Netherlands, 1997; pp. 137–154. [Google Scholar]
- Radosavljević, I.; Antonić, O.; Hruševar, D.; Križan, J.; Satovic, Z.; Turković, D.; Liber, Z. The influence of a seedling recruitment strategy and a clonal architecture on a spatial genetic structure of a Salvia brachyodon (Lamiaceae) population. Plants 2020, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- De Kroon, H.; Huber, H.; Stuefer, J.F.; Van Groenendael, J.M. A modular concept of phenotypic plasticity in plants. New Phytol. 2005, 166, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, H.; Yang, Y. Physiological integration increases sexual reproductive performance of the rhizomatous grass Hierochloe glabra. Plants 2020, 9, 1608. [Google Scholar] [CrossRef]
- Vallejo-Marín, M.; Dorken, M.E.; Barrett, S.C.H. The ecological and evolutionary consequences of clonality for plant mating. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 193–213. [Google Scholar] [CrossRef]
- Slade, A.J.; Hutchings, M.J. The effects of light intensity on foraging in the clonal herb Glechoma hederacea. J. Ecol. 1987, 75, 639–650. [Google Scholar] [CrossRef]
- Slade, A.J.; Hutchings, M.J. The effects of nutrient availability on foraging in the clonal herb Glechoma hederacea. J. Ecol. 1987, 75, 95–112. [Google Scholar] [CrossRef]
- Evans, J.P.; Cain, M.L. A spatially explicit test of foraging behavior in a clonal plant. Ecology 1995, 76, 1147–1155. [Google Scholar] [CrossRef]
- Dong, M. Morphological responses to local light conditions in clonal herbs from contrasting habitats, and their modification due to physiological integration. Oecologia 1995, 101, 282–288. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Retuerto, R. Small-scale heterogeneity in soil quality influences photosynthetic efficiency and habitat selection in a clonal plant. Ann. Bot. 2006, 98, 1043–1052. [Google Scholar] [CrossRef]
- Xie, K.; Zhao, X.; Zhang, Y.; Dong, K.; Fenghe, L.; LI, X. Growth characteristics of Potentilla anserina determined by analyzing small-scale patchy habitats. Pak. J. Bot. 2015, 47, 967–978. [Google Scholar]
- Tomimatsu, H.; Matsuo, A.; Kaneko, Y.; Kudo, E.; Taniguchi, R.; Saitoh, T.; Suyama, Y.; Makita, A. Spatial genet dynamics of a dwarf bamboo: Clonal expansion into shaded forest understory contributes to regeneration after an episodic die-off. Plant Spec. Biol. 2020, 35, 185–196. [Google Scholar] [CrossRef]
- Wang, J.; Xu, T.; Wang, Y.; Li, G.; Abdullah, I.; Zhong, Z.; Liu, J.; Zhu, W.; Wang, L.; Wang, D.; et al. A meta-analysis of effects of physiological integration in clonal plants under homogeneous vs. heterogeneous environments. Funct. Ecol. 2021, 35, 578–589. [Google Scholar] [CrossRef]
- Jing, X.; Cai, C.; Fan, S.; Liu, G.; Wu, C.; Chen, B. Effects of rhizome integration on the water physiology of Phyllostachys edulis clones under heterogeneous water stress. Plants 2020, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Eilts, J.A.; Mittelbach, G.G.; Reynolds, H.L.; Gross, K.L. Resource heterogeneity, soil fertility, and species diversity: Effects of clonal species on plant communities. Am. Nat. 2011, 177, 574–588. [Google Scholar] [CrossRef]
- Portela, R.; Barreiro, R.; Roiloa, S.R. Biomass partitioning in response to resources availability: A comparison between native and invaded ranges in the clonal invader Carpobrotus edulis. Plant Spec. Biol. 2019, 34, 11–18. [Google Scholar] [CrossRef]
- Martin, F.-M. Clonal growth strategies of Reynoutria japonica in response to light, shade, and mowing, and perspectives for management. NeoBiota 2020, 56, 89–110. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Rodriguez-Echeverria, S.; Lopez-Otero, A.; Retuerto, R.; Freitas, H. Adaptive plasticity to heterogeneous environments increases capacity for division of labor in the clonal invader Carpobrotus edulis (Aizoaceae). Am. J. Bot. 2014, 101, 1301–1308. [Google Scholar] [CrossRef]
- Cole, P.G.; Weltzin, J.F. Light limitation creates patchy distribution of an invasive grass in eastern deciduous forests. Biol Invasions 2005, 7, 477–488. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Herben, T. Horizontal growth: An overlooked dimension in plant trait space. Perspect. Plant Ecol. Evol. Syst. 2018, 32, 18–21. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, Z.X.; Cui, L.J.; Zou, C.L. Adaptive significance of and factors affecting plasticity of biomass allocation and rhizome morphology: A case study of the clonal plant Scirpus planiculmis (Cyperaceae). Pol. J. Ecol. 2014, 62, 77–88. [Google Scholar] [CrossRef]
- Ruiz-Reynés, D.; Martín, L.; Hernández-García, E.; Knobloch, E.; Gomila, D. Patterns, localized structures and fronts in a reduced model of clonal plant growth. Physica D 2020, 414, 132723. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Fischer, M.; Schmid, B. Effects of intraspecific competition on size variation and reproductive allocation in a clonal plant. Oikos 2001, 94, 515–524. [Google Scholar] [CrossRef]
- Bakacsy, L.; Bagi, I. Survival and regeneration ability of clonal common milkweed (Asclepias syriaca L.) after a single herbicide treatment in natural open sand grasslands. Sci. Rep. 2020, 10, 14222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Chen, D.; Yan, R.; Yu, F.-H.; van Kleunen, M. Invasive alien clonal plants are competitively superior over co-occurring native clonal plants. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125484. [Google Scholar] [CrossRef]
- O’Connor, R.C.; Taylor, J.H.; Nippert, J.B. Browsing and fire decreases dominance of a resprouting shrub in woody encroached grassland. Ecology 2020, 101, e02935. [Google Scholar] [CrossRef] [PubMed]
- Keser, L.H.; Dawson, W.; Song, Y.B.; Yu, F.H.; Fischer, M.; Dong, M.; van Kleunen, M. Invasive clonal plant species have a greater root-foraging plasticity than non-invasive ones. Oecologia 2014, 174, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, P.; Zhao, Z.; Li, Z. Clonal growth of Populus pruinosa Schrenk and its role in the regeneration of riparian forests. Ecol. Eng. 2016, 94, 380–392. [Google Scholar] [CrossRef]
- Colleran, B.; Lacy, S.N.; Retamal, M.R. Invasive Japanese knotweed (Reynoutria japonica Houtt.) and related knotweeds as catalysts for streambank erosion. River Res. Appl. 2020, 36, 1962–1969. [Google Scholar] [CrossRef]
- Martin, F.-M.; Dommanget, F.; Evette, A. Improving the management of Japanese knotweed s.l.: A response to Jones and colleagues. NeoBiota 2020, 63, 147–153. [Google Scholar] [CrossRef]
- Deguchi, R.; Koyama, K. Photosynthetic and morphological acclimation to high and low light environments in Petasites japonicus subsp. giganteus. Forests 2020, 11, 1365. [Google Scholar] [CrossRef]
- Chazdon, R.L. Sunflecks and their importance to forest understorey plants. In Advances in Ecological Research; Begon, M., Fitter, A.H., Ford, E.D., Macfadyen, A., Eds.; Academic Press: London, UK, 1988; Volume 18, pp. 1–63. [Google Scholar]
- Morales, A.; Kaiser, E. Photosynthetic acclimation to fluctuating irradiance in plants. Front. Plant Sci. 2020, 11, 268. [Google Scholar] [CrossRef]
- Parker, G.G.; Fitzjarrald, D.R.; Gonçalves Sampaio, I.C. Consequences of environmental heterogeneity for the photosynthetic light environment of a tropical forest. Agr. Forest Meteorol. 2019, 278, 107661. [Google Scholar] [CrossRef]
- Hartikainen, S.M.; Pieristè, M.; Lassila, J.; Robson, T.M. Seasonal patterns in spectral irradiance and leaf UV-A absorbance under forest canopies. Front. Plant Sci. 2020, 10. [Google Scholar] [CrossRef]
- Miyashita, A.; Sugiura, D.; Sawakami, K.; Ichihashi, R.; Tani, T.; Tateno, M. Long-term, short-interval measurements of the frequency distributions of the photosynthetically active photon flux density and net assimilation rate of leaves in a cool-temperate forest. Agr. For. Meteorol. 2012, 152, 1–10. [Google Scholar] [CrossRef]
- Huber, H.; Wiggerman, L. Shade avoidance in the clonal herb Trifolium fragiferum: A field study with experimentally manipulated vegetation height. Plant Ecol. 1997, 130, 53–62. [Google Scholar] [CrossRef]
- Min, B.-M. Changes in resource allocation among vegetative organs during the clonal growth of Polygonatum humile (Liliaceae) grown in a temperate forest gap. J. Ecol. Environ. 2017, 41, 30. [Google Scholar] [CrossRef]
- Birch, C.P.D.; Hutchings, M.J. Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. J. Ecol. 1994, 82, 653–664. [Google Scholar] [CrossRef]
- Waite, S. Field evidence of plastic growth responses to habitat heterogeneity in the clonal herb Ranunculus repens. Ecol. Res. 1994, 9, 311–316. [Google Scholar] [CrossRef]
- Huber, H.; Fijan, A.; During, H.J. A comparative study of spacer plasticity in erect and stoloniferous herbs. Oikos 1998, 81, 576–586. [Google Scholar] [CrossRef]
- Takenaka, A. Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot. Ecol. Res. 1994, 9, 109–114. [Google Scholar] [CrossRef]
- Huber, H.; de Brouwer, J.; de Caluwe, H.; Wijschede, J.; Anten, N.P.R. Shade induced changes in biomechanical petiole properties in the stoloniferous herb Trifolium repens. Evol. Ecol. 2008, 22, 399–417. [Google Scholar] [CrossRef]
- Huber, H.; Stuefer, J.F. Shade-induced changes in the branching pattern of a stoloniferous herb: Functional response or allometric effect? Oecologia 1997, 110, 478–486. [Google Scholar] [CrossRef]
- Huber, H. Plasticity of internodes and petioles in postrate and erect Potentilla species. Funct. Ecol. 1996, 10, 401–409. [Google Scholar] [CrossRef]
- Wijesinghe, D.K.; Hutchings, M.J. Consequences of patchy distribution of light for the growth of the clonal herb Glechoma hederacea. Oikos 1996, 77, 137–145. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Tammaru, K.; Košnar, J.; Abbas, A.F.; Barta, K.A.; de Bello, F.; Harrison, S.; Degli, E.I.; Kiss, R.; Lukács, K.; Neumann, S.M.; et al. Ecological differentiation of Carex species coexisting in a wet meadow: Comparison of pot and field experiments. Acta Oecol. 2021, 110, 103692. [Google Scholar] [CrossRef]
- Kitajima, K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 1994, 98, 419–428. [Google Scholar] [CrossRef]
- Onoda, Y.; Schieving, F.; Anten, N.P. Effects of light and nutrient availability on leaf mechanical properties of Plantago major: A conceptual approach. Ann. Bot. 2008, 101, 727–736. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, Y.; Zhang, M.; Hong, A.; Yang, H.; Liu, Y. Shade effects on growth, photosynthesis and chlorophyll fluorescence parameters of three Paeonia species. PeerJ 2020, 8, e9316. [Google Scholar] [CrossRef]
- Jeong, H.H.; Kim, K.S. Effects of shading on the growth of Hedera rhombea Bean and Pachysandra terminalis Sieb. et Zucc. Korea J. Hort. Sci. Tech. 1999, 17, 29–32. [Google Scholar]
- Lee, J.S.; Jeong, S.J.; Heo, J.A.; Kang, H.J.; Hwang, S.Y.; Kim, Y.-K. Light intensity levels and growth inhibitors on growth of shade tolerant Japanese spurge (Pachysandra terminalis). J. Korea Soc. Hort. Sci. 2002, 43, 137–142. [Google Scholar]
- Lovett Doust, J.; Lovett Doust, L. Modules of production and reproduction in a dioecious clonal shrub, Rhus typhina. Ecology 1988, 69, 741–750. [Google Scholar] [CrossRef]
- Yakimowski, S.B.; Eckert, C.G. Threatened peripheral populations in context: Geographical variation in population frequency and size and sexual reproduction in a clonal woody shrub. Conserv. Biol. 2007, 21, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Würth, D.G.; Eusemann, P.; Trouillier, M.; Buras, A.; Burger, A.; Wilmking, M.; Roland, C.A.; Juday, G.P.; Schnittler, M. Environment drives spatiotemporal patterns of clonality in white spruce (Picea glauca) in Alaska. Can. J. For. Res. 2018, 48, 1577–1586. [Google Scholar] [CrossRef]
- Isogimi, T.; Matsushita, M.; Nakagawa, M. Species-specific sprouting pattern in two dioecious Lindera shrubs: The role of physiological integration. Flora 2014, 209, 718–724. [Google Scholar] [CrossRef]
- Hosaka, N.; Kachi, N.; Kudoh, H.; Stuefer, J.F.; Whigham, D.F. Compensatory growth of the clonal understory tree, Asimina triloba, in response to small-scale disturbances. Plant Ecol. 2016, 217, 471–480. [Google Scholar] [CrossRef]
- Kimura, M.K.; Nagashima, T.; Kamitani, T.; Sakio, H.; Tsumura, Y. Recent clonal reproduction of Cryptomeria japonica in a snowy region revealed by a survey of small-sized ramets. Silv. Gen. 2020, 69, 152–157. [Google Scholar] [CrossRef]
- Yaegashi, S.; Omura, T.; Watanabe, K. Spatial genetic structure of the invasive tree Robinia pseudoacacia to determine migration patterns to inform best practices for riparian restoration. AoB Plants 2020, 12, plaa043. [Google Scholar] [CrossRef]
- Nakamura, M.; Nanami, S.; Okuno, S.; Hirota, S.K.; Matsuo, A.; Suyama, Y.; Tokumoto, H.; Yoshihara, S.; Itoh, A. Genetic diversity and structure of apomictic and sexually reproducing Lindera species (Lauraceae) in Japan. Forests 2021, 12, 227. [Google Scholar] [CrossRef]
- Ohashi, H.; Kadota, Y.; Murata, J.; Yonekura, K.; Kihara, H. Wild flowers of Japan; Heibonsha: Tokyo, Japan, 2015. [Google Scholar]
- Ishioka, R.; Muller, O.; Hiura, T.; Kudo, G. Responses of leafing phenology and photosynthesis to soil warming in forest-floor plants. Acta Oecologica 2013, 51, 34–41. [Google Scholar] [CrossRef]
- Yoshie, F.; Arai, H.; Nakashima, H.; Kawano, S. Seasonal changes in nitrogen fractions of Pachysandra terminalis, a forest evergreen chamaephyte. Physiol. Plant. 1990, 79, 7–14. [Google Scholar] [CrossRef]
- Zhou, S.; Sauvé, R.J.; Mmbaga, M.T. Adaptation of Pachysandra terminalis Sieb. & Zucc. to freezing temperatures by the accumulation of mRNA and cold-induced proteins. HortScience 2005, 40, 346. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, J. Phylogenetics and biogeography of eastern Asian–North American disjunct genus Pachysandra (Buxaceae) inferred from nucleotide sequences. J. Syst. Evol. 2009, 47, 191–201. [Google Scholar] [CrossRef]
- Jeong, M.I.; Jeong, N.R.; Han, S.W.; Kim, J.S. Analyzing growth reactions of herbaceous plants for irrigation management. J. People Plants Environ. 2020, 23, 255–265. [Google Scholar] [CrossRef]
- Ntawuhiganayo, E.B.; Uwizeye, F.K.; Zibera, E.; Dusenge, M.E.; Ziegler, C.; Ntirugulirwa, B.; Nsabimana, D.; Wallin, G.; Uddling, J. Traits controlling shade tolerance in tropical montane trees. Tree Physiol. 2020, 40, 183–197. [Google Scholar] [CrossRef]
- Dos Santos, V.A.H.F.; Ferreira, M.J. Are photosynthetic leaf traits related to the first-year growth of tropical tree seedlings? A light-induced plasticity test in a secondary forest enrichment planting. For. Ecol. Manage. 2020, 460, 117900. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Ntagkas, N.; Siebenkas, A.; Maenpaa, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, A.; Tateno, M. A novel index of leaf RGR predicts tree shade tolerance. Funct. Ecol. 2014, 28, 1321–1329. [Google Scholar] [CrossRef]
- Pons, T.L.; Poorter, H. The effect of irradiance on the carbon balance and tissue characteristics of five herbaceous species differing in shade-tolerance. Front. Plant Sci. 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U.; Portsmuth, A.; Tena, D.; Tobias, M.; Matesanz, S.; Valladares, F. Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Ann. Bot. 2007, 100, 283–303. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Portsmuth, A.; Tobias, M. Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytol. 2006, 171, 91–104. [Google Scholar] [CrossRef]
- Schmid, B.; Harper, J.L. Clonal growth in grassland perennials: I. Density and pattern-dependent competition between plants with different growth forms. J. Ecol. 1985, 73, 793–808. [Google Scholar] [CrossRef]
- Doust, L.L. Population dynamics and local specialization in a clonal perennial (Ranunculus repens): I. The dynamics of ramets in contrasting habitats. J. Ecol. 1981, 69, 743–755. [Google Scholar] [CrossRef]
- Komamura, R.; Koyama, K.; Yamauchi, T.; Konno, Y.; Gu, L. Pollination contribution differs among insects visiting Cardiocrinum cordatum flowers. Forests 2021, 12, 452. [Google Scholar] [CrossRef]
- Rostás, M.; Bollmann, F.; Saville, D.; Riedel, M. Ants contribute to pollination but not to reproduction in a rare calcareous grassland forb. PeerJ 2018, 6, e4369. [Google Scholar] [CrossRef] [PubMed]
- Al-Qthanin, R.N.; Alharbi, S.A. Spatial structure and genetic variation of a mangrove species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago. Forests 2020, 11. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: https://www.jma.go.jp (accessed on 14 September 2020).
- Anderson, M.C. Studies of the woodland light climate: I. The photographic computation of light conditions. J. Ecol. 1964, 52, 27–41. [Google Scholar] [CrossRef]
- Takenaka, A. CanopOn 2 ver. 2.03c. Available online: http://takenaka-akio.org/etc/canopon2/ (accessed on 26 September 2020).
- Takenaka, A.; Inui, Y.; Osawa, A. Measurement of three-dimensional structure of plants with a simple device and estimation of light capture of individual leaves. Funct. Ecol. 1998, 12, 159–165. [Google Scholar] [CrossRef]
- Yoshie, F.; Kawano, S. Seasonal changes in photosynthetic characteristics of Pachysandra terminalis (Buxaceae), an evergreen woodland chamaephyte, in the cool temperate regions of Japan. Oecologia 1986, 71, 6–11. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. CRAN Repos 2016, 2, R2. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Alcántara, J.M.; Rey, P.J. Conflicting selection pressures on seed size: Evolutionary ecology of fruit size in a bird-dispersed tree, Olea europaea. J. Evol. Biol. 2003, 16, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Shirakawa, H.; Kikuzawa, K. Redeployment of shoots into better-lit positions within the crowns of saplings of five species with different growth patterns. Forests 2020, 11, 1301. [Google Scholar] [CrossRef]
- Staab, M.; Pereira-Peixoto, M.H.; Klein, A.-M. Exotic garden plants partly substitute for native plants as resources for pollinators when native plants become seasonally scarce. Oecologia 2020, 194, 465–480. [Google Scholar] [CrossRef]
- Koyama, K.; Tashiro, M. No effect of selective maturation on fruit traits for a bird-dispersed species, Sambucus racemosa. Plants 2021, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Case, S.B.; Tarwater, C.E. Functional traits of avian frugivores have shifted following species extinction and introduction in the Hawaiian Islands. Funct. Ecol. 2020, 34, 2467–2476. [Google Scholar] [CrossRef]
- Barr, D.J.; Levy, R.; Scheepers, C.; Tily, H.J. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 2013, 68, 255–278. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwabe, R.; Koyama, K.; Komamura, R. Shade Avoidance and Light Foraging of a Clonal Woody Species, Pachysandra terminalis. Plants 2021, 10, 809. https://doi.org/10.3390/plants10040809
Iwabe R, Koyama K, Komamura R. Shade Avoidance and Light Foraging of a Clonal Woody Species, Pachysandra terminalis. Plants. 2021; 10(4):809. https://doi.org/10.3390/plants10040809
Chicago/Turabian StyleIwabe, Risa, Kohei Koyama, and Riko Komamura. 2021. "Shade Avoidance and Light Foraging of a Clonal Woody Species, Pachysandra terminalis" Plants 10, no. 4: 809. https://doi.org/10.3390/plants10040809
APA StyleIwabe, R., Koyama, K., & Komamura, R. (2021). Shade Avoidance and Light Foraging of a Clonal Woody Species, Pachysandra terminalis. Plants, 10(4), 809. https://doi.org/10.3390/plants10040809





